7.1 Theme 2 - introduction to immunology
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What are the three primary levels of immune defence?
The immune system defends through external barriers, innate immunity, & adaptive immunity.
How do innate & adaptive immunity differ?
Innate immunity provides a broad, rapid, non-specific defence with no memory, found in all invertebrates & vertebrates.
Adaptive immunity offers a highly specific, slower response focused on antigen recognition, develops immune memory, & occurs in vertebrates only.
What are the key features of the immune system?
It recognises pathogens & differentiates self from non-self
It has mechanisms to kill or eliminate pathogens
It uses cytokines to coordinate immune activity, including interleukins, interferons, TNFs & CSFs, & chemokines that induce cell movement (chemotaxis)
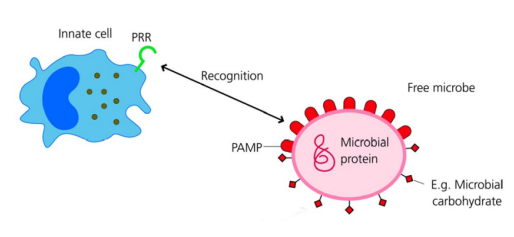
How do innate immune cells recognise non-self?
Innate immune cells, e.g. phagocytes, have pattern recognition receptors (PRRs) that detect pathogen antigens
PRRs can be on the cell surface or inside the cytoplasm depending on pathogen location
Pathogen components recognised by PRRs are called Pathogen-Associated Molecular Patterns (PAMPs)
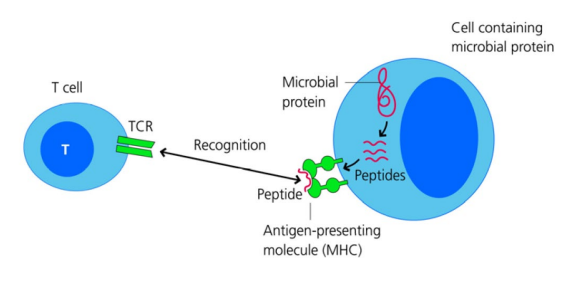
How does adaptive immunity recognise previously unencountered pathogens?
Lymphocytes (T & B cells) express T cell receptors (TCRs) & B cell receptors (BCRs) with randomly generated specificities
Targets are peptides from pathogens (antigens) presented via MHC molecules to the receptors
This allows the immune system to specifically recognise and respond to non-self pathogens
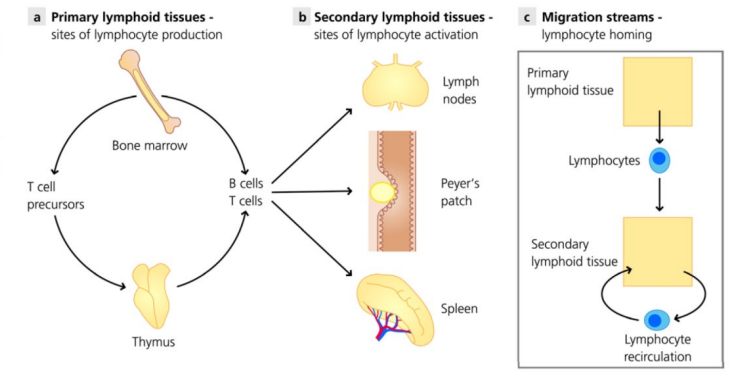
What are the primary & secondary lymphoid organs?
Primary organs: lymphocyte formation & maturation – bone marrow & thymus
Secondary organs: recognise & respond to foreign material – lymph nodes & spleen
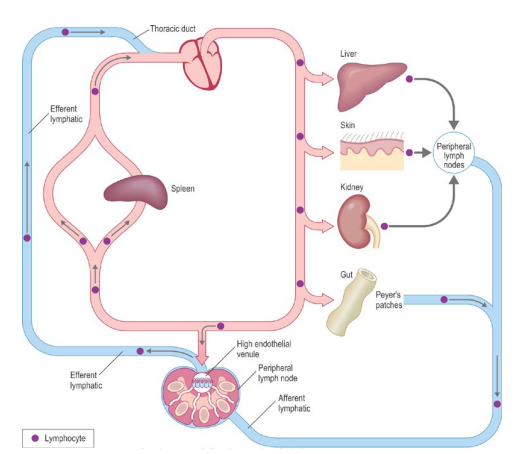
How does the lymphatic system contribute to immune responses?
Antigens from the site of infection enter the lymphatic system
They travel to regional lymph nodes = stimulation of immune response
How does lymphocyte recirculation work?
Recirculation is essential for lymphocytes to encounter antigens anywhere in the body
Lymphocytes flow from blood → tissues → lymph nodes → lymph → back to blood
At infection sites, they are attracted by adhesion molecules & chemokines
Neutrophils only circulate in the blood and do not return
What are the key features of phagocytic cells in innate immunity?
Macrophages & PMNs are main phagocytes
Macrophages need IFNγ activation for digestion
Macrophages present antigens to T cells
What are the main characteristics of neutrophils?
Produced in bone marrow from stem cells in ~2 weeks
Polymorphonuclear (PMN) leukocytes (segmented nuclei)
Granulated, with granules containing antimicrobial substances that kill bacteria, fungi & protozoa
What is neutrophil margination?
In healthy individuals, neutrophils mostly stay in blood, but during inflammation they move to vessel margins (margination) and are attracted to tissue damage or infection by chemical mediators.
The vessel margins is the lining of the endothelium
How do neutrophils move from blood into tissues (diapedesis)?
In response to tissue distress signals (cytokines, e.g. TNF-α), endothelial cells express adhesion molecules (ICAMs) that make neutrophils bind tightly. Chemotaxins then guide neutrophils to cross endothelial junctions (diapedesis) and enter tissues toward the site of infection or injury.
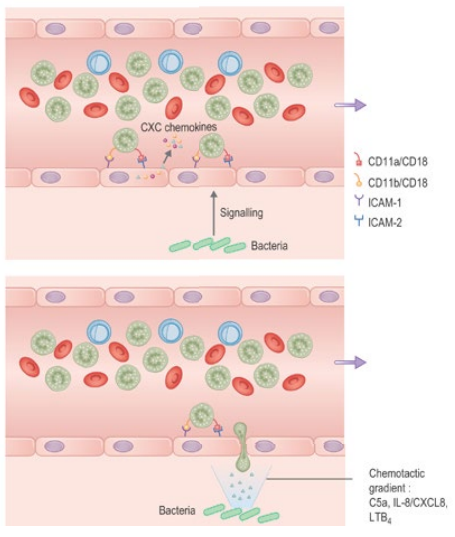
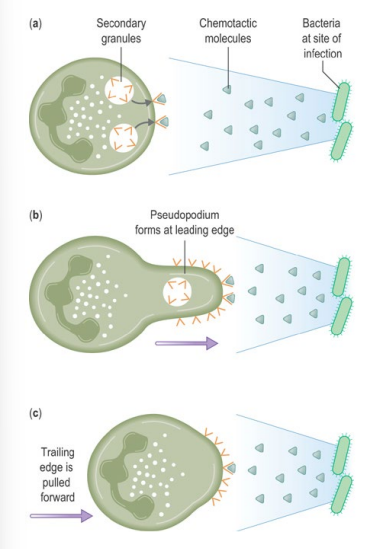
How do neutrophils locate pathogens in tissues (chemotaxis)?
• Neutrophils follow chemotactic gradients to find bacteria or fungi
• E.g. of chemotactic factor: Complement component C5a
• Chemotactic factors bind receptors on one edge of the neutrophil
• This causes pseudopodium formation to move forward and engulf the pathogen
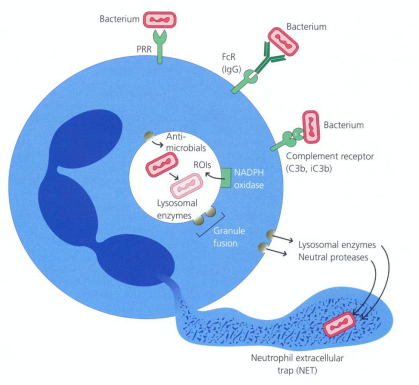
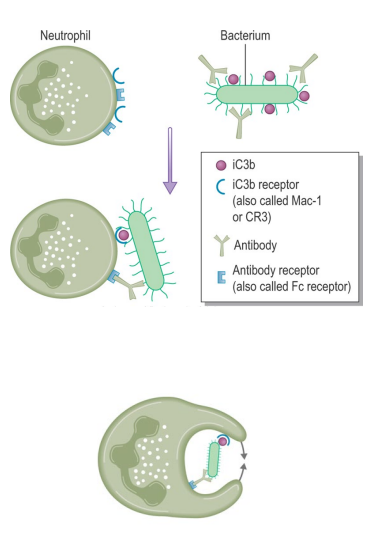
How do neutrophils perform opsonization and phagocytosis?
Neutrophils are inefficient at engulfing bacteria without opsonins e.g. complement C3b
Opsonins bind bacterial surface and interact with neutrophil receptors
This triggers pseudopodia formation to engulf the bacterium into a phagosome
The bacterium is then killed via oxygen-dependent & oxygen-independent mechanisms
How do neutrophils kill pathogens via oxygen-dependent mechanisms?
Respiratory burst and H₂O₂–myeloperoxidase–halide system generate reactive oxygen species
Respiratory burst: NADPH oxidase converts O₂ → superoxide (O2-) → H₂O₂
Myeloperoxidase converts H₂O₂ + Cl⁻ into toxic hypochlorous ions
Neutrophils protect themselves with antioxidants like vitamins C and E
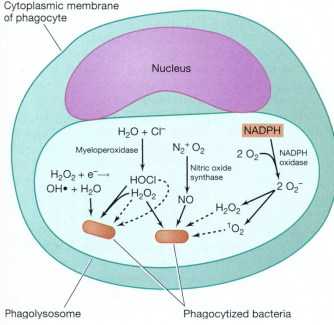
How do neutrophils kill pathogens via oxygen-independent mechanisms?
Examples include lysozyme (breaks bacterial cell walls), lactoferrin (binds iron), and cationic proteins like defensins or phospholipase A2
Important in anaerobic conditions, such as deep abscesses
What are the roles of mast cells and basophils in inflammation?
Mast cells (skin, around blood vessels, gut) and basophils (blood) mediate the acute inflammatory response
They have granules with histamine and leukotrienes to ↑ vascular permeability
IgE (Immunoglobulin E), damage, or complement (C3a, C5a) trigger degranulation
Causes reactions from wheal and flare to anaphylactic shock
Outline the stages of the immune response following initial infection.
Initial infection: Pathogen enters the body, triggering tissue inflammation.
Antigen capture: Dendritic cells in the tissue capture and process antigens.
Migration: Dendritic cells migrate to lymph nodes to present antigens to lymphocytes.
Activation: Lymphocytes (T and B cells) become activated and proliferate.
Response: Antibodies and activated lymphocytes migrate to inflamed peripheral tissue to destroy the pathogen.
Where are dendritic cells found and what do they do?
Found mainly in tissues (low levels in blood) & Langerhans cells (specific type of dendritic cell) in skin. They migrate to lymphoid organs to present antigens and activate lymphocytes.
How do dendritic cells migrate to lymph nodes and activate T cells?
PRRs on dendritic cells recognise pathogens
They internalise and degrade antigens, presenting peptides (from the antigen) on MHC
PRR activation stimulates the DC to migrate to secondary lymphoid tissue
In the lymph node, DCs activate antigen-specific T cells
Dendritic cells can also migrate to the spleen or MALT to activate T cells
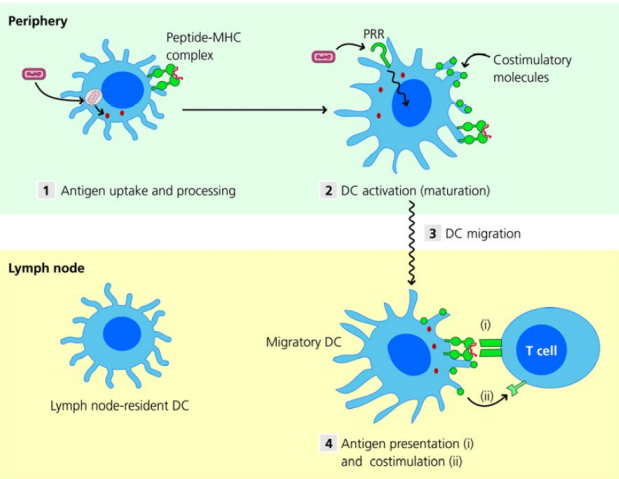
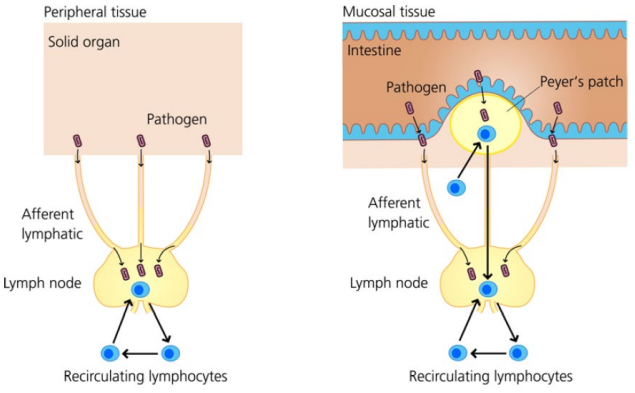
How are tissues monitored in adaptive immunity?
Most solid organs/peripheral tissues are monitored by ________ ________ linked by ________ ________.
The intestine contains ________ ________ for lumen surveillance.
Naïve lymphocytes continually ________ to search for foreign antigens.
Note: Naïve lymphocytes are T/B cells that have left the thymus/BM but have not yet encountered their specific antigen.
Most solid organs/peripheral tissues are monitored by lymph nodes linked by afferent lymphatics.
The intestine contains Peyer’s patches for lumen surveillance.
Naïve lymphocytes continually recirculate to search for foreign antigens.
Note: Naïve lymphocytes are T/B cells that have left the thymus/bone marrow but have not yet encountered their specific antigen.
What are natural killer (NK) cells and their function?
Large granular lymphocytes with cytotoxic activity
Make up 5–20% of mononuclear cells in blood and spleen
Kill tumour cells and virus-infected cells before virus maturation
Resemble cytotoxic T cells but lack classical T-cell receptors and don’t need prior sensitisation
How do NK cells detect infected or abnormal cells?
NK cells use a missing- or altered-self strategy to identify virus-infected or tumour cells
Activator receptors (AR) recognise glycoproteins on many host cells
Inhibitory receptors (IR) detect MHC class I molecules
If MHC I is present, the cell is spared
If MHC I is absent or altered, the NK cell kills the infected or abnormal host cell, preventing pathogen spread
note: MHC I is normally on host cells to display self-peptides. Virus-infected cells may lose, ↓, or alter MHC I. NK cells detect this and kill the infected cell, stopping the pathogen from replicating and spreading.
How do NK cells kill target cells?
High extracellular Ca²⁺ causes perforin to form pores in the target cell membrane = ↑ in permeability = granzymes enter through the pores
Granzymes activate caspases = triggers apoptosis (programmed cell death) of the target cell
What is the role of NK cells in IFNγ production and pathogen control?
NK cells rapidly produce IFNγ
Production and cytotoxic activity are stimulated by cytokines (e.g., IL-12)
IFNγ activates macrophages to kill internalised bacteria (Listeria, Salmonella, Mycobacterium), some viruses (CMV), and protozoa (Leishmania, Toxoplasma)
IFNγ inhibits viral growth in infected cells