mistakes in chemistry
1/144
Earn XP
Description and Tags
re
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
145 Terms
NEET Tricks and Exceptions:
AX₂E₂ = Bent (H₂O, OF₂) – not linear!
AX₃E = Trigonal Pyramidal (NH₃) – lone pair distorts shape.
XeF₂ (AX₂E₃) = Linear – despite 5 electron pairs.
ClF₃ (AX₃E₂) = T-shaped – due to lone pair repulsions.
SF₄ is NOT tetrahedral – it’s seesaw, due to one lone pair.
XeF₄ is square planar (AX₄E₂) – lone pairs opposite each other.
PCl₅ and SF₆ have expanded octets – they exceed 8 electrons.
molecules
Molecule | Expected Shape | Actual Shape | Reason |
|---|---|---|---|
NH₃ | Tetrahedral | Trigonal pyramidal | 1 lone pair repels bonding pairs |
H₂O | Tetrahedral | Bent (V-shaped) | 2 lone pairs on O cause more repulsion |
BeCl₂ | Angular (due to 2 bonds) | Linear | No lone pairs on Be — sp hybridized |
BF₃ | Trigonal pyramidal | Trigonal planar | No lone pair on B — only 3 bonding pairs |
PF₅ | Octahedral | Trigonal bipyramidal | 5 bonding pairs — no lone pair |
SF₄ | Trigonal bipyramidal | See-saw | 1 lone pair → asymmetry |
ClF₃ | Trigonal bipyramidal | T-shaped | 2 lone pairs |
XeF₂ | Trigonal bipyramidal | Linear | 3 lone pairs in equatorial positions |
XeF₄ | Octahedral | Square planar | 2 lone pairs on opposite sides |
ICl₂⁻ | Trigonal bipyramidal | Linear | 3 lone pairs → linear shape |
molecules shape
Molecule | Shape | Symmetrical? | Dipole Moment |
|---|---|---|---|
BF₃ | Trigonal planar | ✅ Yes | 0 |
CCl₄ | Tetrahedral | ✅ Yes | 0 |
CHCl₃ | Tetrahedral | ❌ No | ≠ 0 |
CO₂ | Linear | ✅ Yes | 0 |
H₂O | Bent | ❌ No | ≠ 0 |
NH₃ | Trigonal pyramidal | ❌ No | ≠ 0 |
⚛ 1. Atomic Radius
Across a period: ↓ Decreases (effective nuclear charge ↑)
Down a group: ↑ Increases (new shells added)
🔸 Exception: 3d series (Transition elements) show very slight decrease due to poor shielding of d-electrons.
🔌 2. Ionization Enthalpy (IE)
Across a period: ↑ Increases (harder to remove electrons)
Down a group: ↓ Decreases (outer electrons farther from nucleus)
🔸 Exceptions (due to stability):
Be > B (B has unstable pⁱ electron)
N > O (N is half-filled p³ → more stable)
4. Electronegativity (EN)
Across a period: ↑ Increases
Down a group: ↓ Decreases
F (Fluorine) = Most electronegative (3.98)
Note: EN is not constant for an element — it changes slightly in different oxidation states or bonding environments. ❌
⚗ 5. Nature of Bonding
Beryllium (Be) forms covalent compounds (due to its small size and high ionization energy).
Example: BeCl₂ is covalent in nature.
Does not show properties of typical metals.
🧬 6. Trends in d-Block (Transition elements)
Poor shielding of d-orbitals
Slight decrease in atomic size across the series
Many oxidation states
Complex formation, colored ions, paramagnetism
❌ Common Incorrect Statements (like in NEET):
"Electronegativity is a constant" – ❌ (It varies slightly)
"Be compounds are ionic" – ❌ (Mostly covalent)
"3d series shows large atomic radius variation" – ❌ (Change is small)
✅ TRICK TO REMEMBER:
"AR, IE, EN" →
Across Period: ⬇⬆⬆
Down Group: ⬆⬇⬇
(Atomic Radius ↓, Ionization Energy ↑, Electronegativity ↑ across a period)
bonding and antibonding
MO | Electrons | Type |
|---|---|---|
σ1s | 2 | Bonding |
σ*1s | 2 | Antibonding |
σ2s | 2 | Bonding |
σ*2s | 2 | Antibonding |
σ2p_z | 2 | Bonding |
π2p_x, π2p_y | 4 | Bonding |
π2p_x, π2p_y | 4 | Antibonding |
❌ Octet Rule is NOT Followed In:
✨Correct Answer: NO (Nitric oxide)
💥 Categories of Octet Rule Exceptions:
Category | Example(s) | NEET Point |
|---|---|---|
Odd number of electrons | NO, NO₂, ClO₂ | Cannot pair all electrons |
Incomplete octet | BCl₃, BeCl₂ | Central atom has <8 electrons |
Expanded octet (≥ period 3) | PCl₅, SF₆, ICl₃ | Central atom has >8 electrons |
Concept | Boron (B) | Aluminium (Al) |
|---|---|---|
Group | 13 (III A) | 13 (III A) |
Valence electrons | 3 | 3 |
Electronic configuration | 1s² 2s² 2p¹ | [Ne] 3s² 3p¹ |
Maximum covalency | 4 | 6 or more (can expand octet) |
Reason for covalency limit | No d-orbitals in 2nd period | Has vacant 3d-orbitals |
Forms [BF₄]⁻? | ✅ Yes (tetrahedral) | ❌ Cannot form [AlF₆]³⁻ easily |
Forms [AlF₄]⁻? | ✅ Yes (tetrahedral) | ✅ Yes – even higher coordination |
Can expand octet? | ❌ No | ✅ Yes (due to 3rd period + d-orbitals) |
💡 Quick NEET Tip:
Removing from stable configs (like 2p⁶, full shells) → high IE
Removing from half-filled or less stable → relatively lower IE
Which of the following is the strongest reducing agent?
a) Na
b) Mg
c) Al
d) Zn
a) Na
Sodium (Na) is the strongest reducing agent because it has the lowest ionization energy among the given elements, meaning it can easily lose its electron to become Na⁺. The more easily an element loses an electron, the stronger its reducing power.
1. Ionic vs Covalent Bonding
Charge and Size Relationship:
Smaller the size and higher the charge → higher polarizing power, leading to covalent character.
Larger the size and lower the charge → ionic character remains more.
Quick Trick: Al³⁺ > Mg²⁺ > Na⁺ → AlCl₃ > MgCl₂ > NaCl. The smaller and more charged the cation, the more covalent the bond.
Bond Order Calculation (MO Theory)
Bond Order Formula:
Bond Order=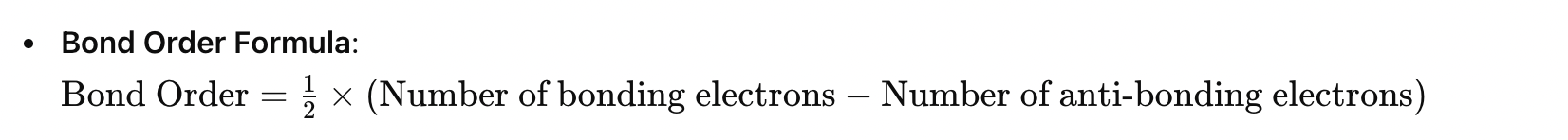
MO Energy Level Trick:
For diatomic molecules like O₂ or F₂, if the molecule has a higher atomic number, anti-bonding electrons come into play, affecting bond order.
Example: In O₂ (Bond Order = 2), in O₂⁻ (Bond Order = 1.5).
Key Tip: Ionic character decreases as covalent character increases.
Trick to remember ionic vs covalent:
High charge density (like in AlCl₃) makes the bond more polar covalent. Low charge density (like in NaCl) keeps the bond more ionic.
NEET Tricks for Dipole Moment & Molecular Polarity 🔺 1. Lone Pair Direction Matters!
🧠 If lone pair dipole & bond dipoles are in the same direction ➡ High dipole moment
🧠 If lone pair dipole opposes bond dipoles ➡ Low dipole moment
✅ NH₃ → Lone pair + 3 N–H bonds all point upward → net dipole is high
❌ NF₃ → Lone pair is up, but 3 N–F bond dipoles are down (towards F) → they cancel each other → net dipole is low
Predict Dipole Moment
Shape | Net Dipole Moment |
|---|---|
Linear (AB₂) | 0 if symmetric |
Trigonal planar | 0 if same atoms |
Tetrahedral | 0 if same atoms |
Bent / Angular | ≠ 0 |
Trigonal pyramidal | ≠ 0 |
Asymmetrical shape | ≠ 0 |
🧪 Special Cases (Must-Know for NEET) 🔹 Scandium (Sc)
Only shows +3
Reason: After losing 3 electrons (1 from 4s, 2 from 3d), it reaches noble gas configuration
✅ So Sc doesn’t exhibit variable oxidation states ❗
🔹 Titanium (Ti)
+2, +3, +4
Common in TiCl₃ (+3), TiO₂ (+4)
🔹 Copper (Cu)
+1 and +2
Cu⁺ is less stable in aqueous solution (disproportionates)
So Cu²⁺ is more common
🔹 Cobalt (Co)
+2, +3
Co²⁺ more stable, but Co³⁺ seen in complexes (like vitamin B₁₂)
📊 Tips to Remember Variable Oxidation States
✅ Lower atomic number → fewer oxidation states
✅ Middle elements → max variability (Mn, Fe, Co)
✅ Higher oxidation states are stabilized by electronegative ligands (F⁻, O²⁻)
✅ d⁵ and d⁰ configurations → stable (Mn²⁺ is d⁵ → stable)
Sc only +3
Zn only +2 (completely filled d-orbitals, so stable)
Cr shows up to +6 (Cr₂O₇²⁻ → +6)
Mn shows +2 to +7 (MnO₄⁻ → +7)
Fe → +2, +3 (ferric & ferrous)
Cu → +1, +2
Co → +2, +3
Ni → mostly +2
🎯 Formula Reminder
n = no. of unpaired electrons
Only d-electrons count
Works for first-row transition metals (Sc–Zn)
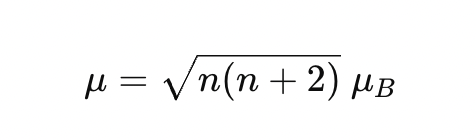
to find d
d⁰ | d¹ | d² | d³ | d⁴ | d⁵ | d⁶ | d⁷ | d⁸ | d⁹ | d¹⁰ |
|---|---|---|---|---|---|---|---|---|---|---|
0 | 1 | 2 | 3 | 4 | 5 | 4 | 3 | 2 | 1 | 0 |
🧠 Shortcut Table of Magnetic Moments
n (Unpaired) | Magnetic Moment (μ<sub>B</sub>) |
|---|---|
0 | 0 |
1 | 1.73 |
2 | 2.84 |
3 | 3.87 |
4 | 4.90 |
5 | 5.92 |
Pro-Tips:
μ = 0 ⇒ all electrons are paired (e.g. d⁰ or d¹⁰)
Aqueous solution: assume high spin (weak field ligands)
Ni²⁺ (d⁸) → 2 unpaired electrons → μ = 2.84 μB
Cu⁺ (d¹⁰) → all paired → μ = 0
Mn²⁺ (d⁵) → 5 unpaired → μ = 5.92 μB
Element | Symbol | Atomic No. | Ground-State Config | Common Ion | Ion Config |
|---|---|---|---|---|---|
Scandium | Sc | 21 | [Ar] 3d¹ 4s² | Sc³⁺ | 3d⁰ (no unpaired) |
Titanium | Ti | 22 | [Ar] 3d² 4s² | Ti³⁺ | 3d¹ |
Vanadium | V | 23 | [Ar] 3d³ 4s² | V³⁺ | 3d² |
Chromium | Cr | 24 | [Ar] 3d⁵ 4s¹ ✅ (exception) | Cr³⁺ | 3d³ |
Manganese | Mn | 25 | [Ar] 3d⁵ 4s² | Mn²⁺ | 3d⁵ ✅ (max unpaired) |
Iron | Fe | 26 | [Ar] 3d⁶ 4s² | Fe²⁺ | 3d⁶ |
Fe³⁺ | 3d⁵ | ||||
Cobalt | Co | 27 | [Ar] 3d⁷ 4s² | Co²⁺ | 3d⁷ |
Nickel | Ni | 28 | [Ar] 3d⁸ 4s² | Ni²⁺ | 3d⁸ |
Copper | Cu | 29 | [Ar] 3d¹⁰ 4s¹ ✅ (exception) | Cu²⁺ | 3d⁹ |
Zinc | Zn | 30 | [Ar] 3d¹⁰ 4s² | Zn²⁺ | 3d¹⁰ ✅ (no unpaired) |
🔥 Actinoids & Their Common Oxidation States
Element | Symbol | Common Ox. States |
|---|---|---|
Thorium | Th | +4 (ONLY) |
Protactinium | Pa | +5 |
Uranium | U | +3, +4, +6 |
Neptunium | Np | +3, +4, +5, +6, +7 ✅ |
Plutonium | Pu | +3, +4, +5, +6, +7 ✅ |
Americium | Am | +3, +4, +5 |
Curium | Cm | +3, +4 |
🧠 Quick Tip for NEET:
+3 is most stable & common for actinoids
+7 is rare and found only in Np and Pu
Actinoids show greater range of oxidation states than lanthanoids due to 5f–6d–7s electron involvement
In the following figure, the Cr-O-Cr bond angle is of X'Y'. What is the most appropriate value of X'?
126°
136°
116°
106°
Cr–O–Cr Bond Angle in [Cr2O7]2-
🔸 The Cr–O–Cr angle is found at the bridging oxygen atom in dichromate.
📌 The most appropriate Cr–O–Cr bond angle = 126°
✅ Correct Answer: 126°
🔬 Why 126°?
Dichromate ion has a structure with two CrO₄ tetrahedra sharing one oxygen (bridge).
Oxygen is sp² hybridized, leading to an angle larger than 109.5° (tetrahedral), but less than 180°.
Experiments and spectroscopy confirm: Cr–O–Cr ≈ 126°
Here's What You Need to Know for NEET:
🔥🔷 Dichromate Ion (Cr₂O₇²⁻):
Two tetrahedral CrO₄ units linked via 1 bridging O.
The angle at bridging O = ~126° (due to repulsion and spatial arrangement).
All Cr–O bond lengths are not equal. Bridging oxygen has slightly different bond length than terminal ones.
📘 Trends & Tips:
Ion | Structure | Bond Angle (M–O–M) | Notes |
|---|---|---|---|
Cr₂O₇²⁻ | Cr–O–Cr bridge | ~126° ✅ | Slightly distorted tetrahedral units |
Mn₂O₇ | Mn–O–Mn bridge | ~120–130° | Strong oxidizer, same family |
Bridging oxides | M–O–M | >109.5° | Because of lone pairs repulsion |
🧠 NEET-TIP:
Cr₂O₇²⁻ (orange) and CrO₄²⁻ (yellow) are interconvertible in acidic/basic medium.
Bridging O always gives a bond angle greater than 109.5° due to lp–bp and bp–bp repulsion.
🔍 Step 2: Identify which other ion has the same structure (tetrahedral)
Compound | Ion | Structure Type | Tetrahedral? | Notes |
|---|---|---|---|---|
MnO₂ | — | Solid, polymeric | ❌ No | Manganese(IV) oxide – not tetrahedral |
K₂Cr₂O₇ | Cr₂O₇²⁻ | Two tetrahedral units joined | ❌ No | Cr₂O₇²⁻ has bridging oxygen, not tetrahedral overall |
KClO₄ | ClO₄⁻ | Tetrahedral | ✅ Yes | Cl in +7 state, same structure as MnO₄⁻ |
None | — | — | — | ❌ Incorrect – there is a match (KClO₄) |
💡 NEET Tips on Isostructural Compounds:
Isostructural = same geometry
Tetrahedral anions:
ClO₄⁻, MnO₄⁻, SO₄²⁻, PO₄³⁻, etc.
Check the oxidation state and lone pairs on central atom using VSEPR theory
Don't get confused with similar formulas – focus on structure & shape
✅ Q.52: Which of the following lanthanoids does not occur naturally?
Element | Atomic Number | Natural/Man-Made |
|---|---|---|
Eu (Europium) | 63 | Naturally Occurring ✅ |
Pm (Promethium) | 61 | ❌ NOT naturally occurring (radioactive, synthetic) |
Gd (Gadolinium) | 64 | Naturally Occurring ✅ |
Lu (Lutetium) | 71 | Naturally Occurring ✅ |
🧠 Lanthanoids (La to Lu, atomic number 57–71) 🔹 General Tips:
f-block elements = inner transition elements.
Lanthanoids = filling of 4f orbitals.
All have +3 oxidation state commonly.
Some like Eu²⁺, Yb²⁺, Ce⁴⁺, Tb⁴⁺ show variable oxidation states.
🔹 Lanthanoid Contraction:
Gradual decrease in atomic & ionic size (Z=57 to Z=71).
Due to poor shielding by 4f electrons.
Causes:
Similar size of 2nd and 3rd transition series
↑ hardness
↑ melting/boiling point trends
↓ basicity of hydroxides
📌 Trick to remember Lanthanoids:
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
👉 "Lazy Ceo Prays, Never Pokes Small Egos, Gets Trouble Doing Homework Every Time You Learn"
Element | Odd Property | Tip |
|---|---|---|
Pm (61) | Synthetic only | ❌ Not natural |
Ce⁴⁺ | Common +4 state | "Cefour" (Ce = +4) |
Eu²⁺, Yb²⁺ | Stable +2 | Eu²⁺ gives red, Yb²⁺ gives pale green |
🧪 Actinoids (Ac to Lr, 89–103)
Fill 5f orbitals.
All are radioactive.
Show more variable oxidation states (+3 to +6).
Example: Uranium (+6 in UO₂²⁺), Np, Pu show up to +7.
📘 Quick NEET Summary Sheet:
Property | Lanthanoids | Actinoids |
|---|---|---|
Electron filling | 4f | 5f |
Reactivity | Less reactive | More reactive |
Oxidation States | Mostly +3 | +3, +4, +5, +6, +7 |
Magnetic Property | Paramagnetic | Strongly paramagnetic |
Occurrence | Mostly natural | Mostly synthetic |
Shielding | Poor (4f) | Very poor (5f) |
Mnemonic: "La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu"
Last Century’s Pretty Nerds Pampered Sexy European Girls, Totally Dripping Hot Every Tuesday, Yearning Lust
🧠 Mini Tip:
💡 pH of buffer ≈ pKa (for acidic buffer)
Use Henderson-Hasselbalch equation if needed:
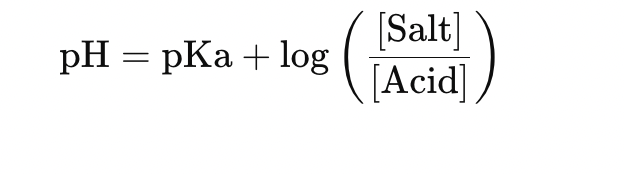
💡 Trick to remember:
🧠 Common Ion = Slight pH shift
🧠 Buffer = Resists pH shift
So if pH changes even slightly on adding a salt, it's due to the common ion effect, not because it's forming a buffer.

Which of the following carbocations is most stable?This is a tertiary carbocation, highly stable due to hyperconjugation and +I effect from 3 methyl groups.
🆚 So now, comparing:
Allyl carbocation: resonance stabilization
Tertiary carbocation: hyperconjugation + inductive
👉 Tertiary carbocation is more stable overall in most cases, unless aromatic or super-delocalized resonance is present.✅ Correct Answer: 1)
Which of the following carbocations will undergo rearrangement to form a more stable carbocation?
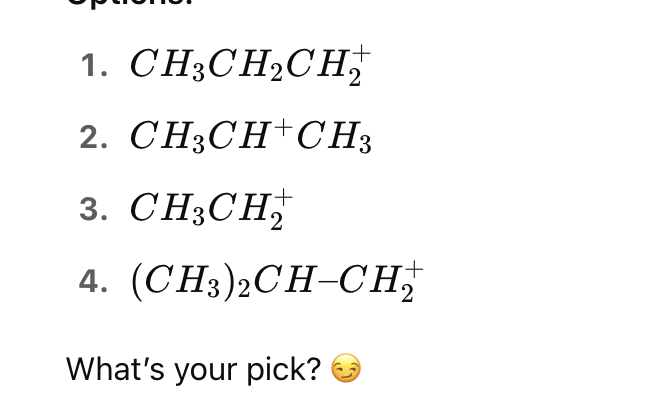
This is a primary carbocation next to a tertiary carbon.
It will rearrange via 1,2-hydride shift to form a tertiary carbocation:
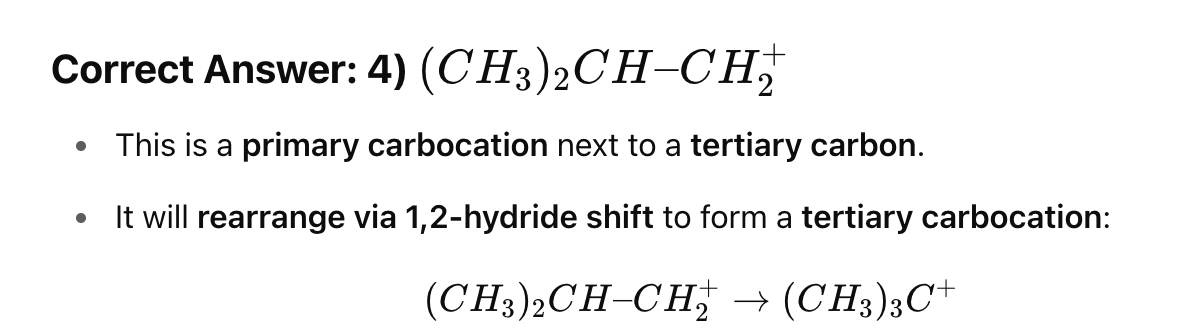
Which of the following carbocations is least stable?
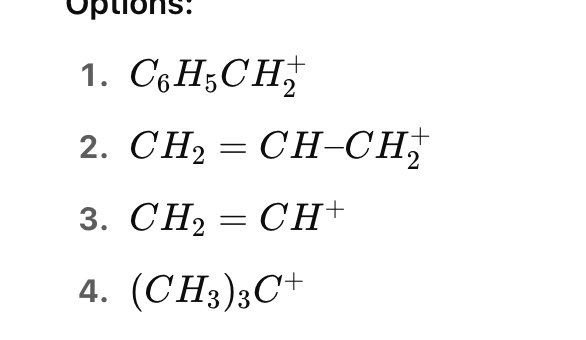

Which of the following carbocations will undergo a hydride shift to form a more stable carbocation?
Options:
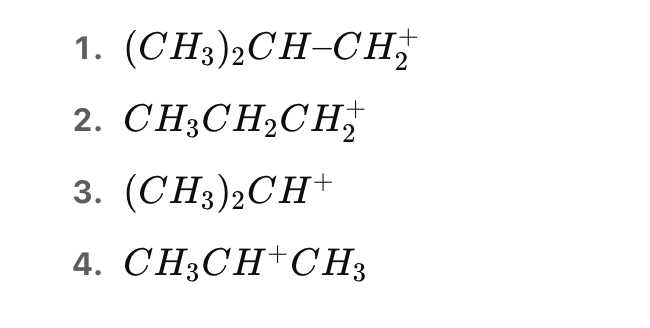
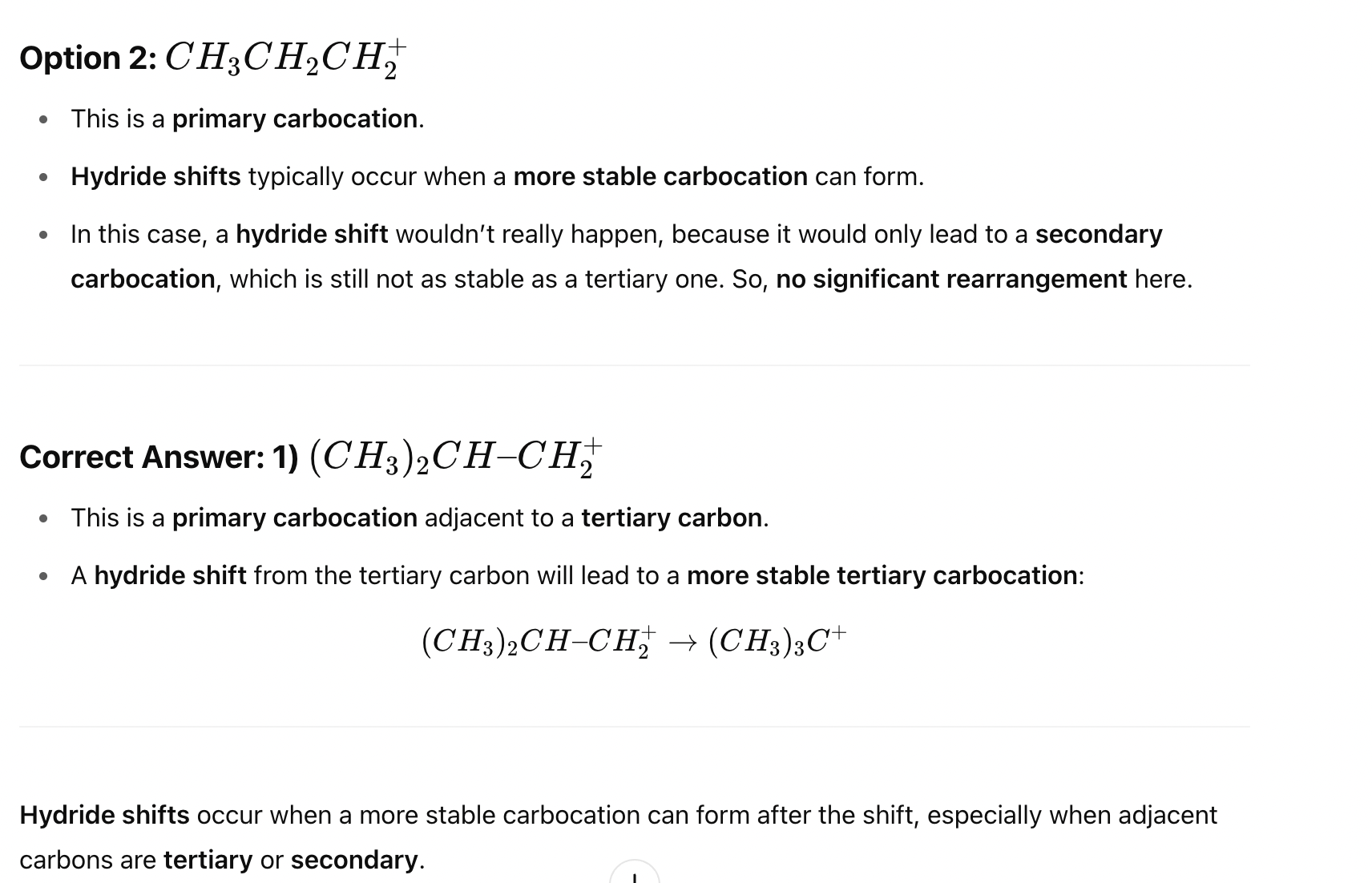
Which of the following halides is most likely to undergo SN2 substitution in polar aprotic solvents like DMSO?
Options:
Explanation:
Methyl iodide (CH3I) is highly reactive in SN2 reactions, especially in polar aprotic solvents like DMSO.
The iodine ion is a very good leaving group, and the methyl group is unstable but unhindered, making it easy for the nucleophile to attack the carbon in an SN2 reaction.
Explanation:
This is an SN2 reaction where ethyl bromide (CH₃-CH₂-Br) reacts with NaI in acetone.
Acetone is a polar aprotic solvent, which favors SN2 reactions because it doesn't solvate the nucleophile (I⁻) too much, allowing it to attack the electrophilic carbon more easily.
Iodide ion (I⁻) is a good nucleophile, and it displaces the bromine atom through backside attack, forming ethyl iodide (CH₃-CH₂-I).

Explanation:
Nitrobenzene (C₆H₅NO₂) is least reactive towards electrophilic aromatic substitution because the nitro group (-NO₂) is a strong electron-withdrawing group, which deactivates the benzene ring and makes it less reactive toward electrophiles.
The NO₂ group withdraws electron density from the ring, making the aromatic system less electron-rich, and therefore less likely to undergo substitution reactions.
Which of the following alcohols reacts fastest with Lucas reagent (HCl + ZnCl₂)?
1° butanol
2° butanol
2-methyl-2-propanol
Ethanol
🔍 Explanation:
Lucas reagent (HCl + ZnCl₂) is used to test alcohol reactivity via the SN1 mechanism, which depends on carbocation stability:
Order of reactivity:
Tertiary alcohol > Secondary alcohol > Primary alcohol
vLet’s look at the options:
1° Butanol → Slow reaction (least stable carbocation)
2° Butanol → Moderate speed
2-methyl-2-propanol → Tertiary alcohol, forms a very stable tertiary carbocation, reacts instantly with Lucas reagent
Ethanol → Primary alcohol, slowest
When Lucas reagent is added:
Tertiary alcohols: immediate turbidity
Secondary: turbidity after 5–10 mins
Primary: no reaction at room temp
Which of the following compounds undergoes the fastest dehydration on heating with conc. H₂SO₄?
Butan-2-ol
2-Methylpropan-2-ol
2-Phenylethanol
Propan-1-ol
2-Methylpropan-2-ol
Let’s break it down:
Dehydration of alcohols (using conc. H₂SO₄ and heat) usually follows the E1 mechanism (except for primary alcohols), and the key step is the formation of a stable carbocation after loss of –OH.
Which of the following compounds will undergo nucleophilic addition with HCN (hydrogen cyanide)?
Acetone (CH₃COCH₃)
Acetaldehyde (CH₃CHO)
Benzaldehyde (C₆H₅CHO)
Cyclohexanone (C₆H₁₀O)
Nucleophilic Addition with HCN:
HCN reacts with carbonyl compounds (aldehydes and ketones) in a nucleophilic addition reaction, where the cyanide ion (CN⁻) adds to the carbonyl carbon, forming a cyanohydrin.
🧠 Reactivity Order (more electrophilic carbonyl group):
Aldehydes > Ketones
What will be the major product when acetanilide is treated with Br₂ in acetic acid?
o-Bromoacetanilide
p-Bromoacetanilide
2,4,6-Tribromoacetanilide
m-Bromoacetanilide
✅ Correct answer: 2) p-Bromoacetanilide 🧠 Explanation:
Acetanilide has a –NHCOCH₃ group, which is moderately activating and directs ortho and para.
Br₂ in acetic acid gives electrophilic bromination.
Para position is preferred due to less steric hindrance compared to ortho.
🧪 So the product is:
Q1. Which of the following reagents is used in Stephen’s reaction to reduce a nitrile to an aldehyde?
A) LiAlH₄
B) SnCl₂ / HCl
C) H₂ / Pd
D) NaBH₄
Answer: B) SnCl₂ / HCl
Q2. The compound CH₃CH₂CN on Stephen’s reaction followed by hydrolysis gives:
A) Propanoic acid
B) Propanal
C) Ethanoic acid
D) Ethanal
Answer: B) Propanal
Q3. Identify the correct product: CH₃–CH₂–CN→HClSnCl2→H3O+ ?\text{CH₃–CH₂–CN} \xrightarrow[\text{HCl}]{\text{SnCl}_2} \xrightarrow{\text{H}_3\text{O}^+} \, ?CH₃–CH₂–CNSnCl2HClH3O+?
A) CH₃CH₂CHO
B) CH₃CH₂NH₂
C) CH₃CH₂COOH
D) CH₃CH₂OH
Answer: A) CH₃CH₂CHO (Propanal)
Q4. Which starting compound will give benzaldehyde on Stephen’s reduction?
A) C₆H₅–COOH
B) C₆H₅–CH₃
C) C₆H₅–CN
D) C₆H₅–CH₂Clx
Q4. Which starting compound will give benzaldehyde on Stephen’s reduction?
A) C₆H₅–COOH
B) C₆H₅–CH₃
C) C₆H₅–CN
D) C₆H₅–CH₂Cl
Answer: C) C₆H₅–CN
Q5. What is the intermediate formed in Stephen’s reaction before hydrolysis?
A) Imine (Schiff base)
B) Amide
C) Aldehyde
D) Alcohol
Answer: A) Imine (specifically: Ar–CH=NH·HCl)
Q6. Which of the following reagents would convert benzyl cyanide (Ph–CH₂–CN) into phenylacetic acid (Ph–CH₂–COOH)?
A) SnCl₂ / HCl
B) H₃O⁺
C) H₂ / Pd
D) NaOH + H⁺ (heat)
Answer: D) NaOH + H⁺ (heat) — acidic hydrolysis of nitrile
1. The product formed when ethyl cyanide (CH₃CH₂CN) reacts with CH₃MgBr followed by hydrolysis is:
Butan-2-one
Propanoic acid
Butanoic acid
Butan-1-ol
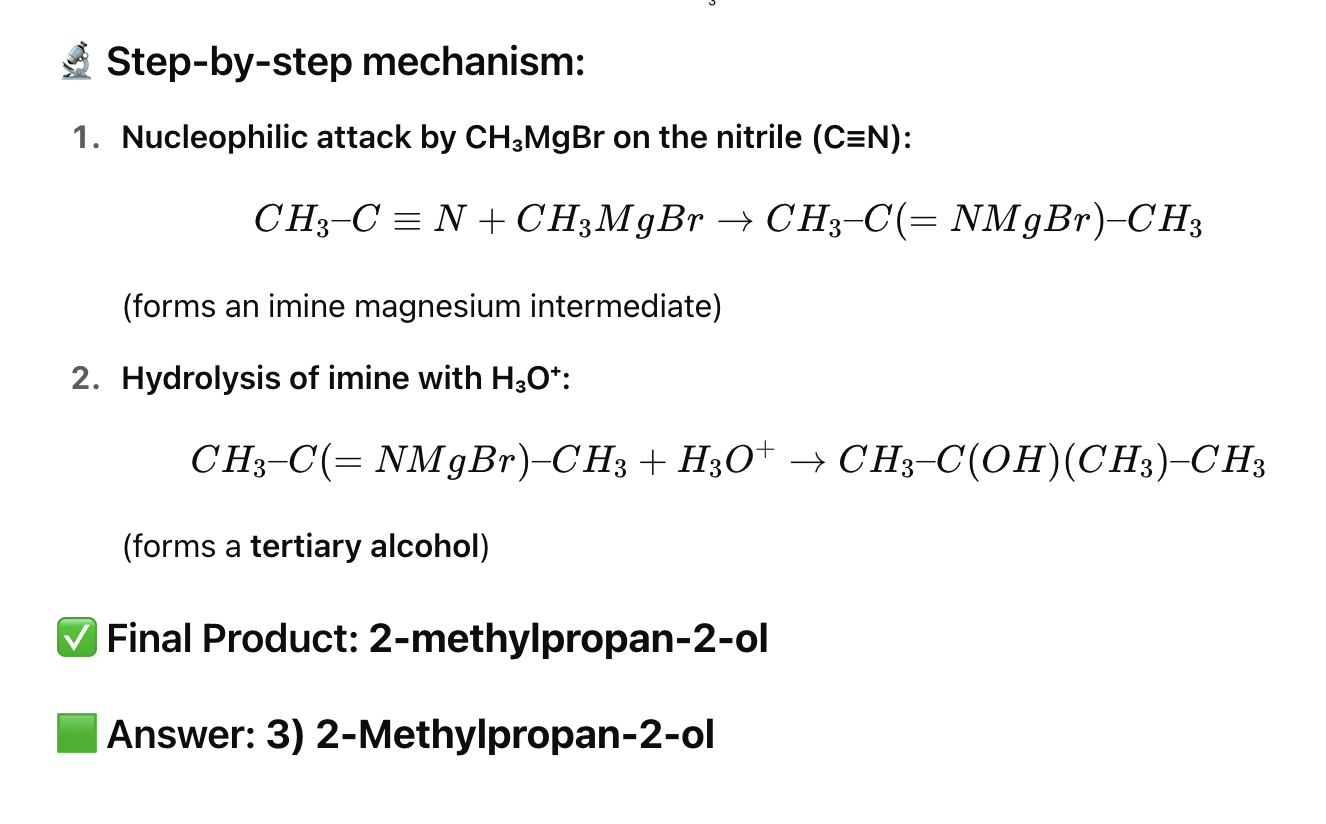
✅ 1. Reaction of ethyl cyanide (CH₃CH₂CN) with CH₃MgBr followed by hydrolysis 🧪 Step-by-step:
CH₃CH₂–C≡N + CH₃MgBr → imine intermediate
Hydrolysis → CH₃CH₂–CO–CH₃ (Butan-2-one)
✅ Answer: 1) Butan-2-one
✅ 3. CH₃–CN + excess CH₃MgBr followed by hydrolysis 🧪 Step-by-step:
CH₃–C≡N + CH₃MgBr → CH₃–C(MgBr)=NCH₃
Hydrolysis → CH₃–C(CH₃)(OH)–CH₃ = 2-methylpropan-2-ol
✅ Answer: 3) 2-Methylpropan-2-ol
🔬 Step-by-step mechanism:
4. The reaction of benzonitrile with acidic KMnO₄ leads to:
Benzaldehyde
Benzyl alcohol
Benzoic acid
Acetophenone
✅ 4. Benzonitrile + KMnO₄ (acidic) 📍 Reaction: Ph–CN→H⁺KMnO4
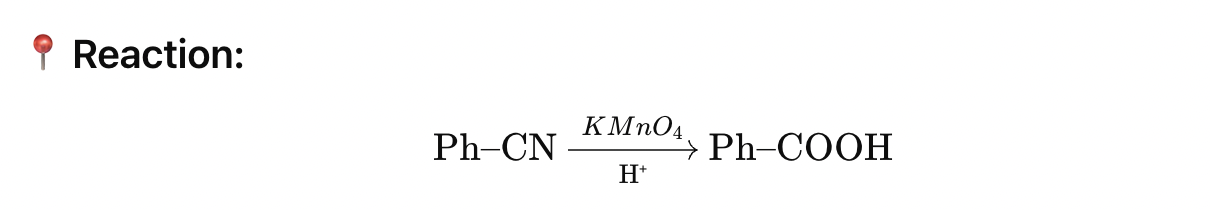
KMnO₄ is a strong oxidizing agent.
It oxidizes the –CN group → –COOH (nitrile → carboxylic acid).
🔄 Transformation:
CN (–C≡N) → COOH (–COOH)
✅ Final Product: Benzoic acid 🟩 Answer: 3) Benzoic acid
5. Identify the correct product in the following reaction: CH₃CH₂CN+CH₃MgBr→H₃O ?
Butan-2-one
Pentan-2-one
Butan-1-ol
Pentan-1-ol
✅ 5. CH₃CH₂CN + CH₃MgBr followed by hydrolysis 🧪 Step-by-step:
CH₃CH₂–C≡N + CH₃MgBr → CH₃CH₂–C(MgBr)=NCH₃
Hydrolysis → CH₃CH₂–CO–CH₃ (Butan-2-one)
✅ Answer: 1) Butan-2-one
Q1. Which of the following is most reactive in nucleophilic addition?
a) CH₃CHO
b) CH₃COCH₃
c) HCHO
d) C₆H₅CHO
🔍 Reason: Formaldehyde has no alkyl groups, so it’s least hindered and most electrophilic, making it the most reactive in nucleophilic addition.
rrange the following in decreasing order of reactivity in nucleophilic addition:
Formaldehyde
Acetone
Benzaldehyde
Acetaldehyde
1 > 4 > 3 > 2
(Formaldehyde > Acetaldehyde > Benzaldehyde > Acetone)
Q3. Which of the following ketones is less reactive in nucleophilic addition?
a) CH₃COCH₃
b) CH₃COC(CH₃)₃
c) CH₃COCH₂CH₃
d) CH₃COCH₂Cl
(b) CH₃COC(CH₃)₃
→ Bulky tert-butyl group increases steric hindrance = less reactive.
. Which one of the following carbonyl compounds reacts fastest with NaHSO₃?
a) Acetone
b) Benzaldehyde
c) Formaldehyde
d) Propanal
🧠 What’s NaHSO₃ Reaction About?
NaHSO₃ adds nucleophilically to the carbonyl carbon of aldehydes/ketones → forming bisulfite addition products.
So, the faster the nucleophile can attack the carbonyl, the faster the reaction.
💡 Factors Affecting Reactivity:
Electrophilicity of carbonyl carbon (How +ve is it?)
Steric hindrance (Are groups blocking the approach?)
Inductive/resonance effects
🔍 Option Analysis:
(a) Acetone (CH₃COCH₃):
It's a ketone with 2 alkyl groups → moderate electrophilicity and some steric hindrance.(b) Benzaldehyde (C₆H₅CHO):
Aldehyde → generally more reactive than ketone.
But phenyl ring has resonance → reduces electrophilicity.(c) Formaldehyde (HCHO):
Has no alkyl or bulky groups. Super electrophilic and zero steric hindrance.(d) Propanal (CH₃CH₂CHO):
Simple aldehyde, but has 1 alkyl group → slightly less reactive than formaldehyde.
✅ Correct Answer: (c) Formaldehyde
👉 Because:
No alkyl groups = no +I effect
Least steric hindrance
Most reactive carbonyl toward nucleophiles like NaHSO₃
Q5. Which of the following gives the product of nucleophilic addition with HCN most rapidly?
a) CH₃COCH₃
b) CH₃CH₂CHO
c) HCHO
d) C₆H₅COCH₃
💡 Concept Recap: Nucleophilic Addition to Carbonyl
In this reaction:
Nucleophile (CN⁻) attacks the carbonyl carbon
Rate depends on:
Electrophilicity of carbonyl carbon (how +ve it is)
Steric hindrance (how crowded it is)
Resonance/inductive effects
🧪 Option Analysis: a) CH₃COCH₃ (Acetone)
It's a ketone with two methyl groups
Moderate electrophilicity, some steric hindrance
❌ Not the fastest
b) CH₃CH₂CHO (Propanal)
Aldehyde → more reactive than ketone
Has one ethyl group (little steric hindrance)
✅ Faster than ketones
c) HCHO (Formaldehyde)
No alkyl groups → maximum electrophilicity
No steric hindrance
✅✅ Most reactive of all
d) C₆H₅COCH₃ (Acetophenone)
It's a ketone and the phenyl ring can delocalize electrons by resonance
Reduces electrophilicity
❌ Slower
✅ Correct Answer: (c) HCHO (Formaldehyde)
Because:
Highest electrophilicity
Least steric hindrance
No +I or resonance effect to reduce reactivity
What is the product when CH₃CHO (acetaldehyde) is treated with NaOH and heated?
a) CH₃CH₂OH
b) CH₃COOH
c) CH₃CH(OH)CH₂CHO
d) CH₃CH=CHCHO
acetaldehyde does have α-hydrogens, so it undergoes an aldol condensation under basic, heated conditions rather than a Cannizzaro reaction.
🔍 Detailed Solution:
Aldol Condensation Conditions:
Base (NaOH) + heat + aldehyde with α-hydrogens ⇒ aldol.
Mechanism Outline:
Step 1: Base abstracts an α-hydrogen from one molecule of CH₃CHO, forming an enolate.
Step 2: The enolate attacks the carbonyl carbon of a second CH₃CHO molecule, giving a β-hydroxyaldehyde (the “aldol”).
Product Structure:
CH3CH(OH)CH2CHO\text{CH}_3\text{CH(OH)CH}_2\text{CHO}
Benzaldehyde on treatment with conc. NaOH followed by heating gives:
a) Benzyl alcohol
b) Benzoic acid
c) Cinnamaldehyde
d) Benzoin
(a) Benzyl alcohol, which is correct for the organic product of the Cannizzaro reaction of benzaldehyde under conc. NaOH and heat (the other product being sodium benzoate, which on acid work‐up gives benzoic acid).
Which of the following converts acetophenone (Ph–CO–CH₃) into 1-phenylethanol (Ph–CHOH–CH₃)?
a) PCC
b) LiAlH₄
c) HNO₃
d) KMnO₄
✅ Q4 Answer Check
You chose (b) LiAlH₄, which is correct.
🔍 Detailed Solution:
Acetophenone (Ph–CO–CH₃) is a ketone.
LiAlH₄ is a strong reducing agent that converts ketones to secondary alcohols.
Thus:
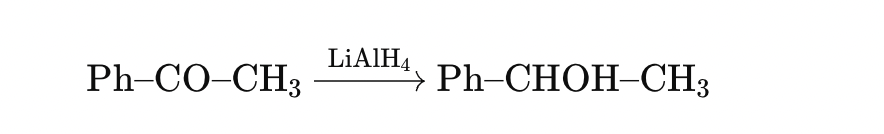
Acetone reacts with 2 equivalents of an alcohol (ROH) in acidic conditions to form:
a) Acetal
b) Ketal
c) Hemiacetal
d) Cyanohydrin
🔍 Detailed Solution:
Nucleophilic Addition to a Ketone
A ketone R–CO–R′\text{R–CO–R′}R–CO–R′ reacts with two equivalents of an alcohol in the presence of acid to form a ketal:
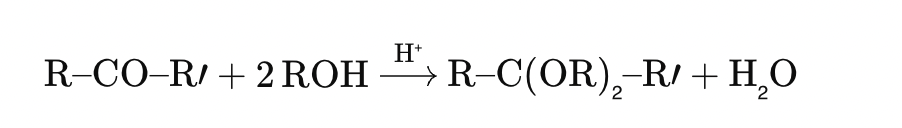
Acetal vs. Ketal
Acetal: product of an aldehyde + 2 ROH
Ketal: product of a ketone + 2 ROH
Which of the following reagents can selectively cleave an acetal back to the aldehyde or ketone?
a) Dilute NaOH
b) Dilute HCl
c) LiAlH₄
d) PCC
Why dilute HCl (acid) works:
Protonation of one –OR group makes it a good leaving group.
Water attacks, kicking out an alcohol.
Repeats until you regenerate the aldehyde/ketone.
Under dilute NaOH, none of that happens—acetals remain intact.
Which of the following is NOT a protecting group for aldehydes or ketones?
a) 1,3-dioxolane
b) Acetal
c) Cyanohydrin
d) Hemiacetal
1,3-Dioxolane (a cyclic acetal) and acetals in general are classic carbonyl protecting groups because they’re stable under basic and neutral conditions and can be cleanly deprotected with acid.
Cyanohydrins, while less common, can also “mask” a carbonyl: the –CN can be later removed (e.g. by mild hydrolysis) to regenerate the aldehyde/ketone.
Why hemiacetals are not:
Hemiacetals (R–C(OH)(OR′)\text{R–C(OH)(OR′)}R–C(OH)(OR′)) are in rapid equilibrium with the free carbonyl and alcohol.
They’re too unstable to serve as an isolable protecting group—they revert back under both acid and base
🌟🌟 Q1. The major product formed when benzene reacts with CH₃Cl in the presence of anhydrous AlCl₃ is:
A) Benzyl chloride
B) Toluene
C) Chlorobenzene
D) Benzaldehyde
🌟 Q1. Benzene + CH₃Cl + AlCl₃ → ?
Correct Answer: B) Toluene
📘 Explanation: This is Friedel-Crafts alkylation. Benzene reacts with CH₃Cl in the presence of AlCl₃ (a Lewis acid catalyst) to form Toluene (C₆H₅CH₃).
🌟 Q2. Oxidation of ethanol with acidified K₂Cr₂O₇ gives:
A) Acetic acid
B) Ethene
C) Methanol
D) Acetone
🌟 Q2. Ethanol + Acidified K₂Cr₂O₇ → ?
Correct Answer: A) Acetic acid
📘 Explanation: Ethanol undergoes oxidation with acidified potassium dichromate (K₂Cr₂O₇/H⁺) to first form acetaldehyde and then further oxidized to acetic acid (CH₃COOH).
🌟 Q3. The product formed when cyclopentene is treated with cold dilute alkaline KMnO₄ is:
A) Cyclopentanone
B) Cyclopentanol
C) cis-1,2-Cyclopentanediol
D) Cyclopentene oxide
🌟 Q3. Cyclopentene + cold dilute alkaline KMnO₄ → ?
Correct Answer: C) cis-1,2-Cyclopentanediol
📘 Explanation: This is Baeyer’s test. Cold dilute KMnO₄ gives cis-1,2-diols via syn-hydroxylation across the double bond.
🌟 Q4. The product of acetylene (C₂H₂) passed through a dilute H₂SO₄/HgSO₄ mixture is:
A) Ethanol
B) Acetaldehyde
C) Acetic acid
D) Methanol
🌟 Q4. Acetylene + Dil H₂SO₄ + HgSO₄ → ?
Correct Answer: C) Acetic acid
📘 Explanation: Acetylene undergoes hydration in the presence of H₂SO₄ and Hg²⁺, forming vinyl alcohol first, which tautomerizes to acetaldehyde and then gets oxidized to acetic acid under acidic conditions.
🌟 Q5. Propene + HBr + Peroxide → ?
Correct Answer: A) 1-Bromopropane
📘 Explanation: Due to the peroxide effect (anti-Markovnikov addition), Br adds to the less substituted carbon, giving 1-Bromopropane.
🌟 Q5. Propene + HBr + Peroxide → ?
Correct Answer: A) 1-Bromopropane
📘 Explanation: Due to the peroxide effect (anti-Markovnikov addition), Br adds to the less substituted carbon, giving 1-Bromopropane.
Q4. The product of acetylene (C₂H₂) passed through a dilute H₂SO₄/HgSO₄ mixture is:
A) Ethanol
B) Acetaldehyde
C) Acetic acid
D) Methanol
Hot alkaline KMnO₄ is a strong oxidizing agent.
It oxidizes side chains attached to the benzene ring if the side chain has at least one benzylic hydrogen (hydrogen attached to the carbon connected directly to the aromatic ring).
This oxidation converts the entire side chain into a carboxyl group (-COOH), forming benzoic acid.
Importantly, the oxidation stops at the acid stage, regardless of how long the side chain is (like ethyl or propyl), as long as there is at least one benzylic hydrogen.
Which reagent selectively oxidizes a primary alcohol to an aldehyde without further oxidizing it to a carboxylic acid?
A) PCC (Pyridinium chlorochromate)
B) KMnO₄ / H⁺ (hot)
C) Jones reagent (CrO₃/H₂SO₄)
D) HNO₃ / heat
Explanation:
PCC selectively oxidizes primary alcohols to aldehydes without further oxidation to carboxylic acids.
Jones reagent (CrO₃/H₂SO₄) and KMnO₄ oxidize primary alcohols all the way to carboxylic acids.
HNO₃ is a strong oxidizer and would typically over-oxidize as well.
Q3. Which of the following reagents is used to convert an aldehyde into a primary alcohol?
A) NaBH4
B) PCC
C) KMnO4
D) H2SO4
NaBH4 (Sodium borohydride) is a reducing agent that converts aldehydes (and ketones) into primary (and secondary) alcohols by adding hydrogen.
PCC (Pyridinium chlorochromate) oxidizes primary alcohols to aldehydes, so it doesn’t reduce aldehydes.
KMnO4 is a strong oxidizing agent that converts aldehydes to carboxylic acids.
H2SO4 is an acid, not a reducing agent.
When phenol is treated with bromine water, the major product formed is:
A) o-Bromophenol
B) p-Bromophenol
C) 2,4,6-Tribromophenol
D) No reaction
Explanation:
Phenol is highly activated towards electrophilic substitution due to the -OH group.
When bromine water is added to phenol, it undergoes multiple substitution at the ortho (2,6) and para (4) positions.
This gives 2,4,6-tribromophenol as the major product, which is a white precipitate.
Q: Which of the following reagents is used for the oxidation of primary alcohols to aldehydes?
A) KMnO₄
B) PCC (Pyridinium chlorochromate)
C) H₂SO₄
D) LiAlH₄
KMnO₄ is a strong oxidizing agent that usually oxidizes primary alcohols all the way to carboxylic acids, not stopping at aldehydes.
PCC is a milder oxidizing agent that stops the oxidation at the aldehyde stage when reacting with primary alcohols.
H₂SO₄ is an acid, not an oxidizing agent.
LiAlH₄ is a reducing agent, not an oxidizer.
The Cannizzaro reaction is a redox reaction where non-enolizable aldehydes (those without α-hydrogens) undergo disproportionation in the presence of a strong base (typically NaOH). One molecule of the aldehyde is reduced to an alcohol, and the other is oxidized to a carboxylic acid (or its salt).
Key point:
Only aldehydes without α-hydrogens undergo Cannizzaro reaction.
Let's look at the aldehydes you've mentioned (interpreting them in textual form):
Formaldehyde (HCHO)
✅ No α-hydrogen → Cannizzaro possibleBenzaldehyde (C₆H₅CHO)
✅ No α-hydrogen → Cannizzaro possibleTrimethylacetaldehyde (CH₃)₃C–CHO
✅ No α-hydrogen (due to bulky tert-butyl group) → Cannizzaro possibleAcetaldehyde (CH₃CHO)
❌ Has α-hydrogens → Cannizzaro NOT possible → Undergoes aldol condensationPropionaldehyde (CH₃CH₂CHO)
❌ Has α-hydrogens → Cannizzaro NOT possible
Oh My Son’s Great Apple Pie
Each word helps you recall one dicarboxylic acid, increasing by one –CH₂– unit each time:
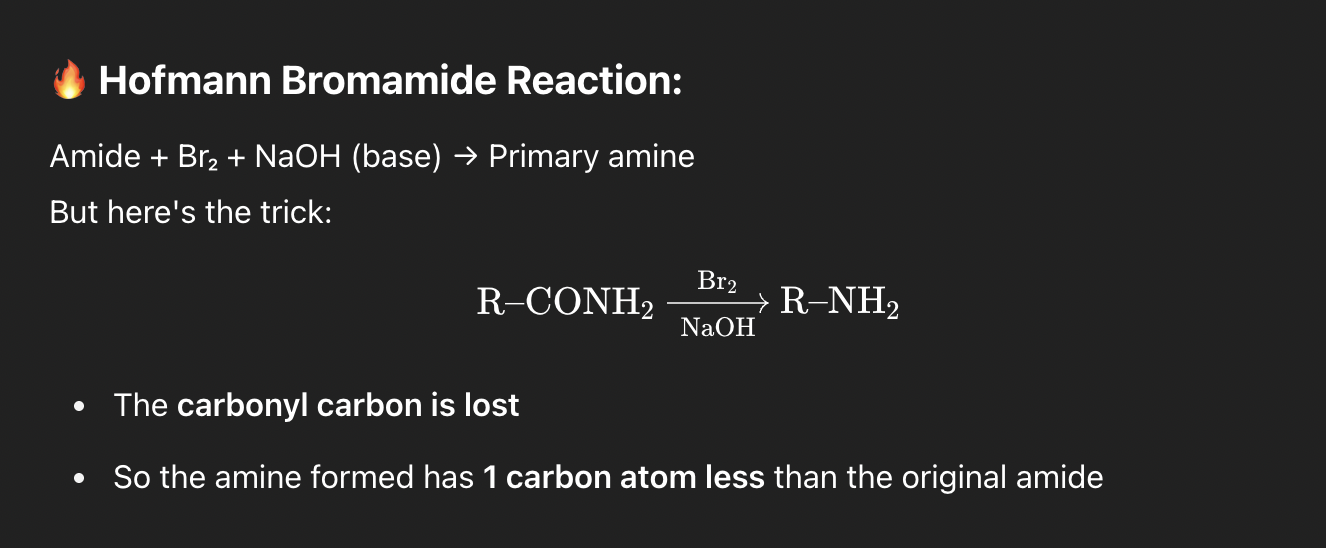
🔥 Hofmann Bromamide Reaction:
Amide + Br₂ + NaOH (base) → Primary amine
But here's the trick:
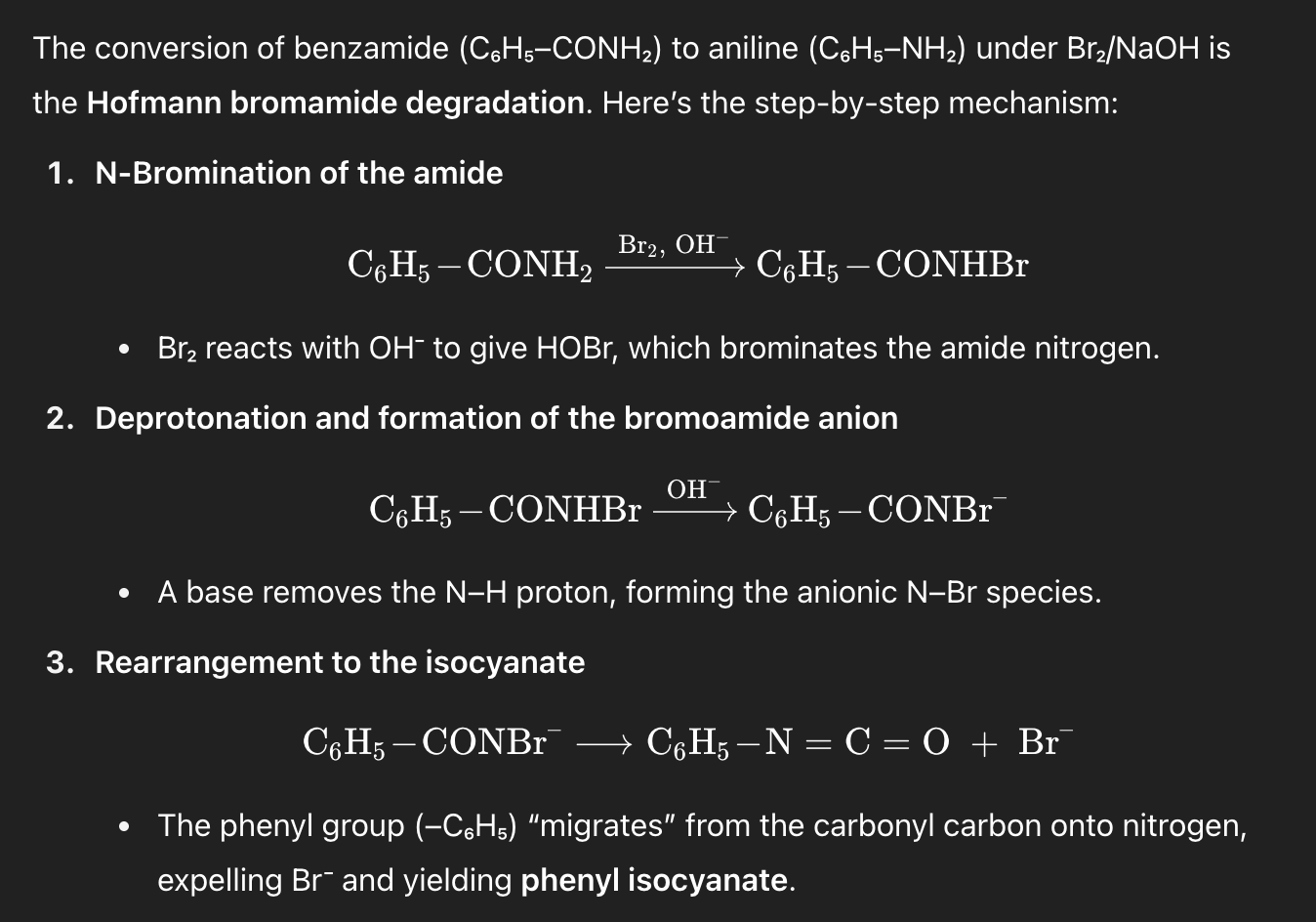
Q1. Hofmann degradation
On treating benzamide with Br₂ and NaOH, the major organic product is:
A. Aniline (C₆H₅–NH₂)
B. Benzylamine (C₆H₅–CH₂–NH₂)
C. Benzoic acid (C₆H₅–COOH)
D. Benzonitrile (C₆H₅–CN)
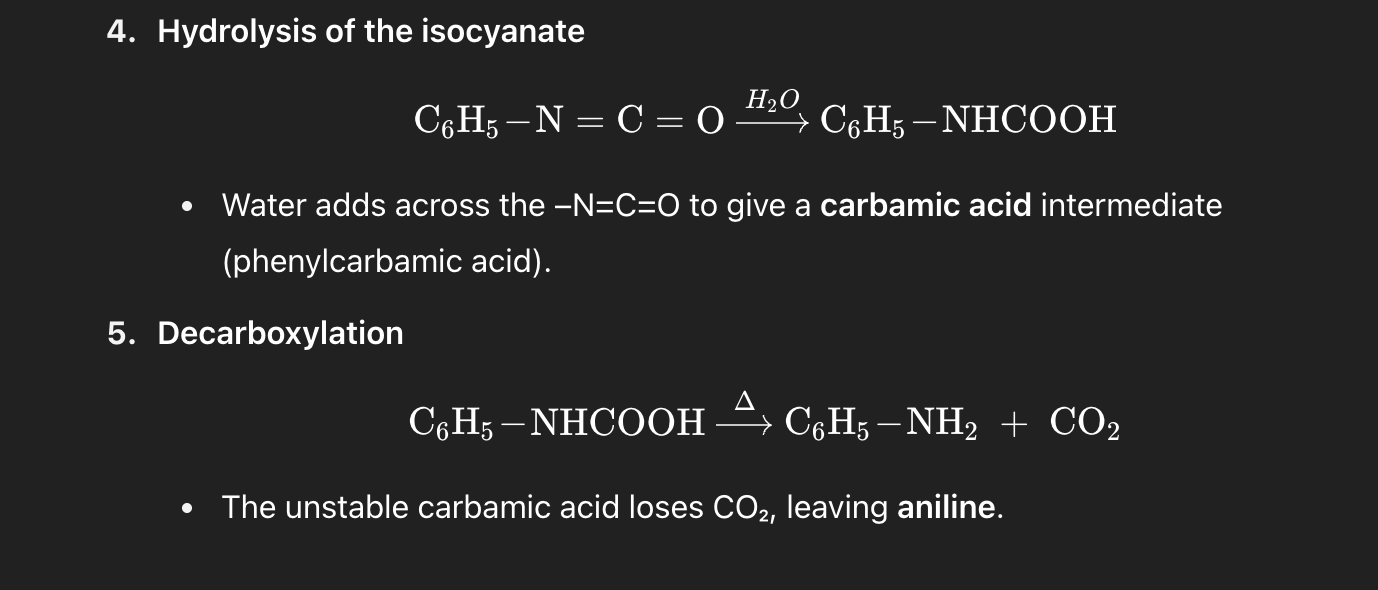
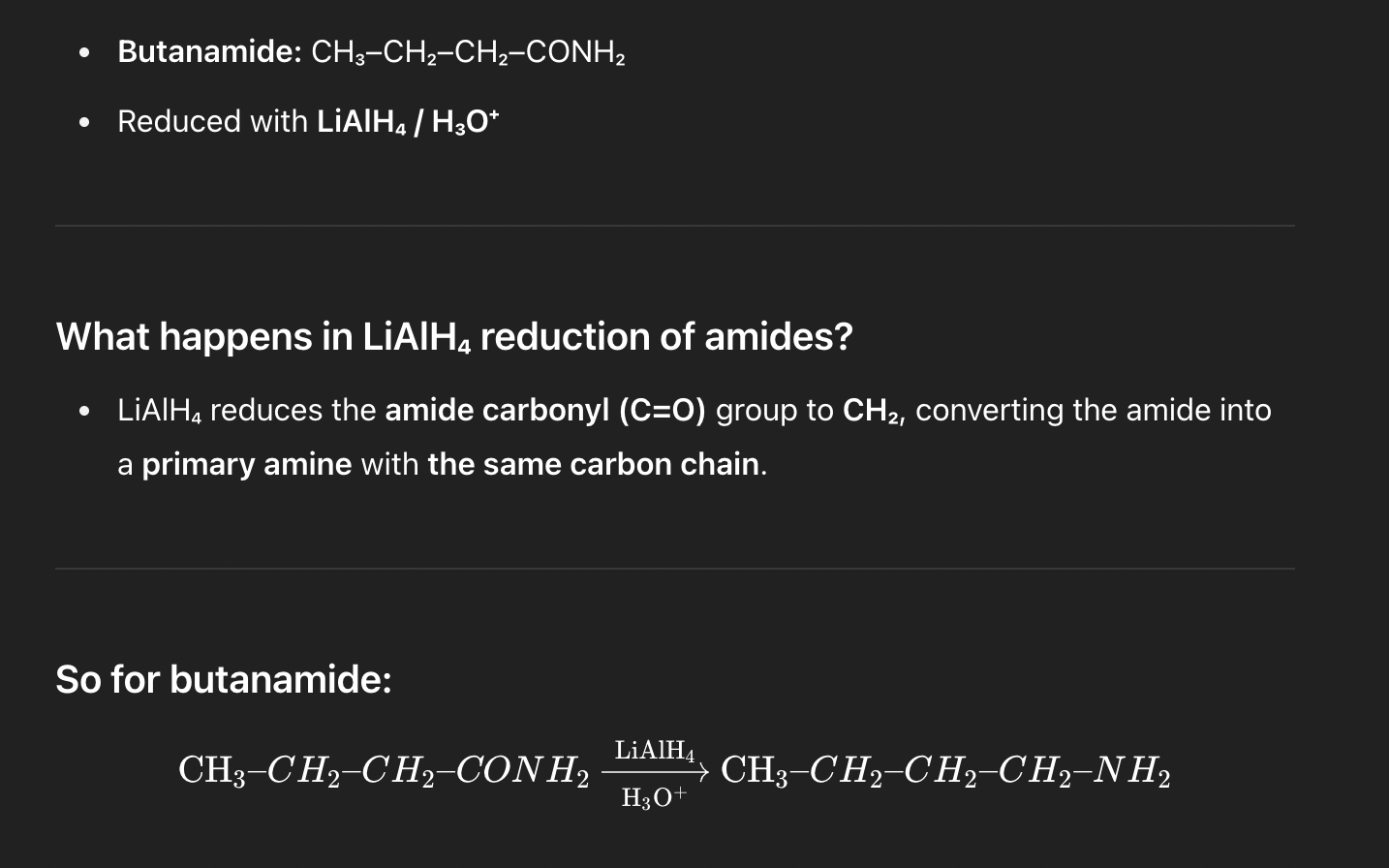
Q2. LiAlH₄ reduction
Butanamide (CH₃–CH₂–CH₂–CONH₂) on exhaustive reduction with LiAlH₄ / H₃O⁺ gives:
A. Butylamine (CH₃–CH₂–CH₂–CH₂–NH₂)
B. Propylamine (CH₃–CH₂–CH₂–NH₂)
C. 2-Amino butane (CH₃–CH(NH₂)–CH₂–CH₃)
D. Ammonia + butanol
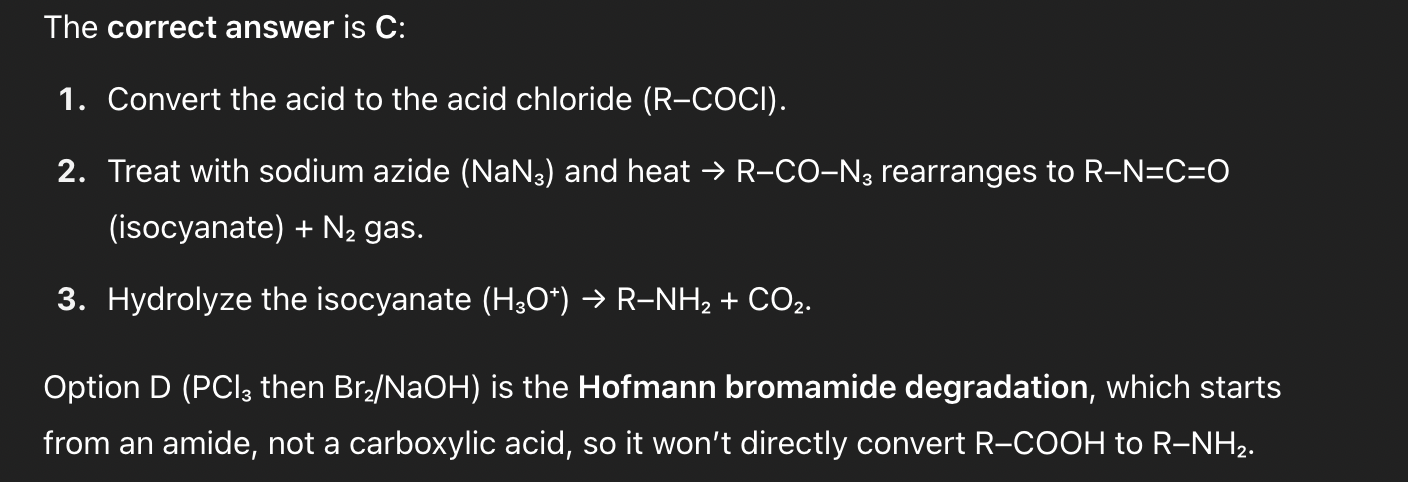
R–COOH → R–NH₂ (same carbon skeleton) via an isocyanate intermediate.
A. SOCl₂ then NH₃, heat
B. PCl₅ then H₂O
C. R–COCl then NaN₃, Δ, H₃O⁺
D. PCl₃ then Br₂, NaOH
Q4. Dehydration of a primary amide R–CONH₂ on treatment with P₂O₅ or SOCl₂ at high temperature gives:
A. Nitrile (R–C≡N)
B. Imine (R–CH=NH)
C. Amine (R–CH₂–NH₂)
D. Amide anhydride (R–CO–O–CO–R)
