N236: Concepts & Methods in Infectious Disease Research
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
Scientific method steps
Observation, Question, Hypothesis, Experiment, Data Collection, Conclusion, and Retest (if needed)
What is the goal of a scientific study?
Describe the relationship between an exposure (E) and a disease (D), after taking into account the effects of other factors (C, control variables)
How to calculate association
calculate measures of disease frequency among “treatment” and “control” groups
Compare the measures of disease frequency to obtain a measure of association
Interpret the measure of association
Rate ratio
rate in treatment/rate in control (no units)
Rate difference
rate in treatment - rate in control (units)
Risk ratio (aka relative risk)
Compares the risk of contracting a disease between the exposed population and the unexposed population
Risk ratio formula
Risk of exposed/risk of unexposed (OVER TIME)
What does a relative risk of > 1 indicate?
The exposure is harmful.
What does a relative risk of < 1 indicate?
The exposure is protective.
What does a relative risk of = 1 indicate?
The exposure is neither harmful nor protective
Risk difference formula
Risk exposed - risk unexposed
What does a relative difference of > 0 indicate?
The exposure is harmful.
What does a relative difference of < 0 indicate?
The exposure is protective
What does a relative difference of = 0 indicate?
There is no association between the exposure and the outcome
Descriptive studies
seek to describe the distribution factors or protective factors in populations, hypothesis-generating
Analytic studies
seek to identify possible risk factors or protective factors using smaller samples, hypothesis-testing
2 types of analytic studies
experimental and observational
Experimental studies
investigator manipulates the exposure status and follows participants to observe the outcome of interest
Observational studies
investigator simply observes the pre-existing exposure status and the outcome of interest
"Crude" analysis
Does not take into account other factors that may influence the relationship between an exposure and a disease
"Adjusted analysis"
Takes into account other factors (control variables) that may influence the relationship between an exposure and a disease
Study design
A set of procedures that are used to collect and analyze data on variables
Randomized clinical trial
An experimental study designed to test the efficacy of a treatment
Preventive trial
A type of randomized clinical trial that evaluates whether an agent reduces the risk of developing a disease (ex: COVID-19 vaccine trials)
Therapeutic trial
A type of randomized clinical trial that evaluates whether a treatment was effective in treating a disease (ex: Paxlovid treatment trials)
Randomized clinical trial study design
Subjects are randomly and blindly assigned by the investigator to either a "treatment" group or a "control" group
What is the point of randomization?
used to put participants in groups that are comparable on all factors except for exposure factor (treatment or placebo)
Unblinded study
Both subjects and investigators are aware of the exposure assignment
Single-blinded study
Investigators are aware but subjects are unaware of the exposure assignment
Double-blinded study
Both subjects and investigators are unaware of the exposure assignment
Cohort studies
Observational study in which subjects who do not have the disease of interest are followed in time to observe if they develop the disease
Cohort study design
Investigator begins with an "exposed" group of subjects and an "unexposed" group of subjects --> then follows them in time to see who develops the disease
Strengths of cohort studies
allows for establishing the temporal sequence of events because it works forwards from exposure to disease, can be used to study several diseases simultaneously, can be used to study rare exposures
Limitations of cohort studies
usually time-consuming and more expensive to conduct than other observational study designs, loss of participants due to withdrawal, migration, or death could result in errors, not efficient for studying rare diseases
Case-control studies
Observational study in which subjects are selected based on their current disease status and then assess them for exposure history
Case-control study design
Compares a group of people with disease (cases) to a group without disease (controls) --> then looks backward in time for prior exposure
Can you measure risk or rate in a case-control study?
No, because you cannot measure incidence (new cases)
Strengths of case-control studies
quicker and relatively less expensive to conduct than cohort studies, can be used to study several exposures simultaneously, can be used to study rare diseases
Limitations of case-control studies
only one disease can be evaluated at a time (in contrast to cohort studies), does not allow for the direct assessment of disease risk because it works backwards from disease to exposure, not efficient for studying rare exposures
Odds ratio
The likelihood of a disease among individuals exposed to a risk factor compared to those who have not been exposed
Odds (cross product) ratio formula
ad/bc
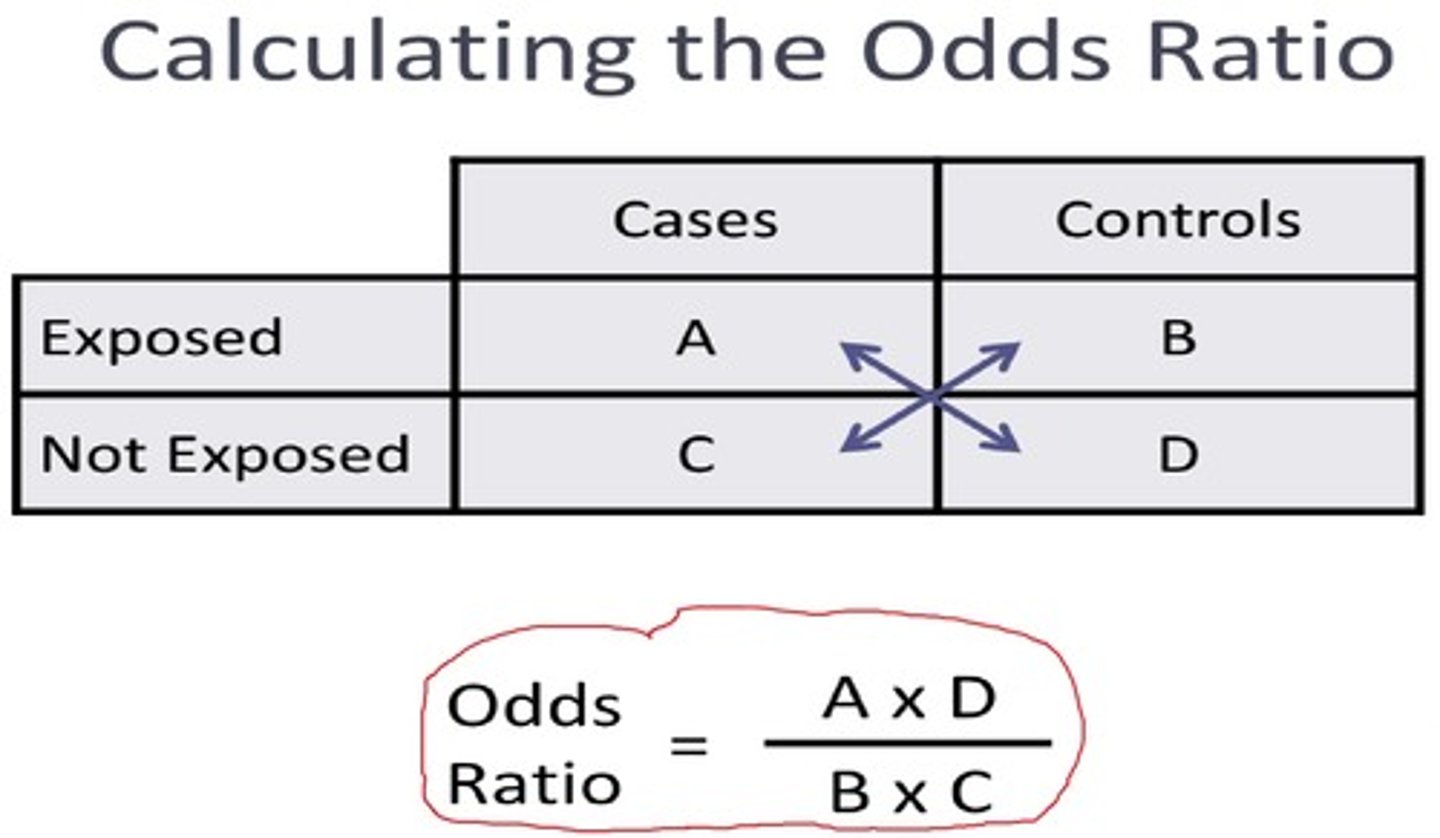
What does a odds ratio of > 1 indicate?
The exposure is harmful
What does a odds ratio of < 1 indicate?
The exposure is protective
What does a odds ratio of = 1 indicate?
There is no association between the exposure and the outcome
Cross-sectional studies
An observational study in which you ask a group of people about their disease status and exposure at the same time
Cross-sectional study design
Investigator interviews group of individuals from a population, and obtains data on their disease status and exposures at the same time (a snapshot in time)
What association can you measure in cross-sectional studies?
Prevalence!
Prevalence ratio
Prevalence (exposed) / Prevalence (unexposed)
What does a prevalence ratio of > 1 indicate?
The prevalence in exposed is higher.
What does a prevalence ratio of < 1 indicate?
The prevalence in exposed is lower
What does a prevalence ratio of = 1 indicate?
The prevalence in exposed and unexposed is similar.
Prevalence difference
Prevalence (exposed) - Prevalence (unexposed)
What does a prevalence difference of > 0 indicate?
The prevalence in exposed is higher
What does a prevalence difference of < 0 indicate?
The prevalence in exposed is lower.
What does a prevalence difference of = 0 indicate?
The prevalence in exposed and unexposed is similar.
Strengths of cross-sectional studies
convenient, quicker, and relatively less expensive to conduct than other study designs, can be used to evaluate several exposures and several diseases at the same time, can help generate clues about potential associations
Limitations of cross-sectional studies
cannot help establish whether the exposure preceded disease or whether the disease influenced exposure, can only identify prevalent (not incident) cases, may miss diseases with shorter durations