L5 Reduction of Carbonyl and Carboxylic Acid Derivatives
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Reduction via hydrogenation
Needs a catalyst to acitvate the H-H bond.
It is liked in industry due to being atom efficient and H2 being cheap
Hydrogenation catalyst types
Heterogeneous - transition metals or salts e.g. Pd,PtO2. They may also be supported by an inert carrier like charcoal or clay. They arenaturally easy to separate.
Homeogeneous - often soluble TM complexes e.g. Wilkinson’s catalyst (Ph3P)3RhCl. Their advantage is ability to tune the ligands for selectivity
Hydride reduction
H- transferred as a nucleophile. However, H- is not nucleophilic and NaH, LiH etc are bases not reducing agents. They deprotonate, they do not attack electrophilic centres.
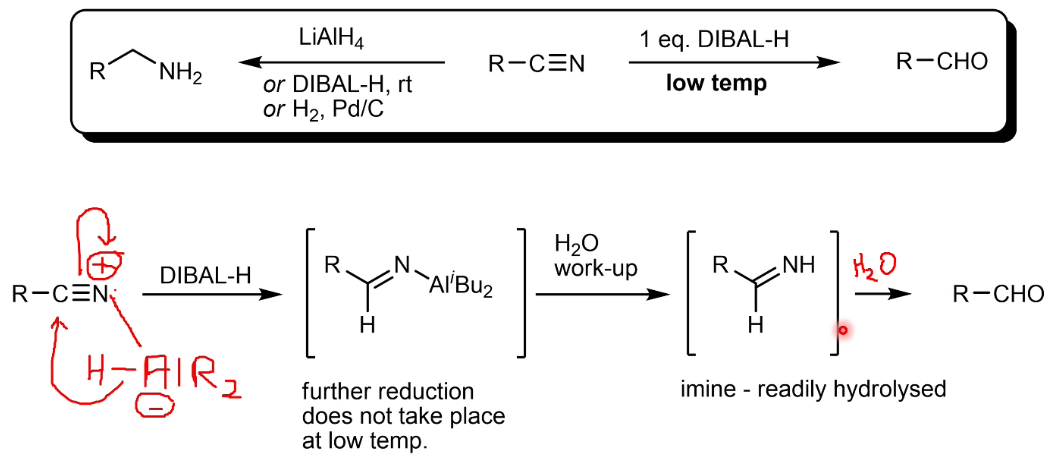
LiAlH4 features
Able to reduce all common polar functional groups. However, it is very moisture sensitive and produces H2 in a violent reaction with air (catches fire). Needs to be used in dry, aprotic solvents like Et2O or THF
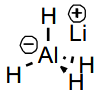
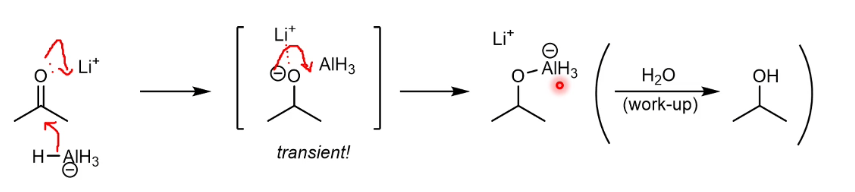
LiAlH4 mechanism for reduction
Li polarises the C=O bond further
NaBH4 features
Easy to handle white powder that can tolerate protic solvents. It is much less reactive than Lith-Al and is used to chemoselectively reduce aldehydes, ketones in the presence of carboxylic acid derivatives. (Not acid chlorides as they are reactive enough)
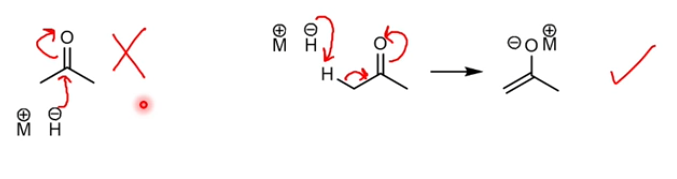
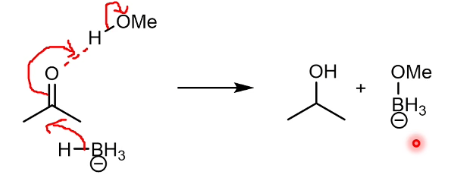
NaBH4 reduction mechanism
DIBAL features
This reagent is electrophilic. It is soluble in organic solvents thanks to the isobutyl.
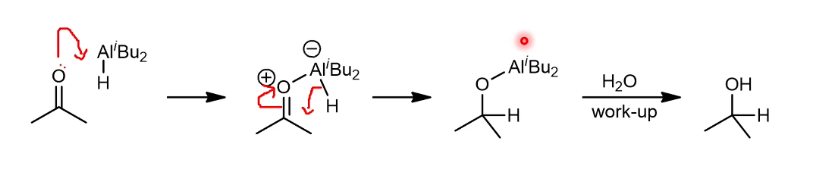
DIBAL reduction of carbonyl mechanism
It must first coordinate to the carbonyl to make it sp3 hybridised and -vely charged to transfer the hydride.
Borane features
Chemoselective for carboxylic acids over esters.
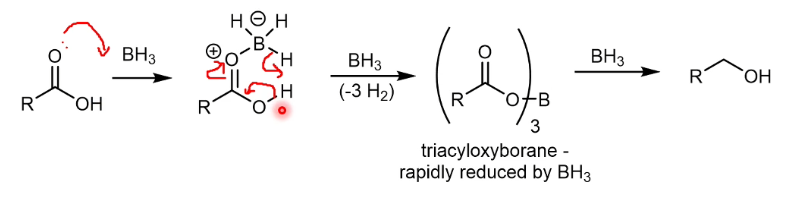
Borane reduction of carboxyl mechanism
Mechanism for borane reduction at the end is not needed - chemoselectivity factor is more relevant.
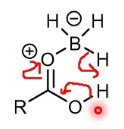
Why is bornae chemoselective for carboxylic acids over esters
The proton movement that happens here is not possible with an alkoxy group - the C-O bond does not break.
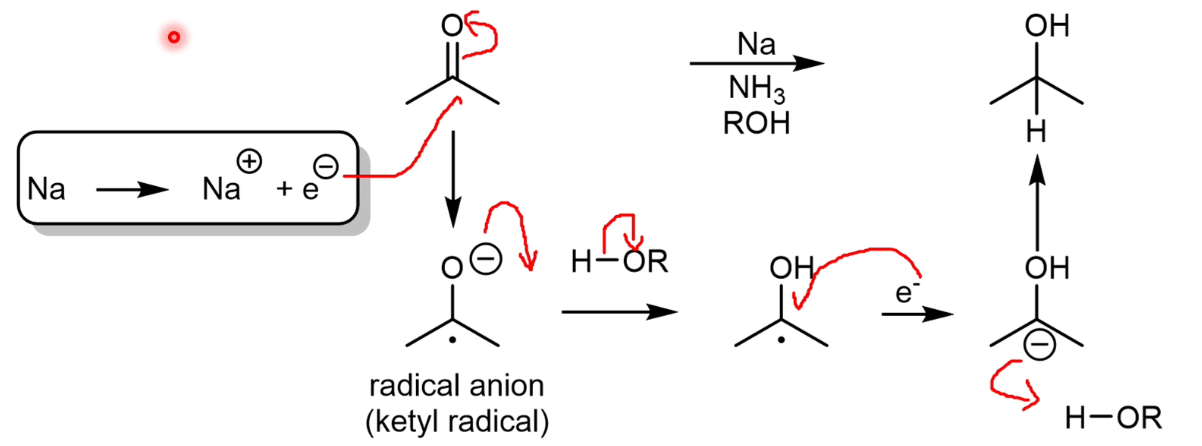
Dissolving metal reductions
Dissolving Na in liquid ammonia liberates free electrons.

Free electron reduction mechanism
Each electron added is followed by the addition of a hydrogen(proton).
Use for dissolving metal reagents given their difficulty to use
Larger reagents will attack on the less hindered face of the carbonyl - in this case preferring the thermodynamically less stable product.
The mechanism using free electrons does not get sterically hindered so we massively favour the thermodynamically more stable product.
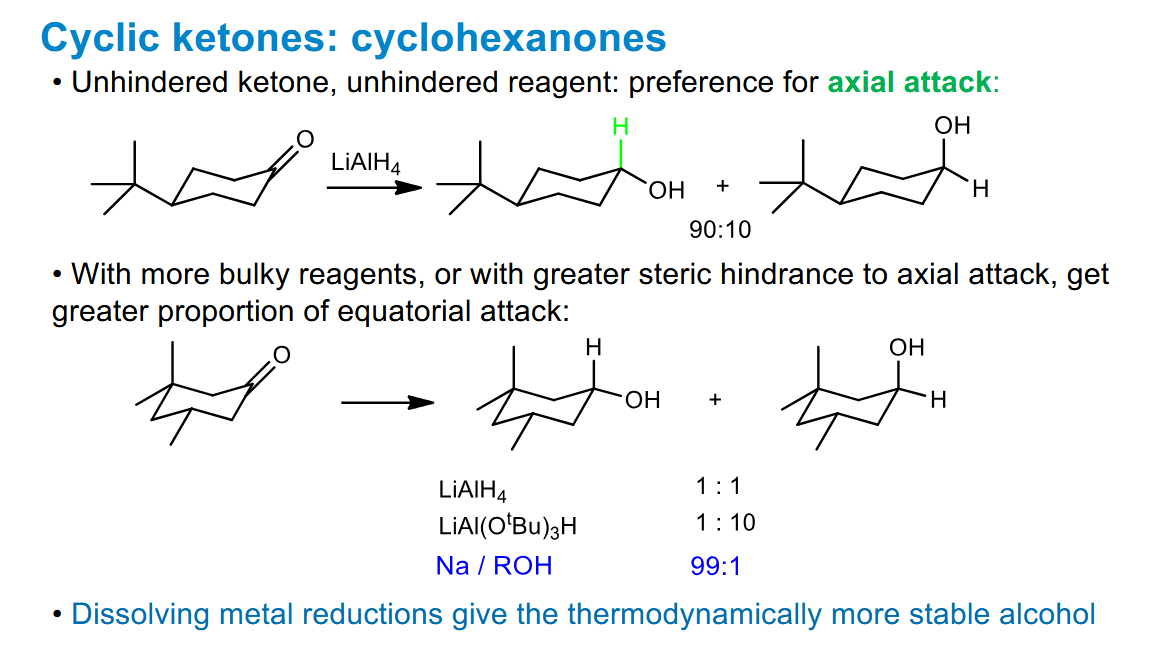
How can Felkin-Anh selectivity be switched using different reagents
You can use large reducing agents to favour the Felkin-Anh product according to sterics, or use a chelating reducing agent to force the other diastereomeric product.
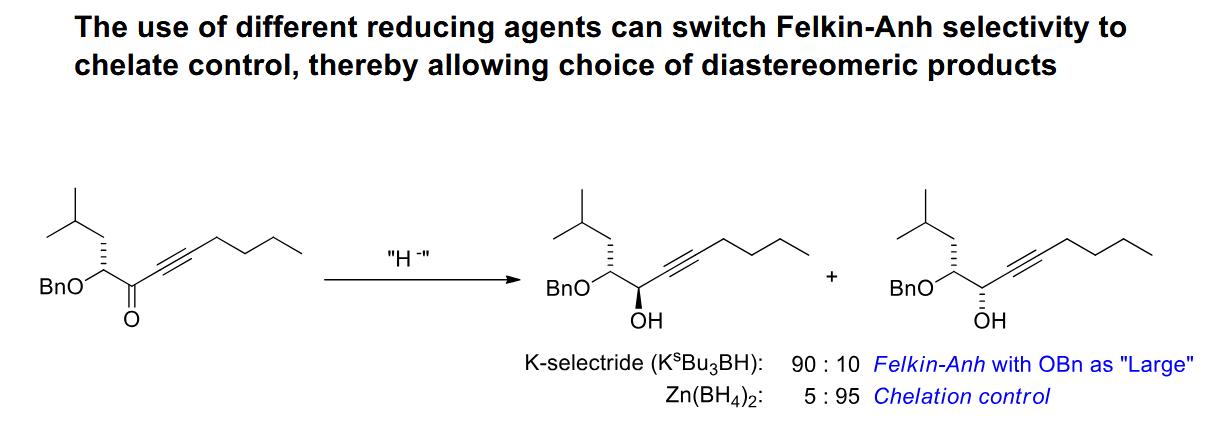
Reduction of a ketone to a secondary amine
We use a modified reducing agetn so it is not storng enough to just reduce the ketone. The ketone can form an imine in presence of acid (although the pH needs to be kept at a point where the amine is not too protonated) and the imine can then be reduced.
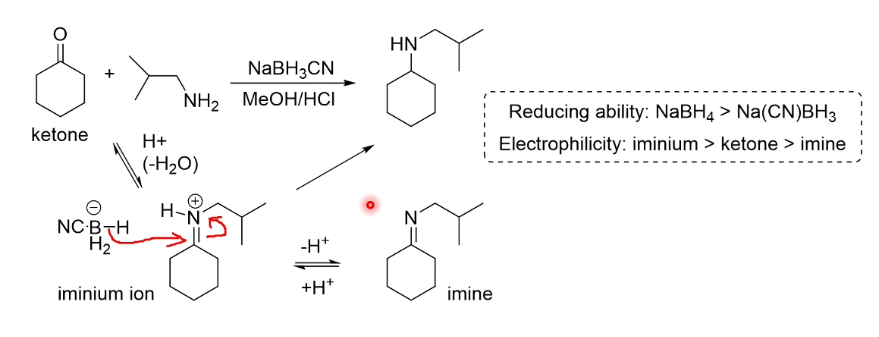
Reduction of a ketone directly to an alkane
3 possible routes
Crucially the top/bottom reactions use acid/base respecitvely, giving some options depending on reagent stability. The last reaction proceeds under neither.
Key properties to tetrahedral intermediates
They will collapse with loss of the best leaving group
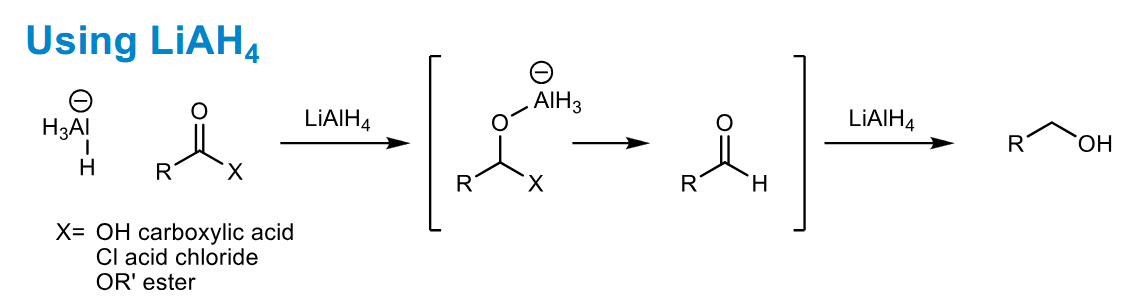
LiAlH4 reduction of carboxylic acid derivatives
Tetrahedral intermediate collapses with loss of X - best leaving group
Aldehydes are mosre reactive than ketones so naturally this instantly reacts again
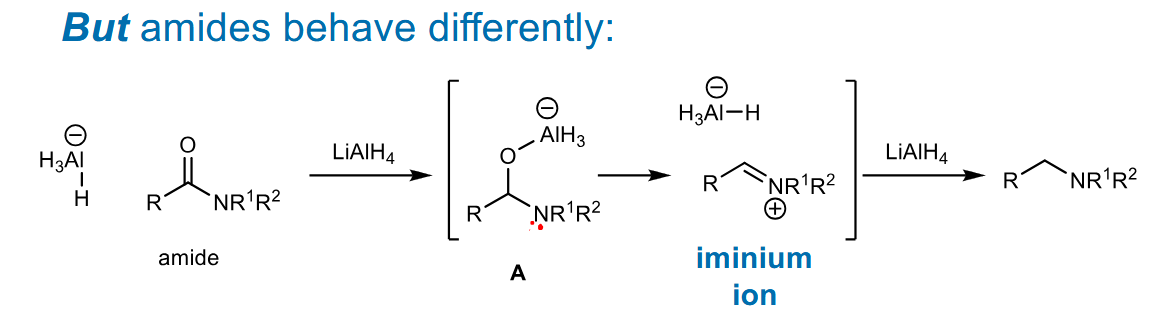
LiAlH4 reduction of amides
The high pKa of the amine means the oxygen is a better leaving group
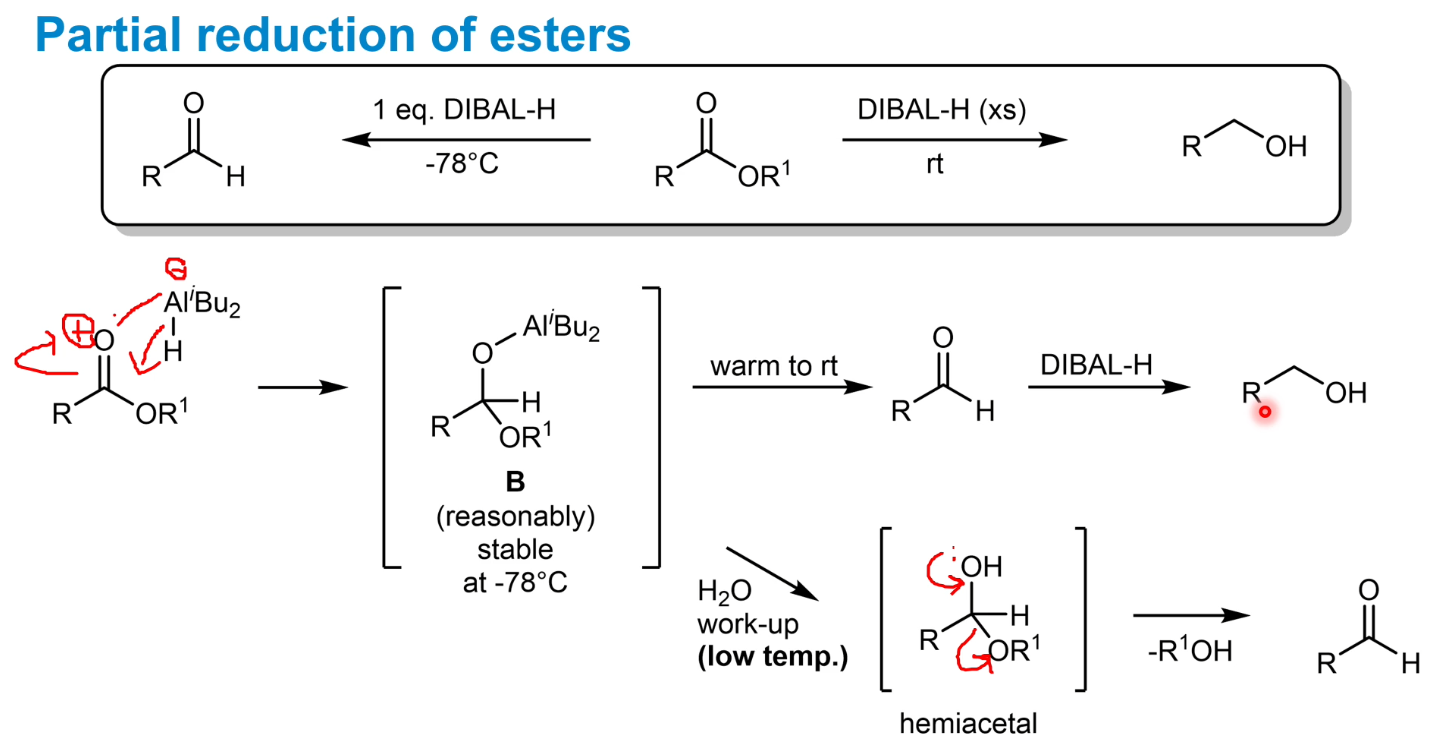
Partial reduction of esters to aldehydes
Crucially at low temps - the intermediate becomes stable. Water can then be added to break the O-Al bond
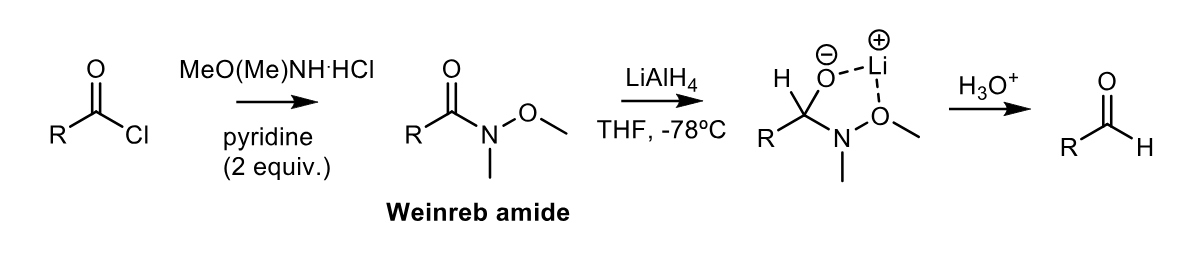
Partial reduction of Weinreb amides to aldehydes (+synthesis)
Reduction of nitriles using DIBAL