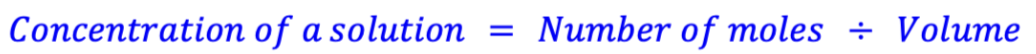formulae; relative masses of atoms and molecules; the mole and the avogadro constant
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 3:26 AM on 7/18/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
molecular formula
gives the type of element and how many are involved
2
New cards
diatomic molecules
exists as molecules made up of two atoms
3
New cards
empirical formula
the most simplified version of a compound (C2H6→CH3)
4
New cards
relative atomic mass
the average mass of the isotopes of an element compared to the 1/12th of the mass of an atom of C-12
5
New cards
relative formula mass
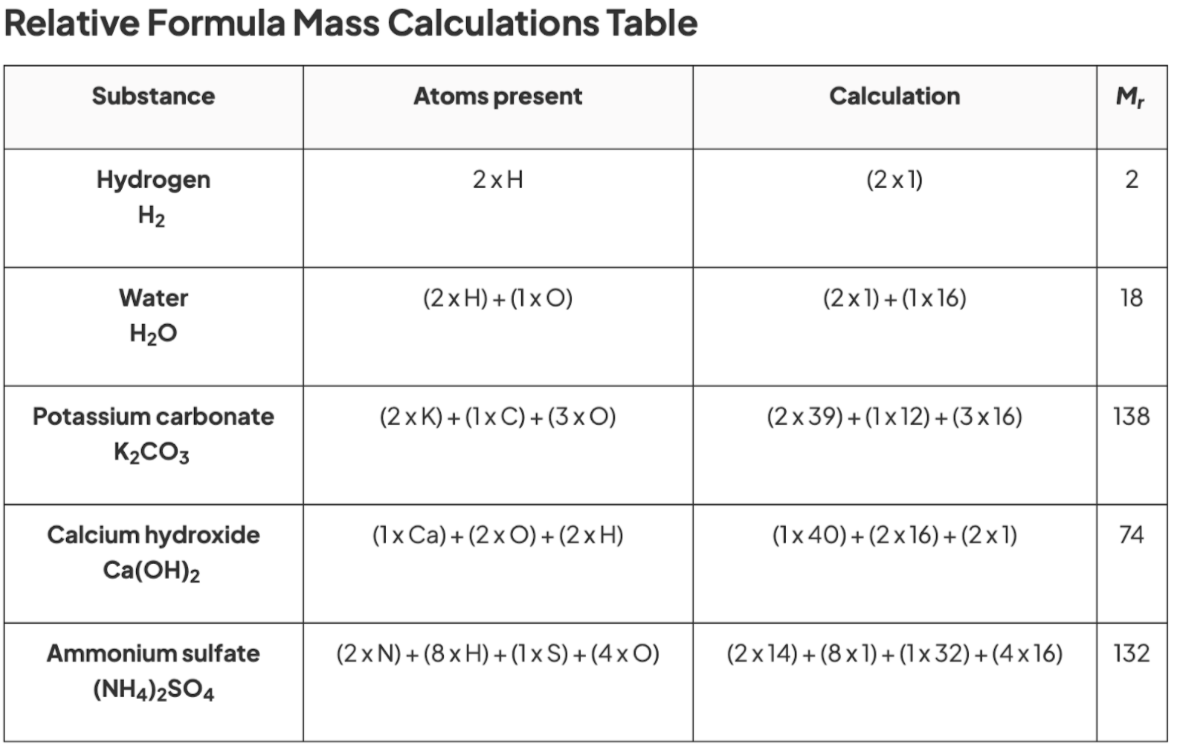
6
New cards
mole
a unit which measures the amount of substance (unit=mol)

7
New cards
molar mass
the mass of 1 mole of a subtance (unit=g/mol)
8
New cards
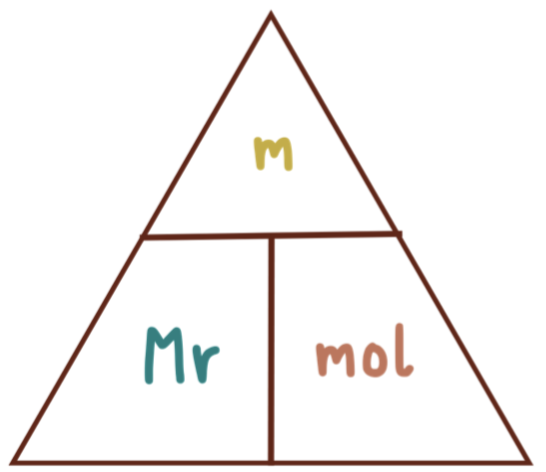
mol/mass/Mr formula
m= mass
Mr= molecular mass
mol= mole
9
New cards
conversion between cm³ and dm³

10
New cards
concentration of a solution formula