Lecture 48: Liquid dosage forms: Suspensions and emulsions 1
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
what are the characteristics of suspensions?
they are dispersions in which the drug is dispersed in the external phase(vehicle)
the solubility of drug in the vehicle is low
diameter of dispersed phase: 0.5 to 100 micrometers
colloidal systems are formed when the particle size is less than 0.5 micrometers
explain the physical stability of suspensions
they are fundamentally unstable, leading to:
sedimentation
particle-particle interactions and ultimately
caking(compaction)
to understand their physical stability, we need to understand the electrical properties of dispersed particles and the effect of distance of separation between particles on their subsequent interaction
this will help us understand how we will have a stable suspension
may need to add components to make them more stable
Electrical properties of dispersed particles:
how can particles obtain a charge following dispersion within an aqueous medium?
ionisation of functional groups on the drug molecule and/or
adsorption of ions to the surface of the particle
following adsorption of ions on to the surface, this establishes the electrical double layer
the boundary of this second layer will possess a potential referred to as the zeta potential. what is the zeta potential?
it measures the degree of electrical charge on particles relative to the bulk medium in which they are suspended
how can pharmaceutical suspensions be stabilised?
adding electrolytes(increasing conc) to suspensions compress the electrical double layer
distance of separation and the interaction between particles: what are the three states of interaction possible?
no interaction: particles are sufficiently distant from one another making them thermodynamically stable (in absence of sedimentation)
coagulation(agglomeration): particles form an intimate contact with each other: this is a pharmaceutically unacceptable formulation as the particles cant redisperse upon shaking
loose aggregation(termed floccules): loose reversible interaction between the particles; enabling the particles to be redispersed upon shaking
what are the opposing forces in particle suspensions?
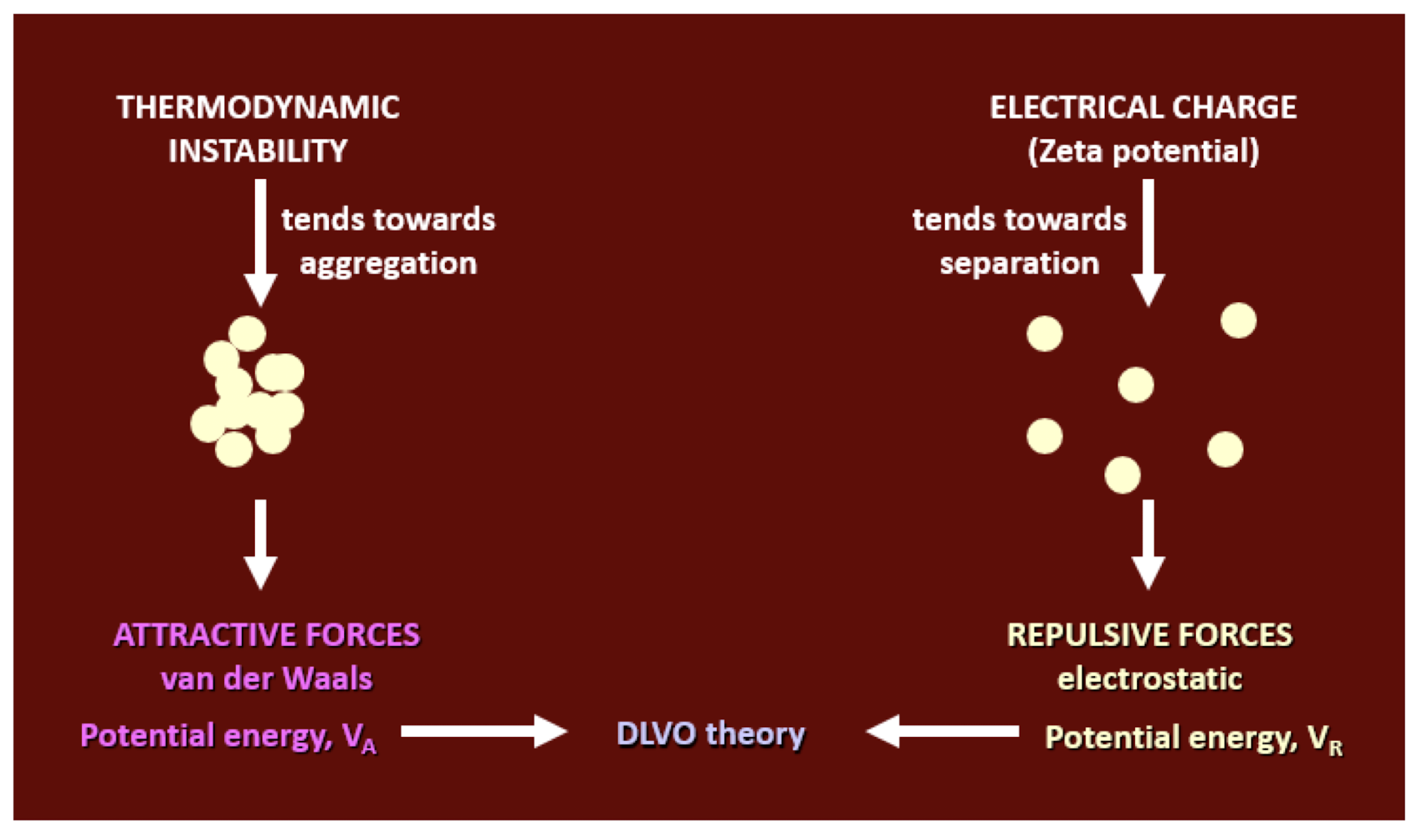
what is the DLVO theory?
when dispersed in a liquid medium, particles will experience repulsive (electrical) forces and attractive(london/vdw) forces

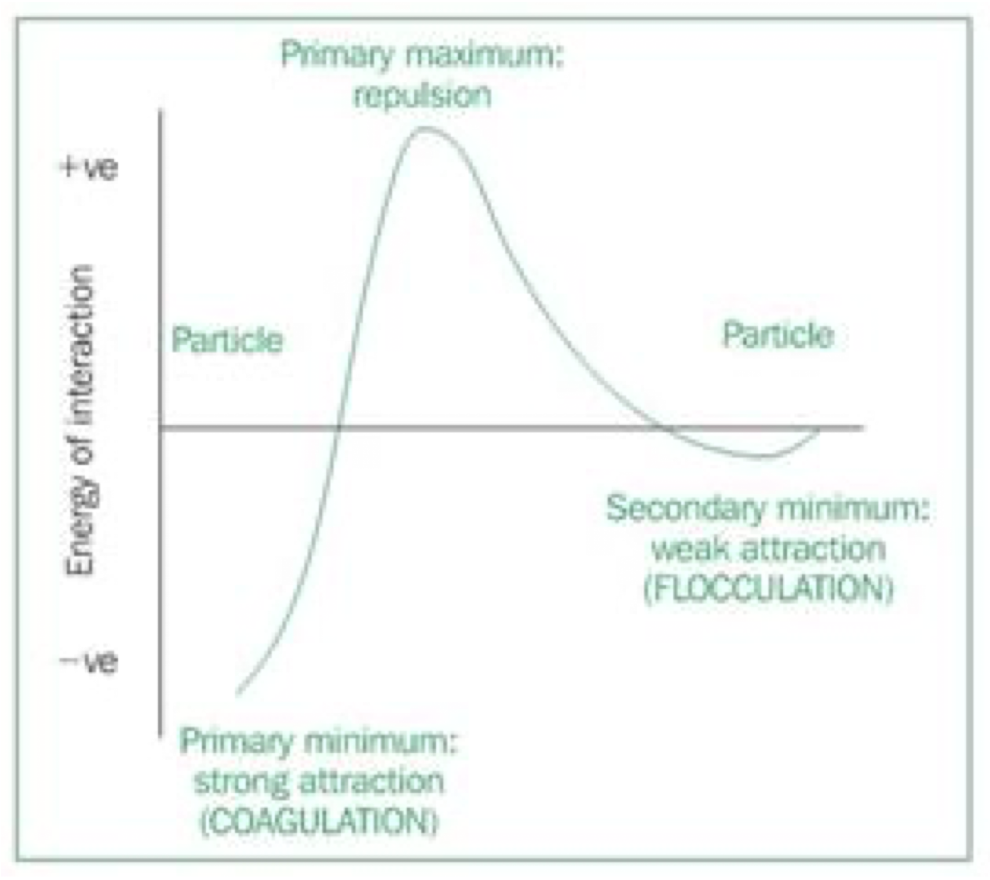
Explain the energy of interaction graph
potential energy curve for particles in suspension shows 2 minima and 1 maximum
at short inter-particle distances, attractive forces predominate(primary minimum) and particles tend to agglomerate
as inter-particle distance increases(sufficient energy added to separate particles) repulsive forces predominate, and particles remain in suspension(maxiumum)
if the inter-particle distance increased further, repulsive force decreases and particles are weakly attracted( secondary minimum)
the depth of the secondary minimum is key to determining the stability of the system
what is sedimentation and what are its features?
particles in a suspension will sediment under the influence of gravity and settle at the bottom of the container
larger particles will reach the bottom initially and the smaller particles occupy the space between the larger particles
what is caking?
particles at the bottom of the container are gradually compressed by the weight of those above and in doing so enough energy is available to overcome the primary maximum(repulsive forces) and the particles become sufficiently close to form an irreversible interaction at the primary minimum.
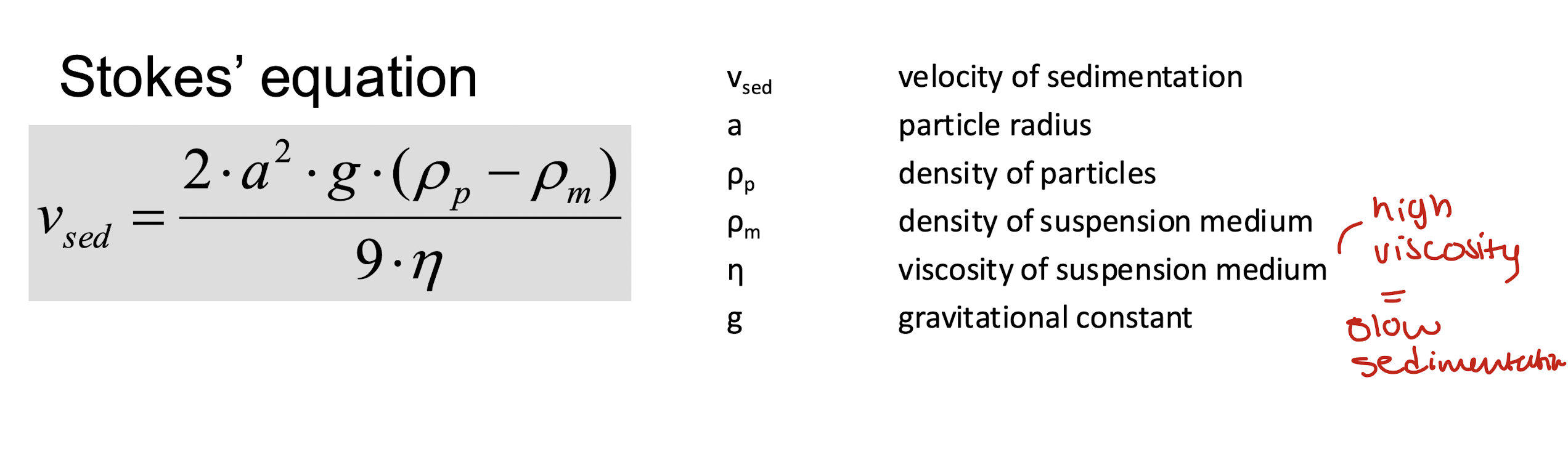
what is the equation for sedimentation and why do we control it?
controlling particle sedimentation may enhance physical stability of the suspension
rate of sedimentation may be practically decreased by reducing average particle diameter and increasing viscosity of vehicle
for particles in which the zeta potential(primary maximum) is high, manipulation of the magnitude of the secondary minimum is required(controlled flocculation)
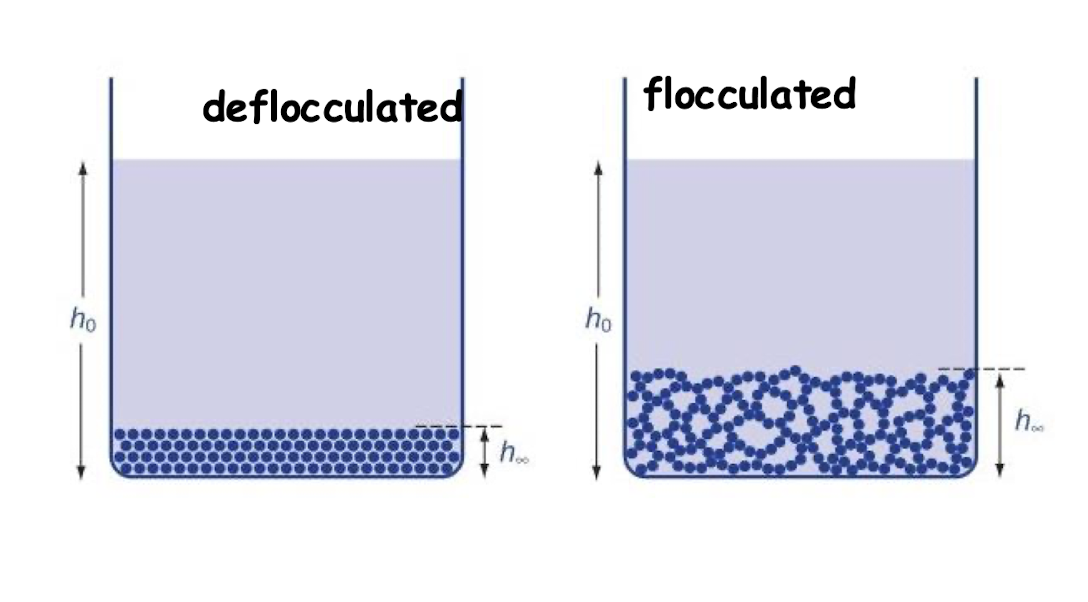
How is flocculation controlled?
by addition of electorlytes or charged surfactants that reduce zeta potential ( hence Vr) to give satisfactory secondary minimum in which flocs can be formed