Silverstein and Hopper Chapter 103: Platelet Disorders
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What are the steps that platelets undergo following vascular injury?
Adhesion, activation, and aggregation
Enables the formation of the platelet plug and facilitates thrombin generation
What is the most common initial presenting sign in dogs with platelet disorders?
Mucocutaneous bleeding leading to petechiae, cutaneous ecchymosis, and melena
What are clinical signs that have been documented in cats in addition to mucocutaneous bleeding?
Hematomas, epistaxis, and hematuria
What may cause thrombocytopenias to arise?
Decreased production
Increased consumption
Increased destruction
Sequestration
Causes of Increased Consumption of Platelets
Disseminated intravascular coagulation
Acute/severe blood loss
Platelet activation/aggregation
Sepsis
Thrombotic microangiopathies
Massive thrombosis
What can cause iatrogenic thrombocytopenia?
Hemodialysis
Postcardiopulmonary bypass
Hemodilution including massive transfusion
EDTA-mediated
What can cause thrombocytopenia due to increased destruction?
Primary immune mediate thrombocytopenia
Secondary immune mediated thrombocytopenia
Anaplasmosis
Babesiosis
Ehrlichiosis
Neoplasia
What can cause thrombocytopenia due to decreased production?
Bone marrow disease
Chemotherapy
What can cause thrombocytopenia due to increased sequestration?
Splenic sequestration
Sepsis/systemic inflammatory response syndrome
What is the most severe form of thrombocytopenia?
Primary or secondary immune-mediated destruction of platelets
Often results in life-threatening hemorrhage
What platelet count can lead to spontaneous hemorrhage? What platelet count indicates the highest risk of fatal bleeding?
Spontaneous hemorrhage can occur when the platelet count is <30 ×109/ml
The highest risk of fatal bleeding occurs when the platelet count is <10 × 109/ml
Does initial platelet concentration correlate with survival in dogs with immune-mediated thrombocytopenia?
No
What does presence of melena at the time of hospital admission associated with in dogs with ITP?
Increased transfusion requirement and mortality
DOGiBAT Score for Dogs with ITP
Novel ITP bleeding score called DOGiBAT was developed to provide an objective and standardized way to clinically assess ITP dogs
Comprises site-specific grades (0 - none, 1 - mild, 2- severe) of nine different anatomic sites
DOGiBAT score is correlated with transfusion requirements and inversely correlated with platelet count
What is the proposed mechanism of ITP in dogs and cats?
Increased phagocytosis by splenic macrophages due to autoantibodies bound to platelet integrin aIIbB3 (fibrinogen receptor) or glycoprotein Ib-IX (von Willebrand factor receptor)
Thrombocytopenia in Sepsis
Thrombocytopenia is a common finding in sepsis and its severity is associated with mortality
Causes of sepsis-mediated thrombocytopenia are multifactorial
Direct microbial-platelet interactions
Bacteria such as E coli and Streptococcus can directly interact with platelets leading to platelet activation and aggregation
Canine platelets directly interact with pathogens by expressing functional TLR 4 which augments platelet activation in the presence of LPS and ADP
Once activated, platelets interact with circulating neutrophils to form platelet-neutrophil aggregates and NETs
Overzealous production of NETs can further exacerbate organ dysfunction and thrombocytopenia by accelerating thrombus formation and reducing fibrinolysis
Platelet-leukocyte aggregate formation
Increased platelet sequestration secondary to microvascular thrombosis
How can acquired platelet disorders manifest?
As platelet dysfunction or hyperactivity resulting in bleeding diathesis or thrombosis
In small animal medicine, acquired platelet dysfunction is more common
Uremia Associated Platelet Dysfunction
Uremia-associated platelet dysfunction is multifactorial
Due to defects in platelet adhesion, secretion, and aggregation
Underlying mechanism in dogs and cats unclear
Dogs with CKD and clinical bleeding were found to have normal platelet aggregation and activation with compromised platelet adhesion
Uremia may directly alter the function of vWF producing a phenotypic resemblance of type II von Willebrand disease in humans
Because vWF supports shear-induced platelet adhesion and aggregation by interacting with platelet glycoprotein (GP) 1ba, defects in vWF may manifest more profoundly in the microcirculation, where shear forces are high
Platelet Disorders Associated with Liver Disease
Associated with both thrombocytopenia and platelet dysfunction
Dogs with hepatic malignancy and cirrhosis have lower platelet counts than those with hepatitis
Decreased platelet aggregation in response to collagen and arachidonic acid has previously been documented in dogs with liver disease but underlying mechanism is unknown
Platelet Disorders Associated with Heart Disease
Studies suggest that cats with occult or overt HCM have hypercoagulable platelets that may predispose them to thrombosis
Platelet Dysfunction due to NSAIDs
COX is a rate-limiting enzyme that converts arachidonic acid to eicosanoids
Platelets express mainly COX-1 which is irreversibly inhibited by acetylsalicylic acid, thereby modulating the biosynthesis of thromboxane A2 (TXA2) and prostaglandin
In theory, COX-2 selective NSAIDs should not inhibit platelet function
There are data suggesting that COX-2 selective NSAIDs possess antiplatelet effects in small animals
A small amount of CO-2 is constitutively expressed in platelets under normal physiologic conditions, it is upregulated during thrombopoiesis or hematopoietic hyper-regenerative conditions (post chemotherapy) and might play a prominent role in the biosynthesis of TXA2
Selective COX-2 inhibitors may induce clinically significant platelet dysfunction in these conditions
Effect of Anticoagulants and Fibrinolytic Drugs on Platelet Function
Anticoagulants and fibrinolytic drugs have been documented to have a direct or indirect impact on platelet function
Elevation in plasmin activity by strepotokinase or tPA may impair platelet aggregation by direct degradation of fibrinogen, which mediates platelet-to-platelet aggregation, or by cleavage of integrin a1IbB3
Activation of plasmin, a protease enzyme, also cleaves thrombin receptors and thus induces platelet aggregation
How can drugs that elevate intracellular cyclic nucleotides affect platelet function?
Drugs that elevate intracellular cyclic nucleotides such as cAMP or GMP, both critical inhibitory secondary messengers that modulate fundamental platelet pathways, can inhibit platelet receptor activation, degranulation, shape change, and aggregation
How can phosphodiesterase inhibitors affect platelet function?
Platelets express three isoforms of phosphodiesterase (2, 3, and 5) and thus in theory, platelet function can be inhibited by nonselective or isoenzyme-selective phosphodiesterase inhibitors
Mechanisms of Platelet Dysfunction Secondary to Hydroxyethyl Starch (HES)
Mechanisms of platelet dysfunction secondary to hydroxyethyl starch (HES) administration are multifactorial
Binding of colloidal molecules on the extracellular domains of integrin aIIbB3 or glycoprotein 1b inhibits their conformational changes and subsequently, platelet aggregation and adhesion
Binding of colloidal molecules also interferes with factor VIII/vWF complex formation causing their accelerated elimination
Can further inhibit platelet adhesion upon vascular injury
Slowly degradable HES may further exert its inhibitory effects in platelets by interfering with intracellular signaling function
Clinicians should weight the risks and benefits when administering HES in patients and should avoid HES in those with preexisting platelet disorders
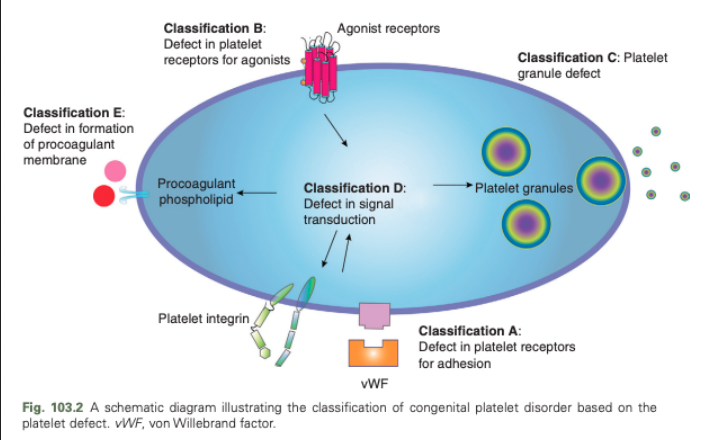
Congenital Platelet Disorders - Classification A
Defect in platelet receptors for adhesion
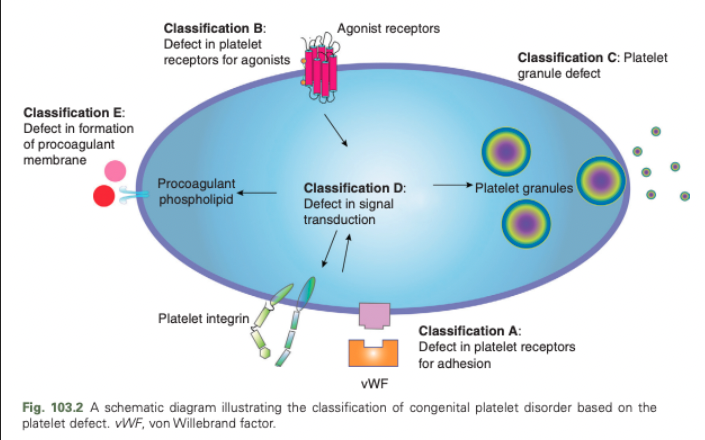
Congenital Platelet Disorders - Classification B
Defect in platelet receptors for agonists
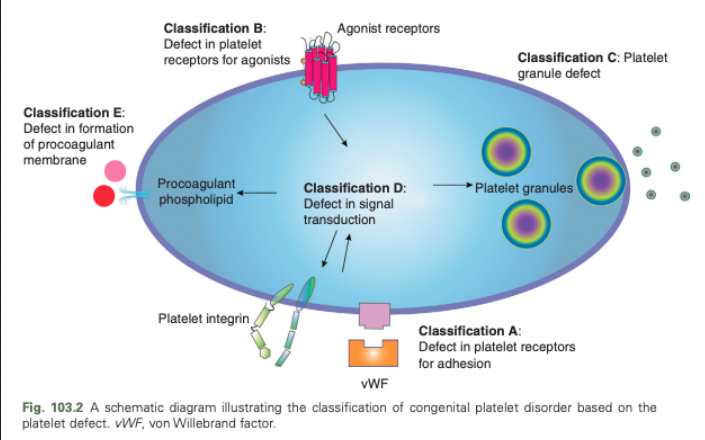
Congenital Platelet Disorders - Classification C
Platelet granule defect
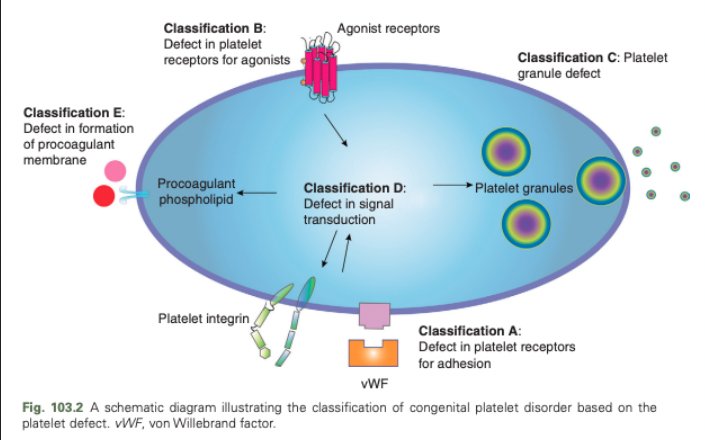
Congenital Platelet Disorders - Classification D
Defect in signal transduction
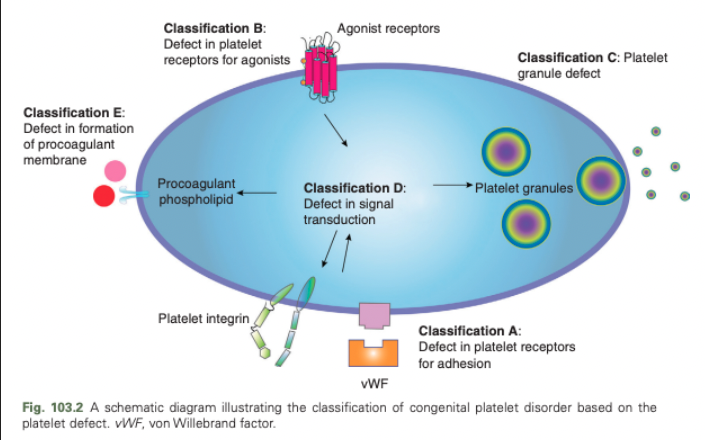
Congenital Platelet Disorders - Classification E
Defect in formation of procoagulant membrane
Recommendations for Discontinuation of Antiplatelet Therapy Prior to Elective Procedures
Not recommended in patients at high risk of thrombosis due to the potential rebound of platelet reactivity and acute thrombotic events
Discontinuation of one antiplatelet drug (preferably clopidogrel) 5-7 days prior to elective procedures is recommended in patients considered high risk of surgical bleeding receiving dual antiplatelet therapy
Antiplatelet drugs should be discontinued at least 5-7 days prior to elective procedures in animals with low to moderate risk of bleeding
What are the two general principles of the treatment of platelet disorders?
Prevention of bleeding diathesis
Control of major hemorrhagic events
Prevention to Avoid Catastrophic and Life-Threatening Hemorrhages in Animals with Platelet Disorders
Avoid IM injections
Discontinue medications with confirmed antiplatelet or antithrombotic properties
Commonly prescribed antiplatelet drugs have short half-lives but their irreversible inhibitory effects on platelet function can persist beyond the reported platelet life span (4-6 days)
Hemorrhage is rare following SQ injections but direct pressure should be applied following injections
To prevent bleeding diatheses in patients with congenital platelet disorders, discourage animals from engaging in strenuous exercise or high impact activities that may result in unnecessary trauma
Preventative treatments may be indicated prior to invasive or surgical procedures
Indications for Whole Blood-Derived Platelets
Thrombocytopenia
Prophylaxis in patients with severe thrombocytopenia (10×109/L or less) or requiring invasive procedures (50 × 109/L or less)
Severe thrombocytopenia causing anemia, shock, or intracranial/pulmonary hemorrhage
Congenital/acquired platelet disorders
Severe or uncontrolled bleeding
Prophylaxis in animals prior to invasive procedures
Indications for Trehalose Stabilized Cryopreserved Platelet Concentrate
Severe thrombocytopenia causing anemia, shock, or intracranial/pulmonary hemorrhage
Indications for Fresh Whole Blood with Platelet Dysfunction
Hemorrhage due to severe thrombocytopenia, congenital/acquired platelet disorders, and vWD with concurrent anemia
Indications for Cryoprecipitate
Type 1, 2, or 3 vWD
Indications for Desmopressin with Platelet Disorders
Congenital or acquired platelet disorders
Mild bleeding
Adjunctive therapy with platelet transfusions
Aspirin-induced platelet dysfunction
Platelet dysfunction caused by liver disease ± uremia
Type 1 or 2 vWD
Indications for Antifibrinolytic Drugs (Epsilon-aminocaproic acid and tranexamic acid) with Platelet Disorders
Thrombocytopenia
Associated hemorrhage
Congenital or acquired platelet disorders
Prophylactic treatment prior to elective noninvasive procedures
Mild to severe bleeding diathesis
Adjunctive therapy with platelet transfusions
Indications for Fresh Frozen Plasma
Platelet disorders with concurrent consumptive coagulopathy and hypofibrinogenemia
Types 1, 2, or 3 vWD
What can you use to control hemorrhagic events due to acquired and congenital platelet disorders?
Platelet transfusions, antifibrinolytic therapy, desmopressin (DDAVP), and fresh frozen plasma transfusion
What should platelet transfusions be reserved for?
Patients with major hemorrhagic events including intracranial and pulmonary bleeding secondary to severe thrombocytopenia, irreversible antiplatelet drugs, and severe platelet disorders
May temporarily stop or slow down bleeding in dogs with severe ITP to allow time for immunosuppressive therapies to take effect
What are alternatives to fresh platelet products?
Cryopreserved or lyophilized platelet products using either dimethylsulfoxide or trehalose have been extensively studied in human medicine
Trehalose prevents cell damage caused by lyophilization that induces temperature-mediated membrane reorganization, calcium fluxes, and morphological changes