Check 1 Redo Q's
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
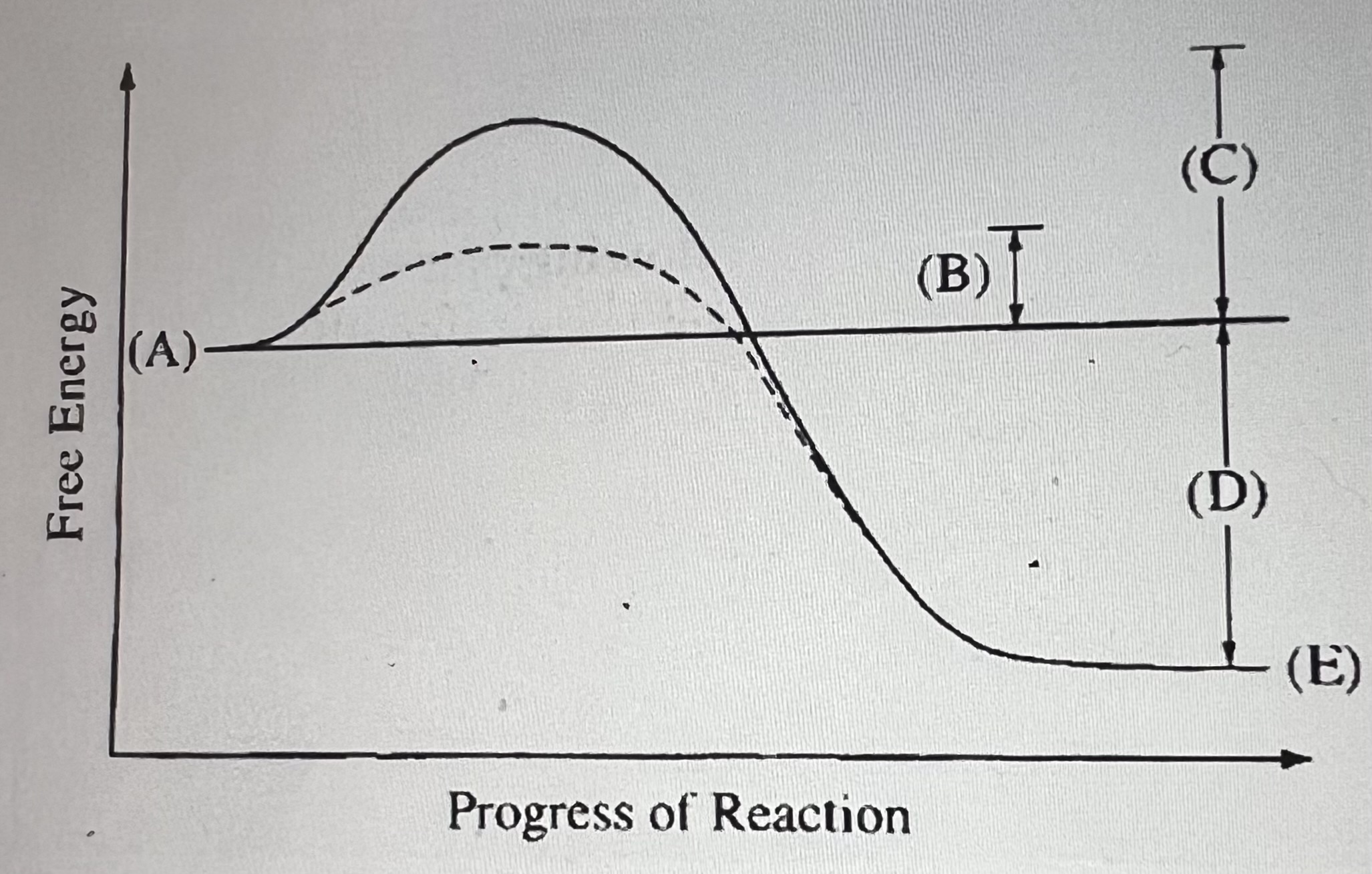
What does B represent?
Activation energy of the enzyme-catalyzed reaction
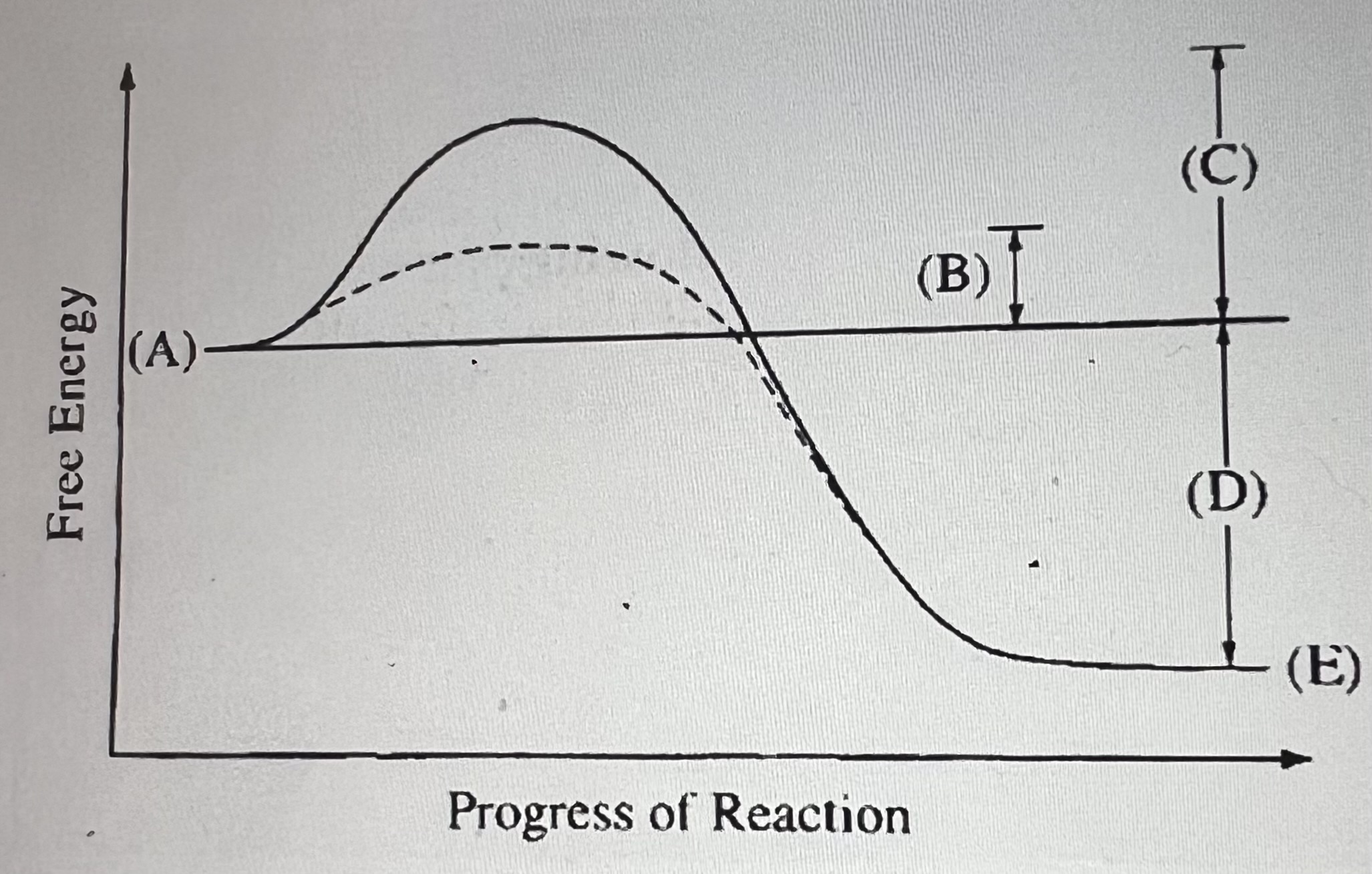
What does C represent?
Activation energy required for the non-catalyzed reaction
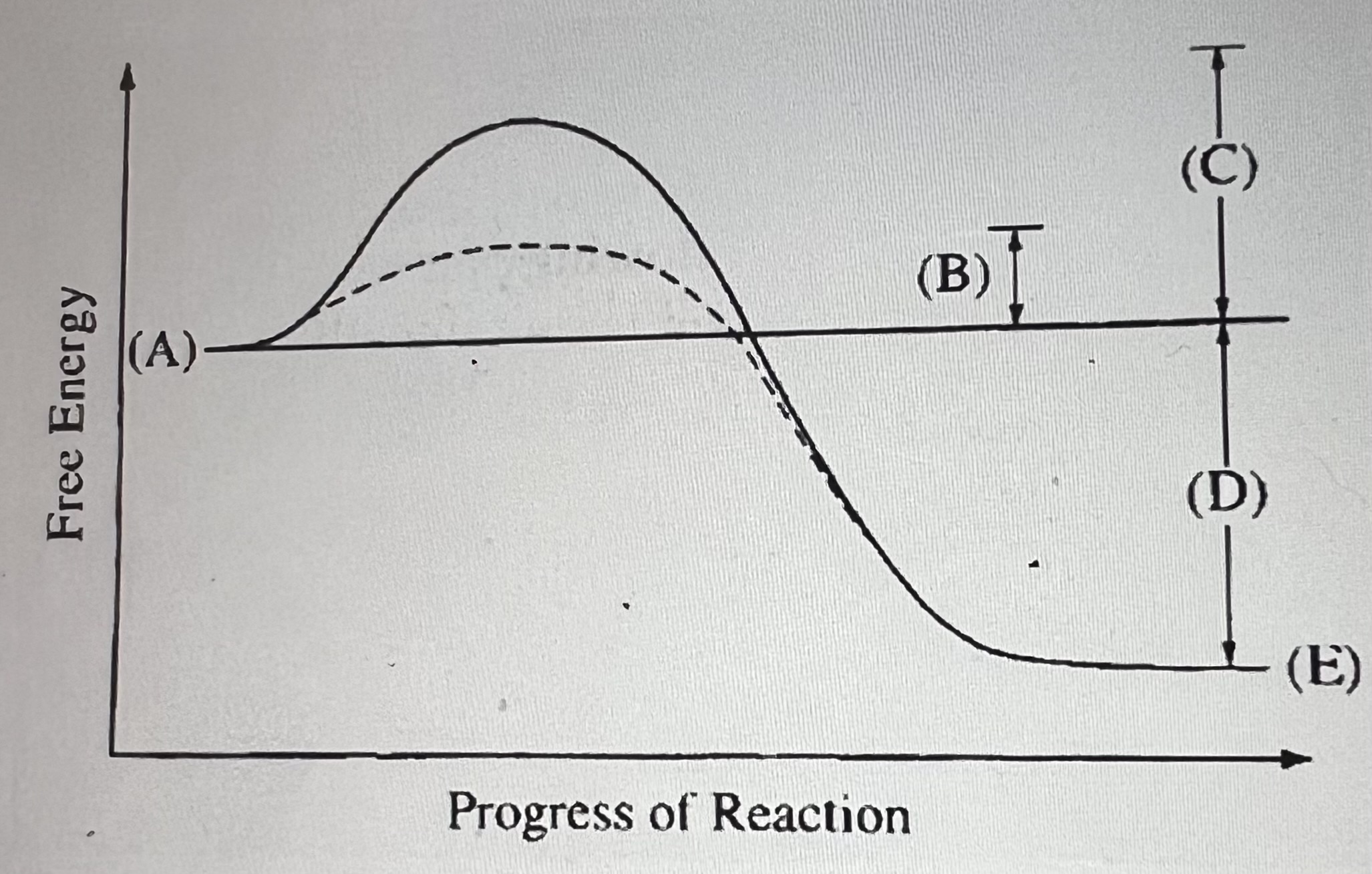
What does D represent?
The net change, ΔG, of the reaction
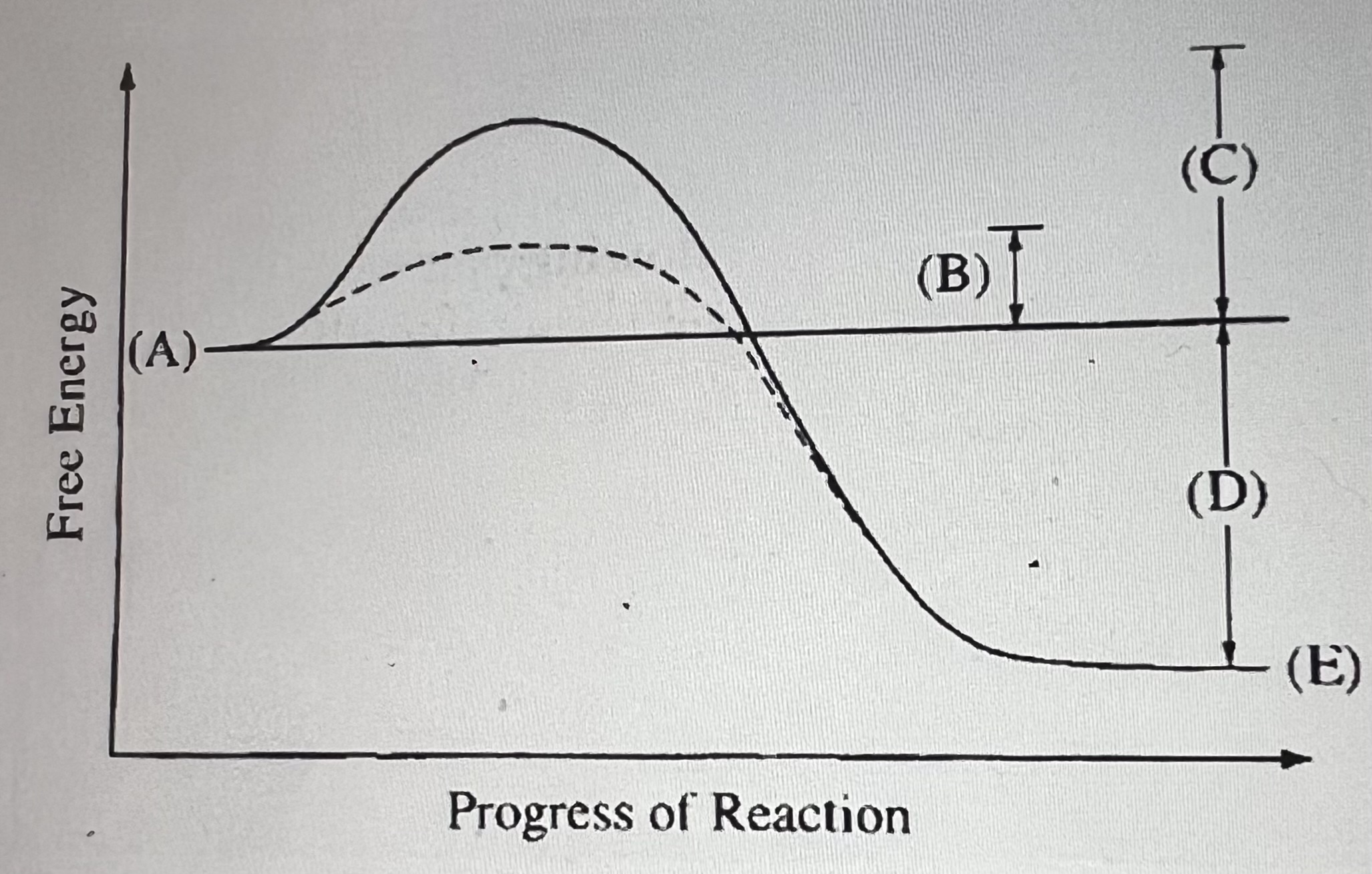
What does E represent?
the energy state of the products of the enzyme-catalyzed reaction
Which of the following best characterizes the reaction: Glucose + Galactose + energy → Lactose
exergonic
Enzymes are required by all living things because enzymes….
properly orient reactants and lower activation energy
Only small amounts of enzymes are required for reactions within cells because enzymes…
are reused
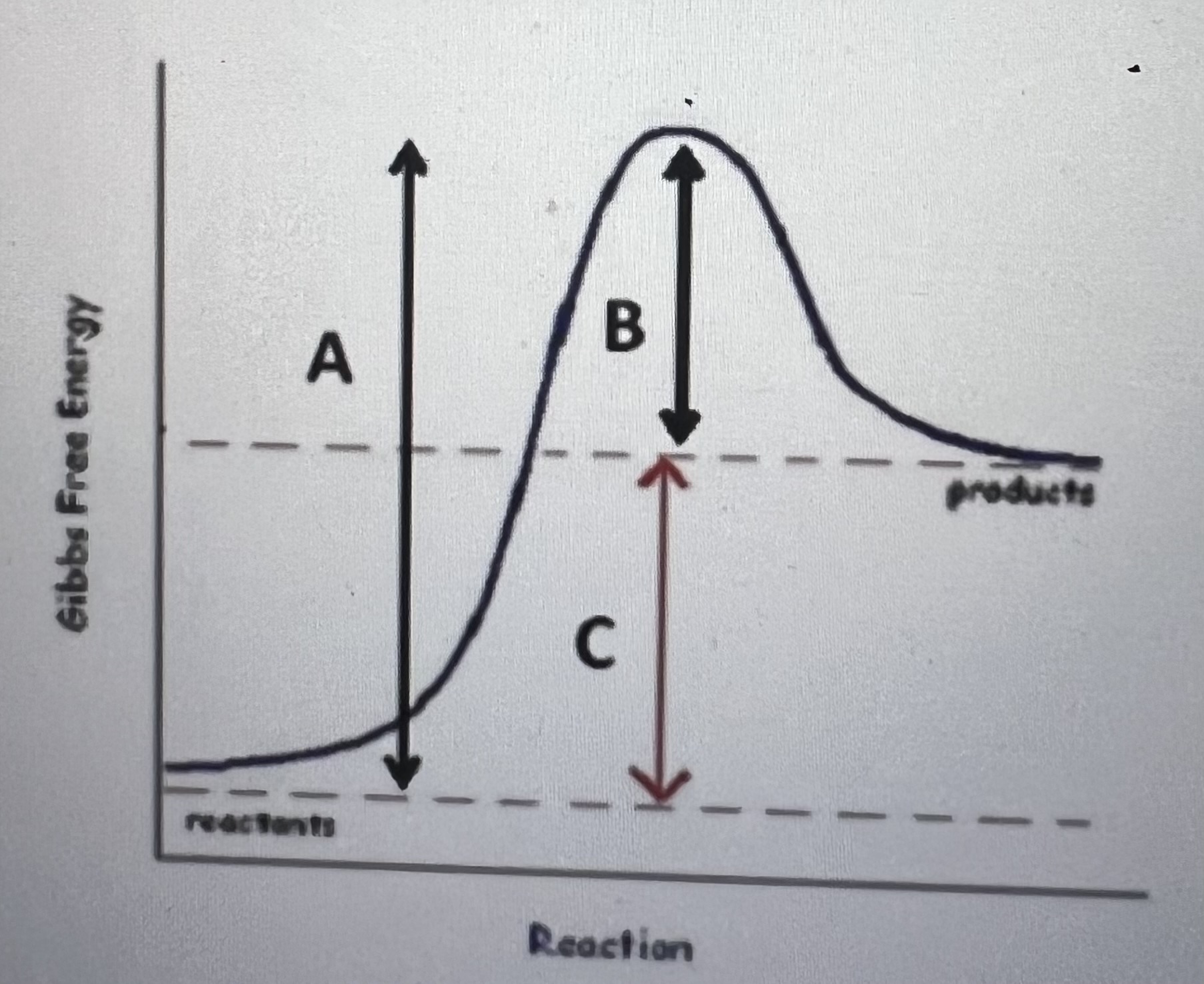
The graph below illustrates the course of a chemical reactions as it progresses. What is TRUE regarding this reaction
This is an endergonic reaction and arrow C labels the activation energy of the reaction
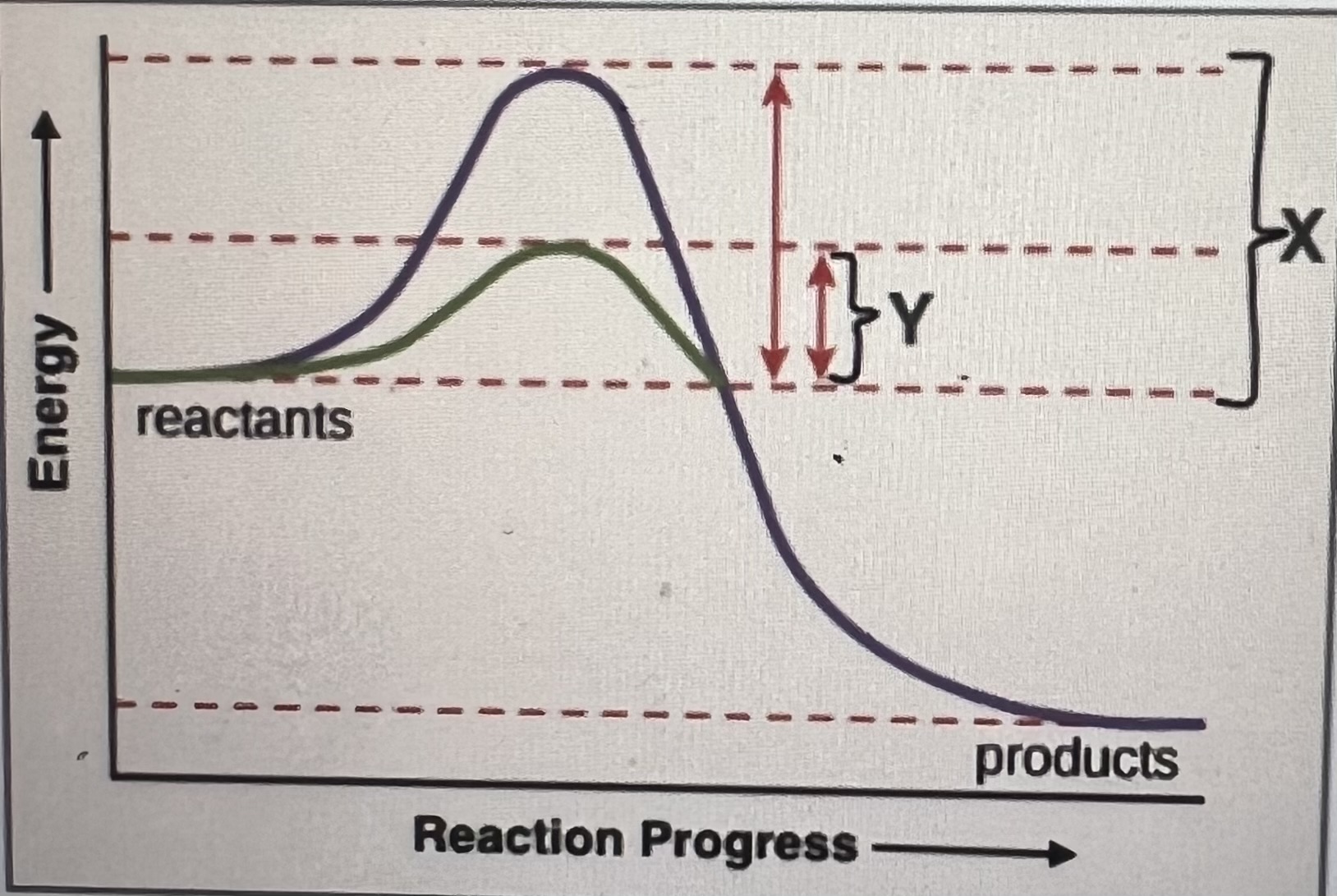
In the graph below, what might cause a reaction that normally proceeds through the path of Y to change to proceed through the path of X?
The addition of a catalyst
Catabolism
Exergonic, hydrolysis, breaking
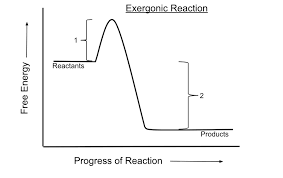
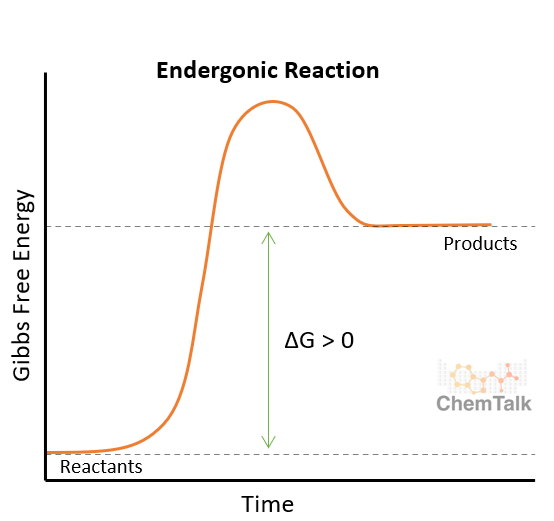
Anabolism
Endergonic, dehydration synthesis, building