The Standard Hydrogen Electrode and cell EMF
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What is the standard hydrogen electrode?
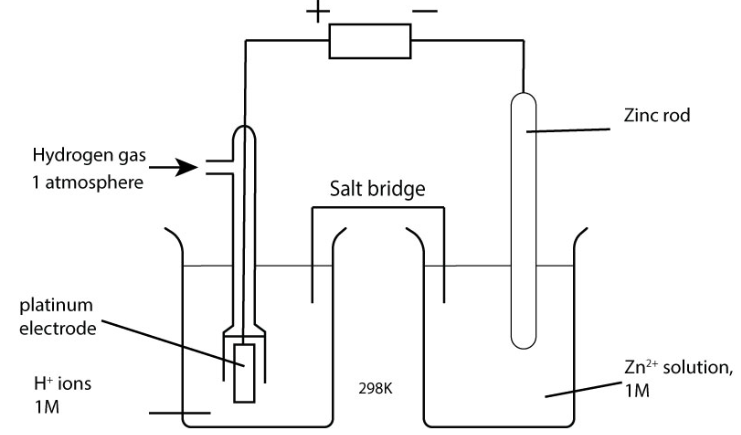
The standard hydrogen electrode is a half-cell which uses H2 gas at 1 atm, a solution of H+(aq) ions of concentration 1 moldm−3 (usually using 0.5 mol dm-3 sulfuric acid), and a platinum electrode.
How is the standard hydrogen electrode set up?
The standard hydrogen electrode (SHE) is connected to the positive terminal of the voltmeter and is placed on the left hand side of the circuit.
The half-cell being tested would be connected to the negative terminal of the voltmeter and be placed on the right.
The two half-cells would be connected by a salt bridge.
Why is a platinum electrode used when setting up the standard hydrogen electrode?
It is inert i.e doesn’t react much with hydrogen, and allows electrons to follow easily.
What standard conditions are required for the standard hydrogen electrode?
Temperature at 298K.
Pressure at 1 atm.
1 moldm−3 in respect to H+.
What equilibrium does the standard hydrogen electrode involve?
2H+(aq)+2e−⇌H2(g)
What is the standard electrode potential?
The potential difference of a cell when the electrode is connected to the standard hydrogen electrode under standard conditions.
What is the standard electrode potential for the SHE?
0V- it is used as a reference half cell to measure the electrode potentials of different half cells.
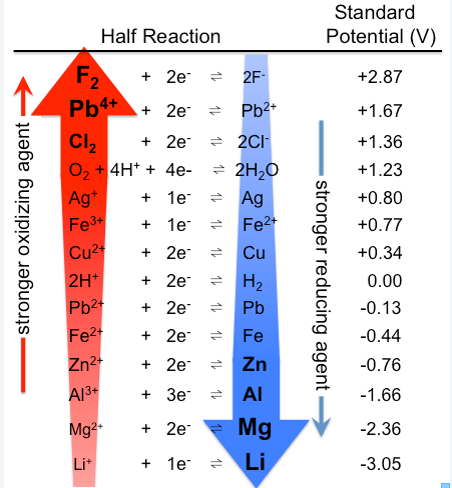
What is the electrochemical series?
A series where species are arranged in order of their standard electrode potentials; it can be used to determine oxidising / reducing powers of different species.
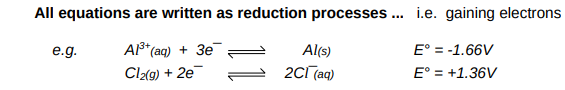
How do you calculate the EMF of a cell?
EMF= E°(more positive) - E°(less positive)
Electrons will flow from the more negative half cell (cell that is being oxidised) to the more positive half cell (cell which is being reduced).
The more positive the E°…
The more likely the system is to gain electrons i.e. reduced.
What redox process occurs at the anode (positive electrode)?
Oxidation.
What redox process occurs at the cathode (negative electrode)?
Reduction.
How do you remember what redox process occurs at which electrode?
Oxidation = Anode
Reduction= Cathode
Which species are better oxidising agents and which are better reducing agents?
The species on the LHS are better oxidising agents e.g. Cl2
There is a greater tendency for these species to gain electrons and are therefore, more easily reduced.
The species on the RHS are better reducing agents e.g. Mg.
There is a greater tendency for these species to lose electrons and are therefore, more easily oxidised.
What is an oxidising agent?
A species that oxidises another species by removing electrons from it. It therefore becomes reduced itself in the process.
What is a reducing agent?
A species that reduces another species by donating electrons to it. It therefore becomes oxidised itself in the process.
How can we determine whether or not a reaction is feasible?
Electrode potential values can be used to predict whether a reaction is feasible.
For a reaction to be feasible, what must the value of the EMF be?
EMF must be positive.