BIOL 216 Exam 2
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
78 Terms
How can we study the brain to determine behaviour?
by looking at the mechanisms and neurophysiology
What is methodological reductionism?
understanding a system by analyzing its constituent parts
what are the three components of methodological reductionism?
decomposition: taking something apart to understand what parts it has
internal causes: investigating relationships between parts while simplifying the broader environment
isolating components: studying the features and properties of the parts individually or in limited arrangements
what is the problem with methodological reductionism in neuroscience?
it can be mistaken for explanatory reductionism; interaction of components does not mean mechanism
what are possible explanations for phylogenetic trees having organisms that do not have nervous systems coming after ones that do have them?
nervous system evolved twice, evolutionary reversals, or the phylogeny is wrong
do you need a nervous system to have behaviour?
no, single-celled organisms can show behaviour too
organisms without brains
have less specialized neurons that can detect many things (instead of highly specialized neurons)
two principle cell types in brain
neurons and neuroglia
ways to define neurons
shape (unipolar, bipolar, multipolar), connectivity (sensory neurons, motor neurons, interneurons), neurochemistry (glutamatergic, dopaminergic, cholinergic)
what is the direction of signal flow in neurons?
from dendrite/soma to axon
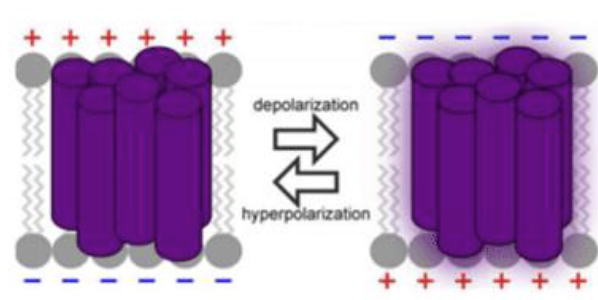
is the inside of the neuron more positive or more negative than the outside?
negative
what is resting membrane potential of a neuron?
-70 mV
what is the threshold potential of a neuron?
-55 mV
what is the potential when the neuron repolarizes?
+40 mV
what are the cations that contribute to resting potential?
K+, Na+, Ca2+
what are the anions that contribute to resting potential?
Cl-, OA- (organic acids, proteins)
ion channels
Determine the permeability of the membrane to each ion:
Selectivity
K+ channel
Cation channel
Gating
Leak channels (always open)
Ligand gatedLigand-gated
Mechanically gated
What are the forces that control the flow of ions?
diffusion and electrical force (and goes until the electrical force is equal to the diffusion force)
ion permeability and membrane potential
the ions that are more permeable have a larger effect on the membrane potential
Nernst Equation
used to calculate equilibrium potential
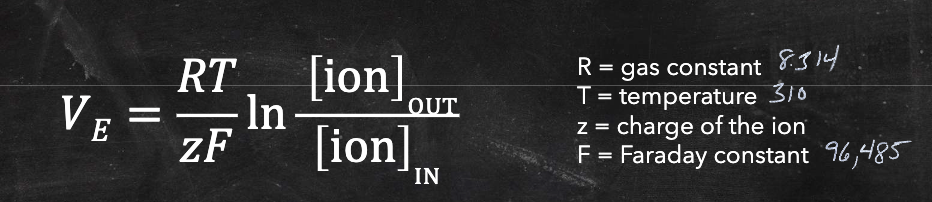
what do pumps and transporters do?
move ions against their electrochemical gradients to maintain resting potential
what are cotransporters?
membrane proteins that harness the energy of an ion moving in a direction that it wants to across the membrane to move another ion in a direction that it doesn’t want to
cotransporter example
K-Cl symporter, Na-Ca antiporter
where is the synaptic cleft?
between axon terminal and dendrites
example of neurotransmitter and receptor
excitatory: glutamate (neurotransmitter) and AMPA receptor
inhibitory: GABA (neurotransmitter) and GABA receptor
what is a graded potential?
local depolarization (positive charge) of the membrane
what is temporal summation?
when repeated inputs to the synapse give many graded signals, and they get added up (if EPSP). If there are enough they will trigger an action potential.
what is spatial summation?
when multiple dendrites add together spatially to reach threshold
voltage-gated sodium channels
closed when below threshold (-55mV), and open when above. Large increase in membrane potential (to +40 mV) from influx of Na+ ions
voltage-gated potassium channels
at +40 mV, voltage-gated sodium channels close and voltage-gated potassium channels open, bringing a large efflux of K+, and bringing the membrane potential below the resting potential (hyperpolarization)
absolute refractory period
period between opening of voltage-gated potassium channels and hyperpolarization
relative refractory period
period between hyperpolarization and return to resting potential
what is an EPSP?
excitatory post-synaptic potential: increases membrane potential
what is an IPSP?
inhibitory post-synaptic potential: decreases membrane potential
myelination in CNS
oligodendrocytes
myelination in PNS
Schwann cells
space between myelin sheaths
nodes of Ranvier
what does myelination do?
increases the speed of action potentials
voltage-gated calcium channels
rapid influx of calcium allows for vesicles to fuse with the membrane and transmit neurotransmitters. Calcium is in high density in pre-synaptic terminals
what does the calcium cause in a synapse?
it causes a conformational change that allows the vesicle (from readily releasable pool) to fuse with the membrane (slamming it)
what is exocytosis in a synapse?
recovering of vesicle proteins, reforming the vesicle
what is the recycling pool?
the vesicle pool that gets reused by inserting new neurotransmitters to vesicles that had just been used
frequency coding
magnitude of APs stays the same but the frequency of firing changes
Neurotransmitter removal
Re-uptake: neurotransmitter re-uptake occurs through membrane proteins, so they do not remain in the synaptic cleft and quickly clear out the cytoplasm. Calcium moves from the synaptic cleft into the axon terminal to close/recycle the vesicle. Drugs can block re-uptake to allow the neurotransmitter to remain in the synaptic cleft for longer (and diffuse more)
Degradation: enzymes like acetylcholinesterase will remove neurotransmitters from the synaptic cleft
Diffusion: neurotransmitters get diffused to various target receptors on the other side of the synaptic cleft
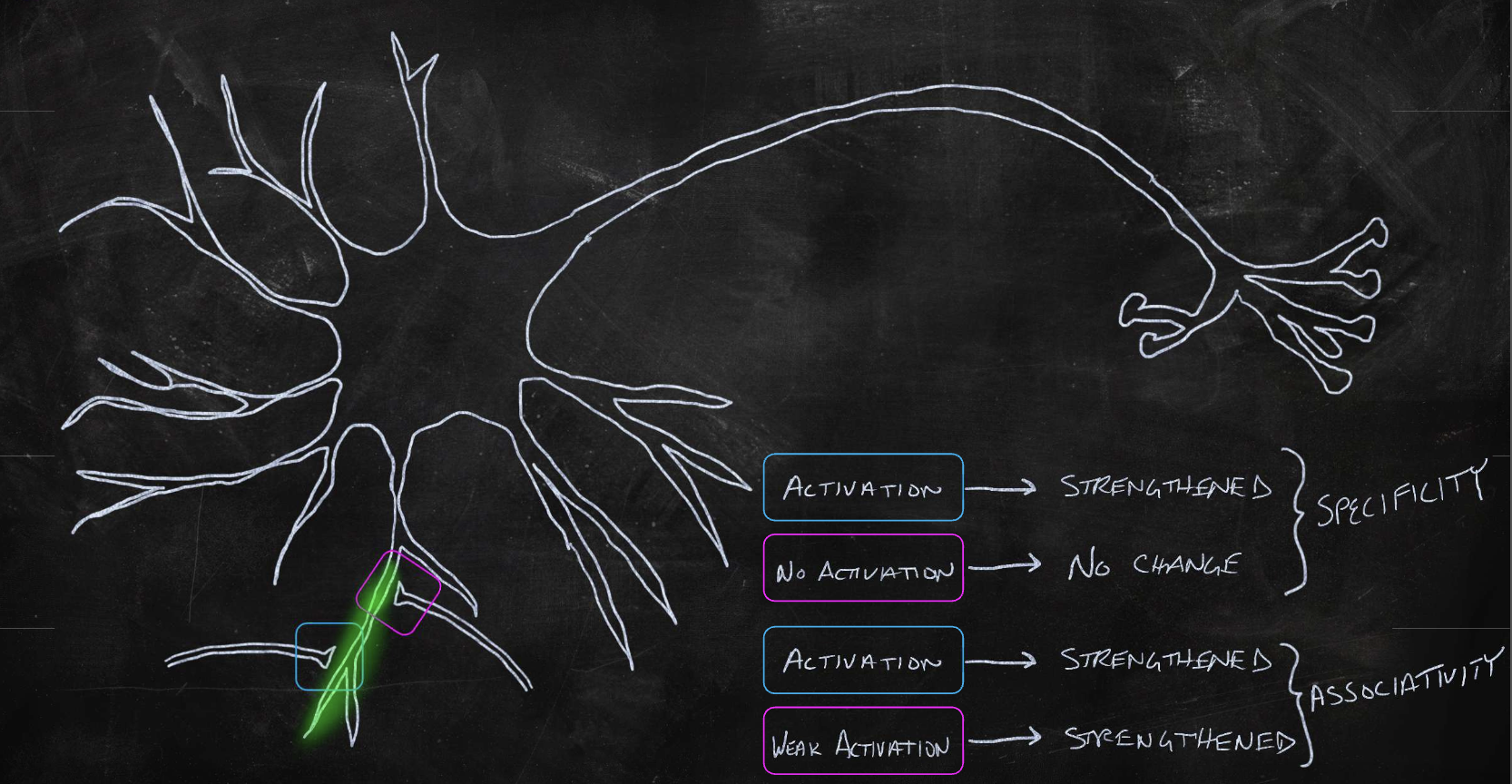
synaptic plasticity
when an axon of cell A is near enough to excite cell B, and repeatedly fires it, the efficiency of cell A will increase (to fire cell B more too)
what is channelrhodopsin?
a light-gated cation channel, and it stimulates activity when exposed to blue light
what is halorhodopsin?
a light-gated chloride pump that inhibits activity when exposed to yellow light
how to get specificity when studying neurons
optogenetic excitation (as opposed to electrical stimulation)
specific promoters (cell type)
viral injections (spatial)
drug-inducible expression (temporal)
what is GCaMP?
a calcium indicator used to study brain activity
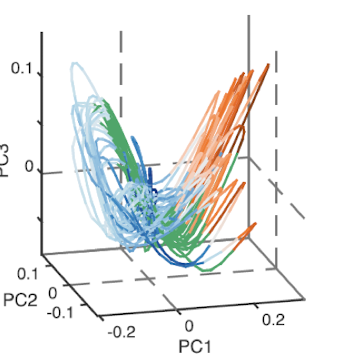
phase trajectory
plot of how a system evolves in time
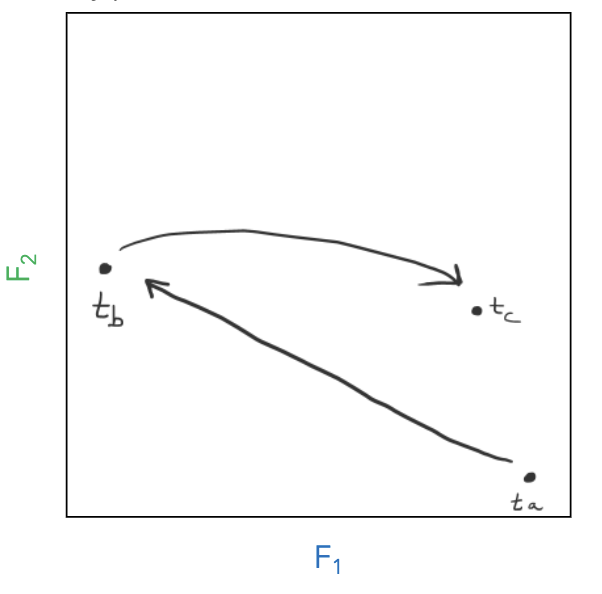
attractor
space that phase trajectory tends to evolve toward
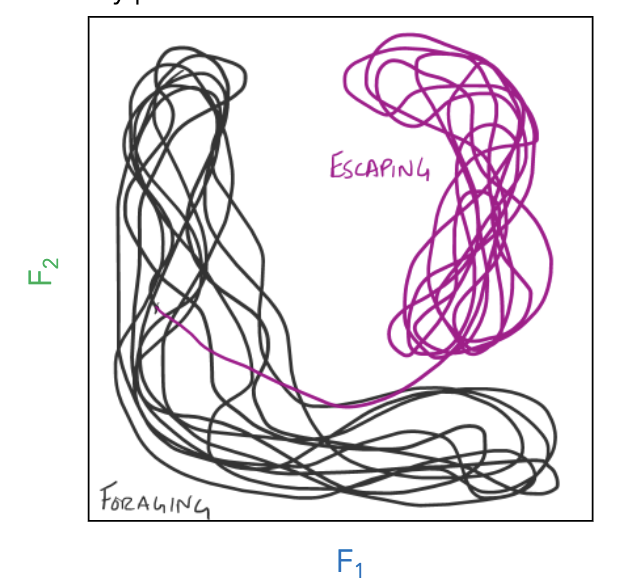
dimensionality reduction
When highly correlated dimensions can be reduced to another new dimension, that is a better description of the variance in the data (principal component).
It allows us to describe the activity states of large populations of neurons
what is a manifold?
the shape of the structure when mapping the principal components. It can be coloured by behaviour to show specific parts of phase-based trajectory
it is a lower dimensional surface embedded in a higher dimensional space
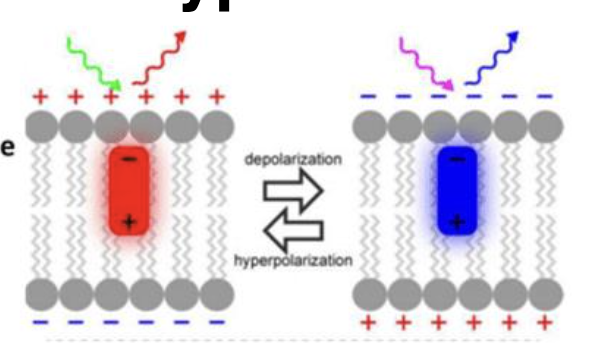
what is membrane potential?
the difference in voltage due to the constant presence of ion gradients
small molecule voltage sensitive dyes
change fluorescence in response to changes in membrane potential
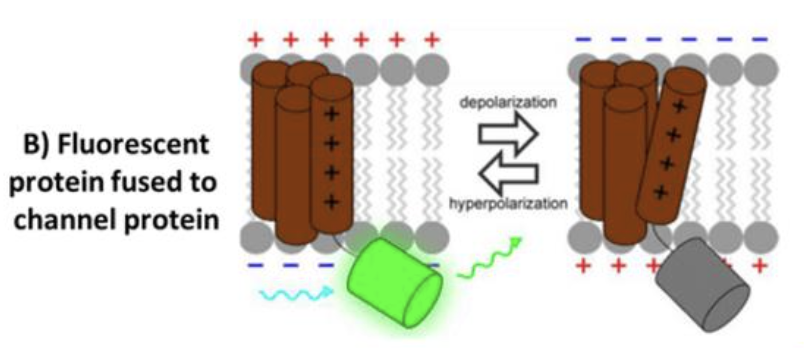
fluorescent protein fused to channel protein
fuse fluorescent protein to voltage-sensing domains, fluorescence changes based on membrane potential
fluorescent opsins
light sensitive channel proteins
ex vivo extracellular electrophysiology
electrodes placed near cell bodies of neurons to record the electrical signals. The electrodes can detect changes in the extracellular voltage and provide information about the rate and timing of spikes
ex vivo dual recording electrophysiology
electrodes placed near the cell body and axon of neurons to measure axonal fidelity (propagation of action potential down axon)
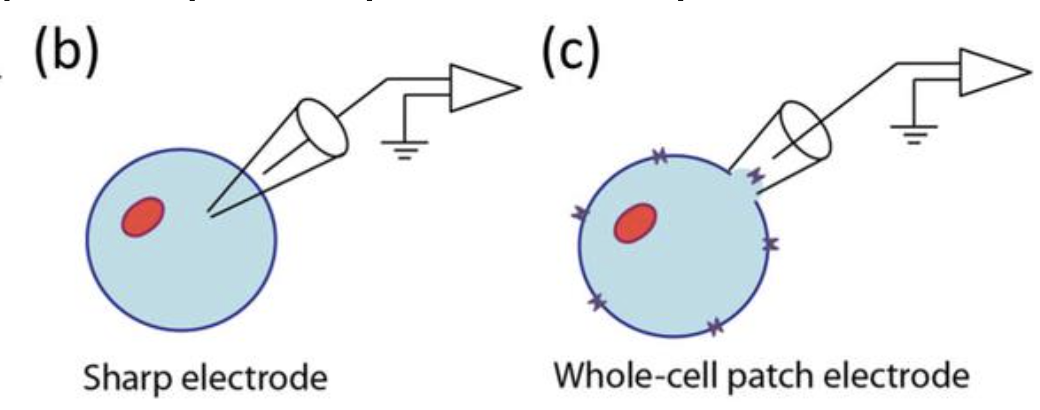
Ex vivo intracellular electrophysiology
Electrode inside the cell, allowing you to measure the membrane potential and ionic currents across the membrane. This provides information about a single cell’s electrical properties.
Patch clamp: intracellular activity of single neurons with a high signal-to-noise ratio
Sharp electrode: large leak currents, making voltage-clamp recordings difficult
Voltage clamp
clamps cell membrane at a desired constant voltage and records what currents are being delivered to achieve that voltage (measured in amps). can be used to study ion channel function
current clamp
electrode that records and injects current inside a neuron. You then measure the resulting voltage in response to the injected current. This is used for mimicking electrical signal coming from a synapse or neurotransmitters
Multi-electrode arrays
done while awake
measures:
spiking activity from multiple neurons in a region
local field potentials which reflect summed synaptic activity
Network activity (synchronization and rhythmic patterns)
neuropixels probe
picks up readings of separate neurons
what can GFP be used for?
labelling specific aspects/proteins to focus on and ignoring the rest
what happens to calcium channels as the neuron is depolarized?
they open, causing a huge increase in calcium throughout the cytoplasm
NMDA receptor
when bound to Mg2+, it prevents glutamate from binding. If enough Na+ flows in from an AMPA receptor, NMDA changes conformation (in response to voltage change), removing Mg2+, binding glutamate, and allowing an influx of calcium
methods for large-scale neuronal activity data collection
multi-channel electrodes and calcium imaging
multi-channel electrodes
measure action potentials from hundreds or thousands of neurons
allows study of behaviour for large animals
invasive
too big for small brain animals
calcium imaging
non-invasive
slower time scales (able to detect single APs)
Depth issues for imaging
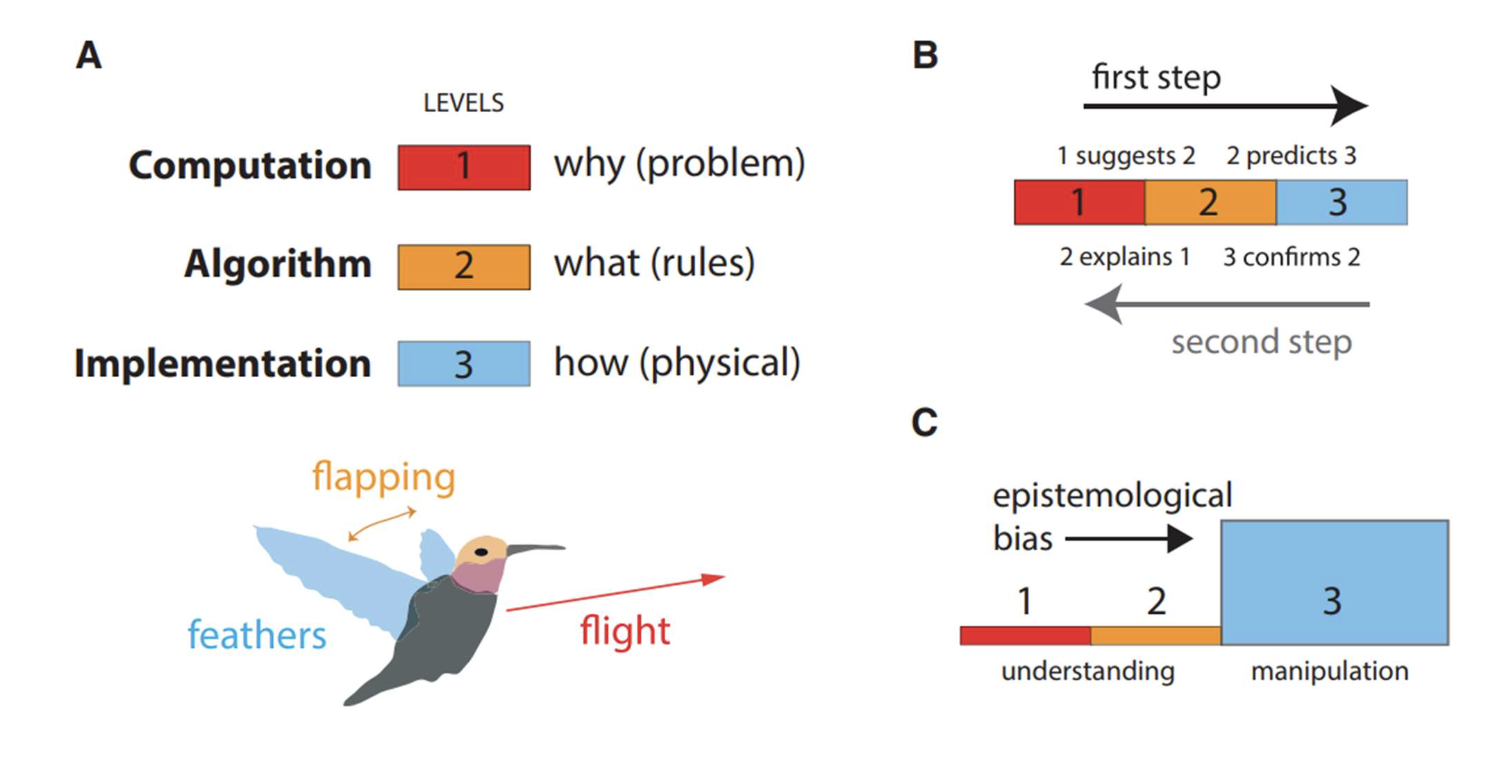
Marr’s levels
computation (why), algorithm (what), implementation (how)
why do behavioural states not correspond to synaptic connections?
because the neurons receive input with many neurons, and because the same circuit structure can produce many outcomes
what do neuromodulators (like G-protein receptors and RTKs) do?
they alter the neural signalling properties to produce changes (on a slow time-scale) in circuit dynamics. When a neuromodulator enhances the activity of a synapse, it increases the number of docked synaptic vesicles, to allow for more neurotransmitter release
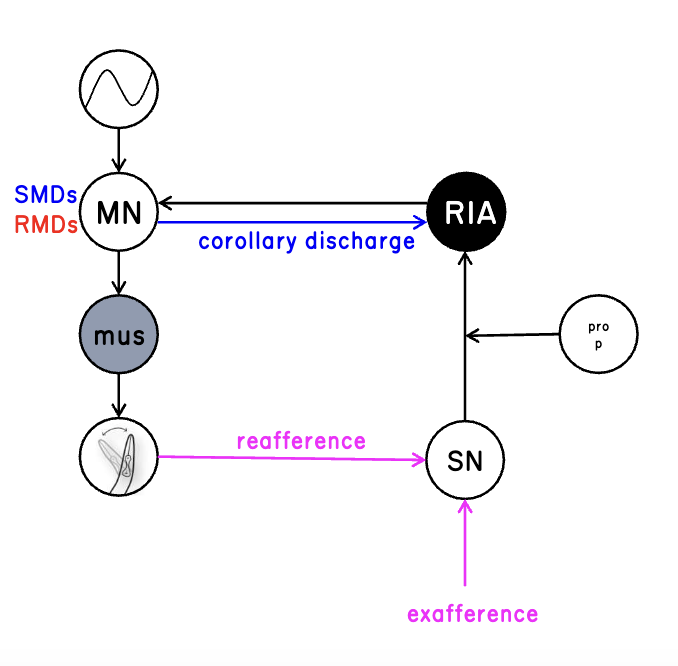
exafferent sensory input
input from outside world
reafferent sensory input
caused from our own movement
sensory filtration
inhibiting one part of body when doing one behaviour
ex: crickets chirping, desensitize the sound when chirping, when it isn’t chirping it can hear its surroundings
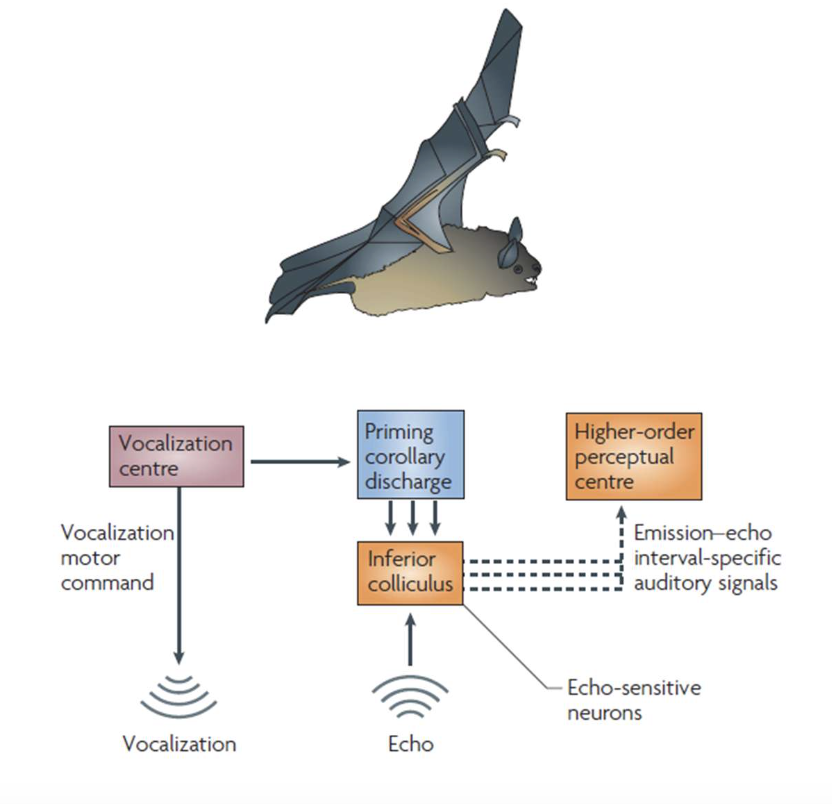
active sensing
create a copy of sensory input and differentiate between input from outside world and from within.
ex: bats; difference between its sound and echo to help with visualization
c. elegans circuit
integrates head position and sensory input to respond to the spatial distribution of the stimuli
sensory and motor signals converge on a single interneuron where they are encoded through separate calcium signalling pathways
“self” representations (corollary discharge) are fundamental to sensory perception