MCAT General Chemistry - Bonding and Chemical Interactions
1/54
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
Maillard reaction
a nucleophilic reaction between the amino terminus of the peptide chain of a protein and the carbonyl functionality of a sugar to form an N-substituted glycosylamine; a complex series of rearrangements and other reactions to produce a set of compounds that gives cooked food its pleasing color and delectable flavor
ex. browning meat, crisping cookies
molecules
combinations of bonded atoms
chemical bonds
strong attractive forces between atoms in a molecule formed via the interaction of the valence electrons of the combining atoms
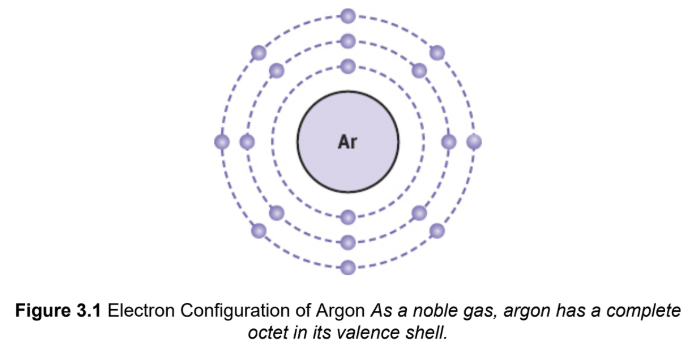
octet rule
an atom tends to bond with other atoms so that it has eight electrons in its outermost shell, thereby forming a stable electron configuration similar to that of the noble gases
Incomplete octet
stable with fewer than 8 electrons in their valence shell
hydrogen (2 electrons)
helium (2)
lithium (2)
beryllium (4)
boron (6)
Expanded octet
Any element in period 3 and greater can hold more than 8 electrons
phosphorus (10)
sulfur (12)
chlorine (14)
Odd numbers of electrons
Any molecule with an odd number of valence electrons cannot distribute those electrons to give eight to each atom
ex. nitric oxide (NO) has eleven valence electrons
common elements that almost always abide by the octet rule
carbon, nitrogen, oxygen, fluorine, sodium, magnesium
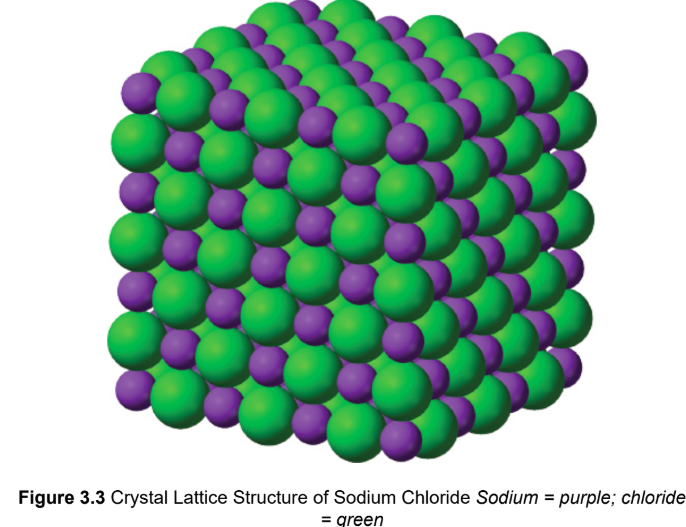
ionic bonding
one or more electrons from an atom with a low ionization energy, typically a metal, are transferred to an atom with a high electron affinity, typically a nonmetal; difference in electronegativity must be greater than 1.7 on the Pauling scale

ionic crsytalline lattice
repeating rows of cations and anions; attractive forces between oppositely charged ions are maximized, and the repulsive forces between ions of like charge are minimized
covalent bonding
an electron pair is shared between two atoms, typically nonmetals, that have relatively similar values of electronegativity
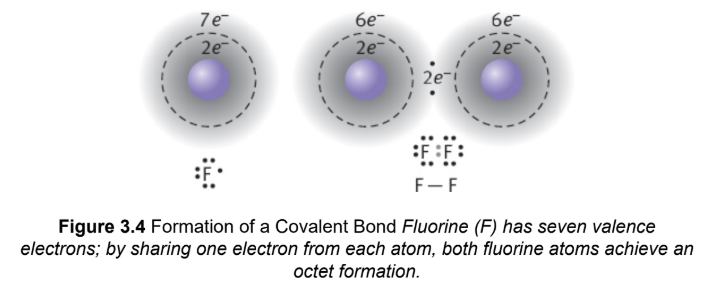
nonpolar covalent bond
the electron pair is shared equally; no separation of charge (dipole) across the bond; difference in electronegativity less than 0.5
polar covalent bond
the electron pair is shared unequally; separation of charge (dipole) across the bond; difference in their electronegativities between 0.5 and 1.7
coordinate covalent
both of the shared electrons are contributed by only one of the two atoms; a lone pair of one atom attacked another atom with an unhybridized p-orbital to form a bond, Lewis acid–base reactions
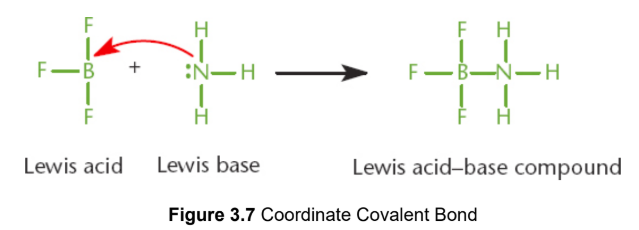
cation
positively charged ion; loses electron(s)
anion
negatively charged ion, gains electron(s)
ionic compounds
very high melting/boiling points
sissolve readily in water/polar solvents
molten/aqueous - good conductors
crystalline lattice
single bond
covalent bond sharing 2 electrons
double bond
covalent bond sharing 4 electrons
triple bond
covalent bond sharing 6 electrons
bond order
the number of shared electron pairs between two atoms
Bond length
average distance between the two nuclei of atoms in a bond; as bond order increases, bond length decreases;
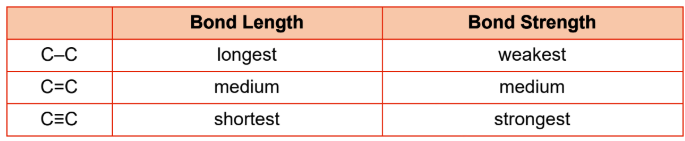
Bond energy
energy required to break a bond by separating its components into their isolated, gaseous atomic states; higher bond order, more energy required
polarity (bond)
two atoms have a relative difference in electronegativities; higher electronegativity gets larger share of electron density, creating a dipole
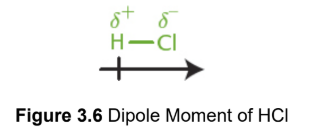
partial negative charge (δ–)
more electronegative element acquiring a greater portion of the electron density
partial positive charge (δ+)
less electronegative element acquiring a smaller portion of the electron density
dipole moment
vector quantity of separation of charge in a molecule, from positive to negative
p = qd
p is the dipole moment
q is the magnitude of the charge
d is the displacement vector separating the two partial charges.
measured in Debye units
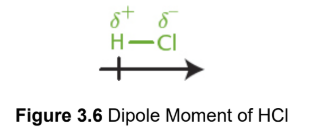
Debye units
unit of dipole moment, also coulomb-meters
bondingelectrons,
valence electrons involved in a covalent bond
nonbonding electrons
valence electrons not involved in a covalent bond, aka lone pairs
Lewis structure/dot diagram
system of notation developed to keep track of the bonded and nonbonded electron pairs; number of dotsThe difference
between the number of electrons assigned to an atom in a Lewis
structure and the number of electrons normally found in that atom’s
valence shell comes from group numbers
formal charge
The difference between the number of electrons assigned to an atom in a Lewis structure and the number of electrons normally found in that atom’s valence shell
formal charge = V - Nnonbonding - ½ Nbonding = valence - dots - sticks
V is the normal number of electrons in the atom’s valence
shell
Nnonbonding is the number of nonbonding electrons
Nbonding is the number of bonding electrons
A Lewis structure with small or no formal charges is preferred over a Lewis structure with large formal charges.
A Lewis structure with less separation between opposite charges is preferred over a Lewis structure with a large separation of opposite charges.
A Lewis structure in which negative formal charges are placed on more electronegative atoms is more stable than one in which the negative formal charges are placed on less electronegative atoms.
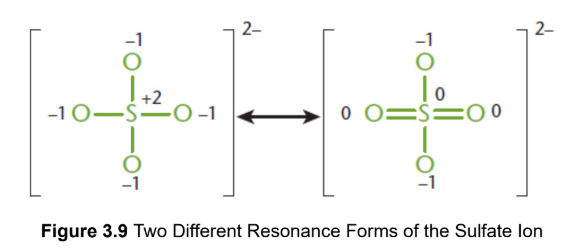
resonance structures
two or more Lewis structures that demonstrate the same arrangement of atoms but that differ in the specific placement of the electrons; represented with a double headed arrow between them; actual electronic distribution in the compound is a hybrid, or composite, of all of the possible resonance structures
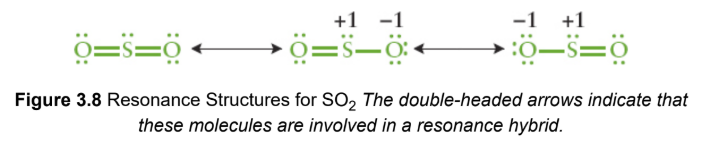
resonance hybrid
actual structure of a compound with multiple resonance structures; more stable structure contribute more to the character
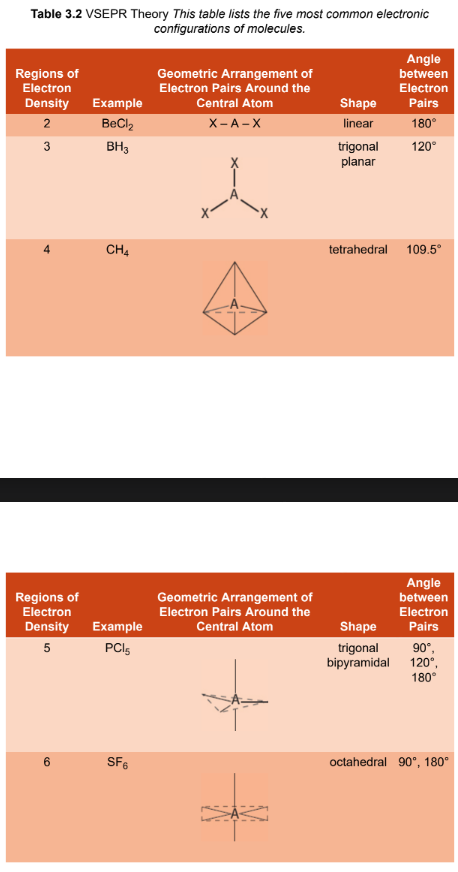
valence shell electron pair repulsion (VSEPR) theory
uses Lewis dot structures to predict the molecular geometry of covalently bonded molecules; the three- dimensional arrangement of atoms surrounding a central atom is determined by the repulsions between bonding and nonbonding electron pairs in the valence shell of the central atom
Electronic geometry
the spatial arrangement of all pairs of electrons around the central atom, including both the bonding and the lone pairs
molecular geometry
the spatial arrangement of only the bonding pairs of electrons
coordination number
number of atoms that surround and are bonded to a central atom
bond angle
the angle formed between three atoms across at least two bonds
linear electronic geometry
2 regions of electrons density
180°

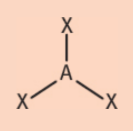
trigonal planar electronic geometry
3 regions of electrons density
120°
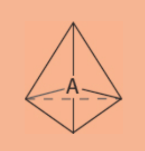
tetrahedral electronic geometry
4 regions of electrons density
109.5°
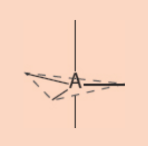
trigonal bipyramidal electronic geometry
5 regions of electrons density
90°, 120°, 180°
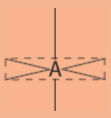
octahedral electronic geometry
6 regions of electrons density
90°, 180°
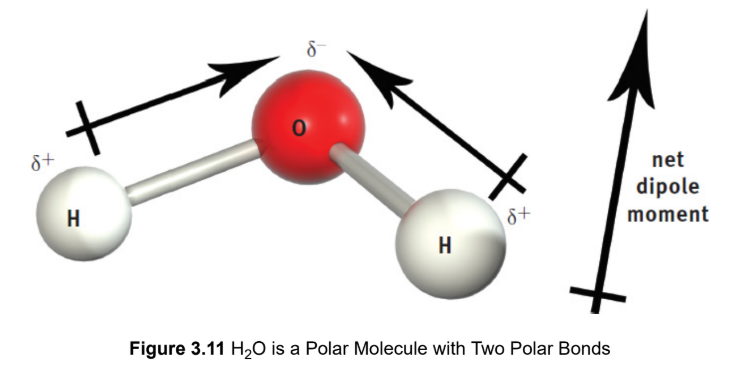
polarity (molecule)
a molecule with polar bonds that do not cancel each other out, causing a net dipole moment
molecular orbital
atomic orbitals interact to form a model that describes the probability of finding the bonding electrons in a given space; obtained by combining the wave functions of the atomic orbitals
bonding orbital
the signs of the two interacting atomic orbitals are the same
bonding orbital
the signs of the two interacting atomic orbitals are different
sigma (σ) bond
orbitals overlap head-to-head; allow for free rotation about their axes; the electron density of the bonding orbital is a single linear accumulation between the atomic nuclei
pi (π) bond
overlap in two parallel electron cloud densities; do not allow for free rotation; electron densities of the orbitals cannot be twisted in such a way that allows continuous overlapping of the clouds of electron densities
intermolecular forces
force that mediates interaction between molecules; relatively weak
London dispersion forces
type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; very weak and temporary
van der Waals force
distance-dependent interaction between atoms or molecules
Dipole–dipole interactions
the positive region of one molecule aligns with the negative region of another molecule to form an attractive electrostatic force; present in solid and liquid phases
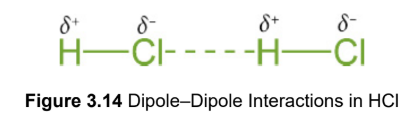
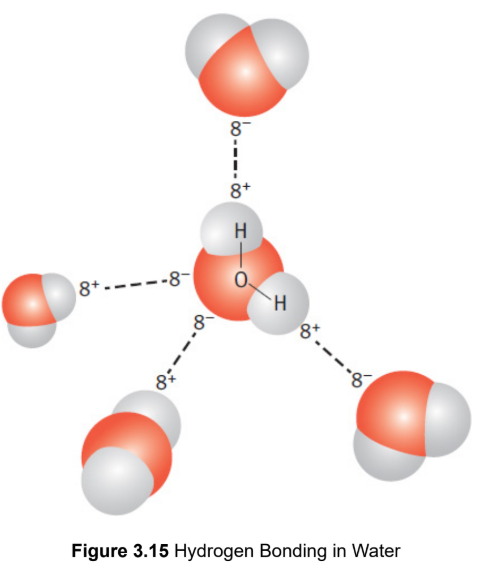
hydrogen bond
specific, unusually strong form of dipole–dipole interaction; positively charged hydrogen atom interacts with the partial negative charge of fluorine, oxygen, or nitrogen on nearby molecules
ex. nitrogenous bases