Cell Bio Exam Review Topic 7 RNA Translation
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
RNA translation overvoew
mRNA is translated into a protein in the cytoplasm (once exported from nucleus). An mRNA sequence is decoded in sets of three nucleotides, named codons. Each triplet (codon) codes for a single aminoacid in protein sequence. 4 nucleotides code for 20 different aminoacids -> code is redundant
tRNA role
tRNA molecules match amino acids to codons in mRNA -> specific shape with a set of three nucleotides exposed to the outside = anticodon, whose sequence is complementary to codon sequences. Bound to an aminoacid on the opposite anticodon side. More than one TRNA for the same amino acid
Correct tRNA binding
Recognition and attachment of the correct amino acid depend on enzymes called aminoacyl-tRNA synthetases, which covalently couple each amino acid to its appropriate set of tRNA molecules. Each aminoacid has o
mRNA message decoded by what?
The mRNA message is decoded by ribosomes. They are located in the cytoplasm and formed by rRNA and ribosomal proteins
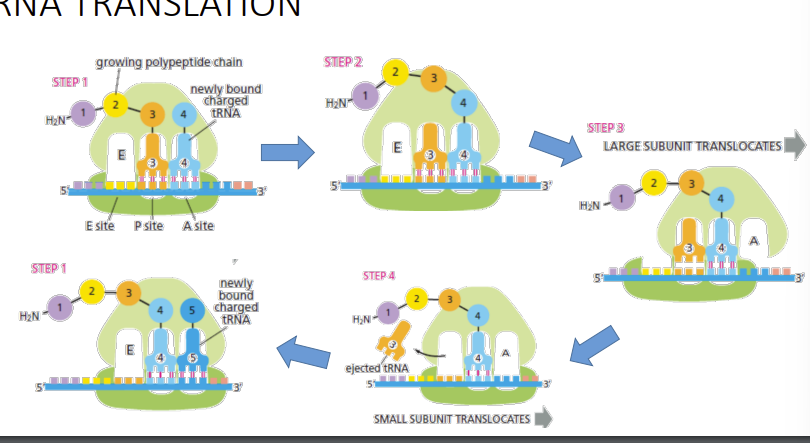
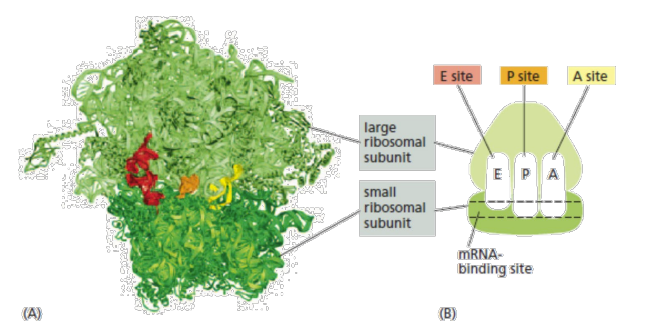
ribosome structure
Ribosome domains: binding site for an mRNA molecule + three binding sites for tRNA molecules, called the A site, the P site, and the E site
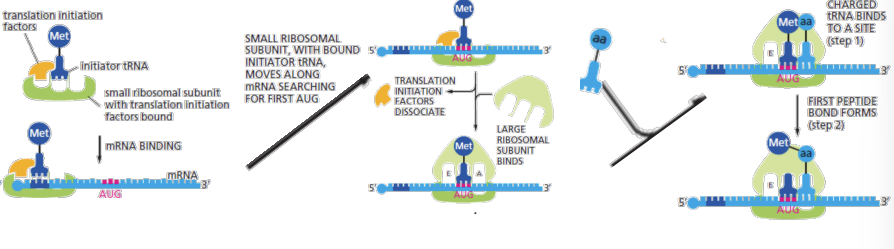
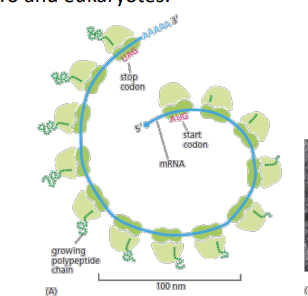
start codon
AUG amino acid methionine, translation initiation factors + initiator tRNA-met loaded in P site (distinct to the tRNA that normally carries Methionine
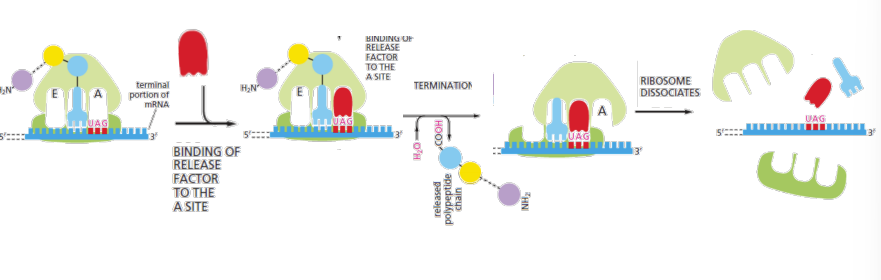
Stop codons
UAA, UAG, UGA, Release factors (proteins) bind to stop codons in A site and peptidyl transferase adds water ->release of polypeptide. And ribosome dissociation.
proteins made in?
Proteins are made in polyribosomes (polysomes): multiple ribosomes bound to a single mRNA and translating into a protein. Existing in both pro and eukaryotes.
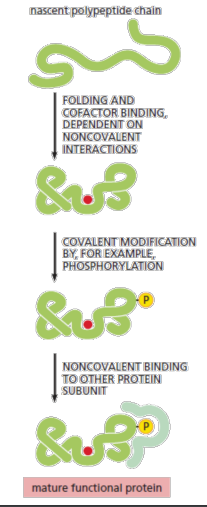
post translational modifications
Many proteins—once they leave the ribosome—require further attention before they are useful to the cell. Examples of such post-translational modifications include covalent modification ,the binding of smallmolecule cofactors, or association with other protein subunits, which are often needed for a newly synthesized protein to become fully functional.
proteolysis
Cells possess specialized pathways that enzymatically break proteins: proteolysis. The enzymes that degrade proteins, first to short peptides and finally to individual amino acids, are known collectively as proteases. They act by cutting (hydrolyzing) the peptide bonds between amino acids: • In proteins whose life span is supposed to be short • Proteins misfolded
proteins that need to be destroyed are marked with?
ubiquitin