Oncology 5 (endocrine tumors)
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
is thyroid neoplasia a common occurrence in dogs?
relatively common, accounts for 1-4% of all tumors in dogs
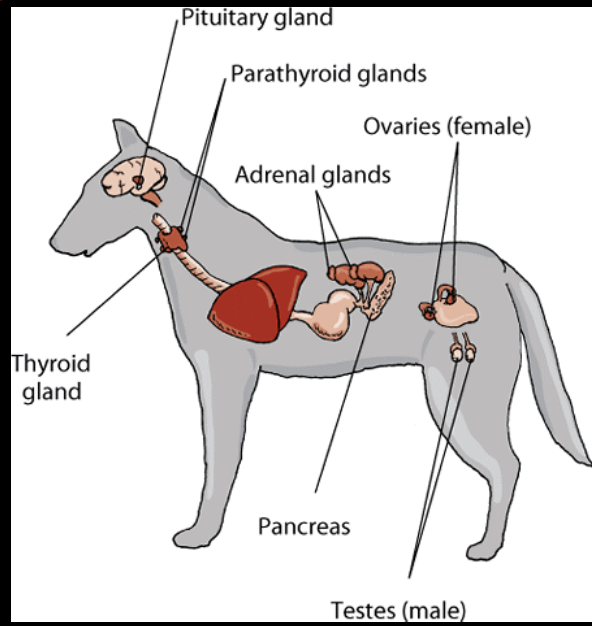
are most thyroid tumors in dogs benign or malignant?
majority of tumors associated with clinical signs are malignant
benign adenomas are generally small, noninvasive, and clinically silent
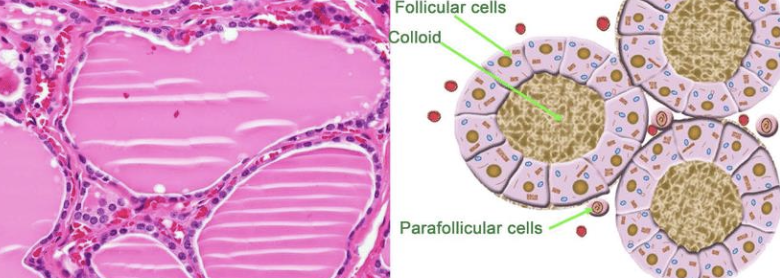
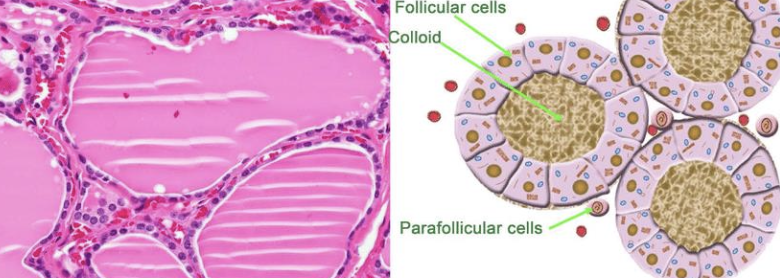
what are the 2 cellular origins of thyroid tumors in dogs?
1. follicular cell origin (most common)
2. parafollicular cell origin (medullary, uncommon)
what is the typical signalment of thyroid neoplasia in dogs?
older dogs (9-11 yrs)
breeds: goldens, beagles, boxers, siberian huskies (familial medullary thyroid CA in alaskan malamute)
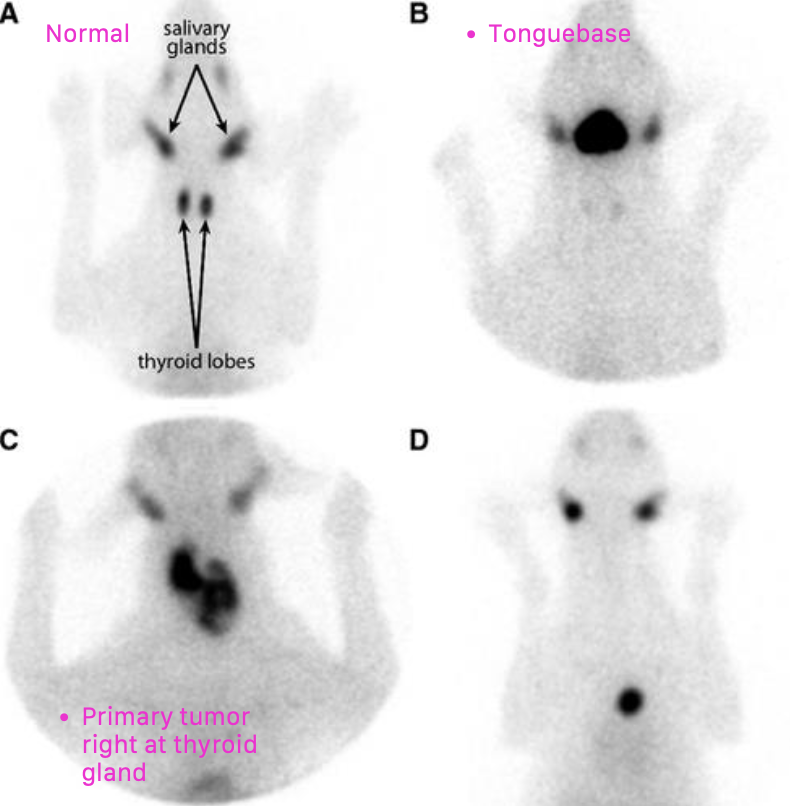
what is the location of thyroid tumors in dogs?
~60% of cases have bilateral disease
can also have ectopic thyroid neoplasia (can occur anywhere from base of tongue to base of the heart)
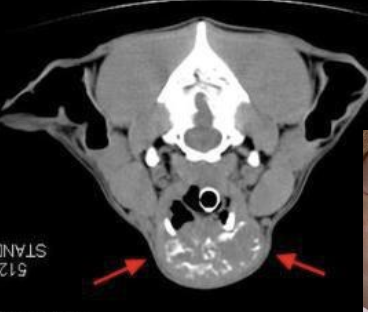
what is the metastatic potential of thyroid tumors in dogs?
metastasis noted at diagnosis in ~35% of cases
80% of cases will eventually develop mets (so fairly high metastatic potential)
what are the common sites of metastasis for thyroid tumors in dogs?
local lymph nodes
lungs
other tissues
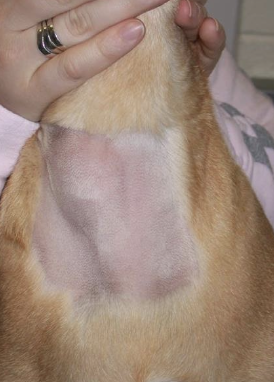
how do most dogs with thyroid carcinoma present?
most dogs present with palpable ventral cervical mass (may be incidental findings on routine PE)
what are the less common clinical presentations of dogs with thyroid carcinoma?
cough
dyspnea
dysphagia
change in bark
laryngeal paralysis
Horner's syndrome
facial edema
are the majority of canine thyroid carcinomas functional or non-functional?
majority of canine thyroid carcinomas are non-functional
-60% are euthyroid
-30% are hypothyroid
-10% hyperthyroid (functional)
how is canine thyroid carcinoma diagnosed/staged?
1. routine CBC/chem/UA, 3V-CXR
2. cervical ultrasound
3. cytology
4. CT of neck
5. scintigraphy
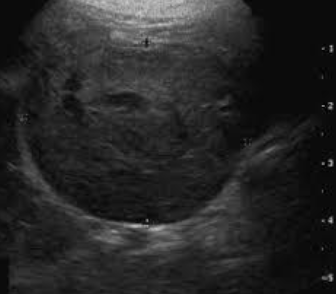
how can cervical ultrasound help diagnose/stage canine thyroid carcinoma?
used to confirm mass of thyroid origin, assess invasiveness and vascularity
lymph node assessment for potential mets
how can cytology help diagnose/stage canine thyroid carcinoma?
may be helpful to rule out other differentials
-indicates thyroid origin 50% of time
-local lymph nodes for mets
what may be a complication of doing cytology on a thyroid carcinoma?
these are vascular tumors and hemodilution of the sample is a common problem
how can CT be helpful for staging/diagnosing thyroid carcinoma?
not entirely necessary, but may be useful for surgical planning of fixed/invasive tumors
also useful for radiation planning
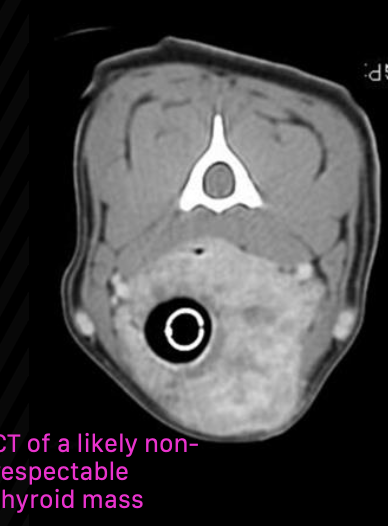
when is surgery indicated as treatment for canine thyroid carcinoma?
surgical excision indicated when mass is freely moveable (least morbidity)
what is the median survival time of canine thyroid carcinoma treated with surgical excision of the tumor?
if freely moveable, MST is 3 years
if tumor is invasive, MST is 6-12 months
what are the treatment options for canine thyroid carcinoma with non-resectable tumors or patients with metastatic disease?
1. radiation therapy
2. chemotherapy
3. combination treatments
4. I131 ablation
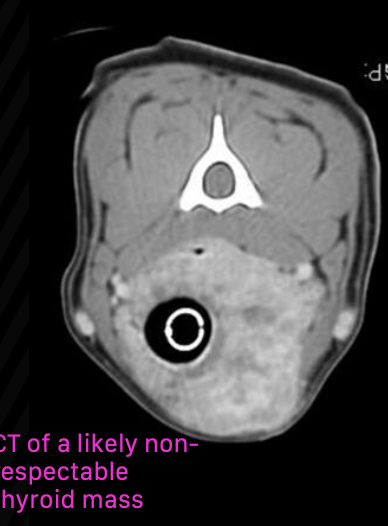
how effective is radiation therapy for canine thyroid carcinoma patients with non-resectable tumors?
most dogs have stable disease or partial response to radiation (radiation is helpful in reducing size)
80% of patients were progression-free at one year
what chemotherapy drugs are used for treatment of non-resectable canine thyroid carcinomas?
palladia
mitoxantrone
doxorubicin
carboplatin

how common are adrenal tumors in dogs and cats?
1-2% of all canine tumors
0.2% of feline tumors (ie rare in cats)
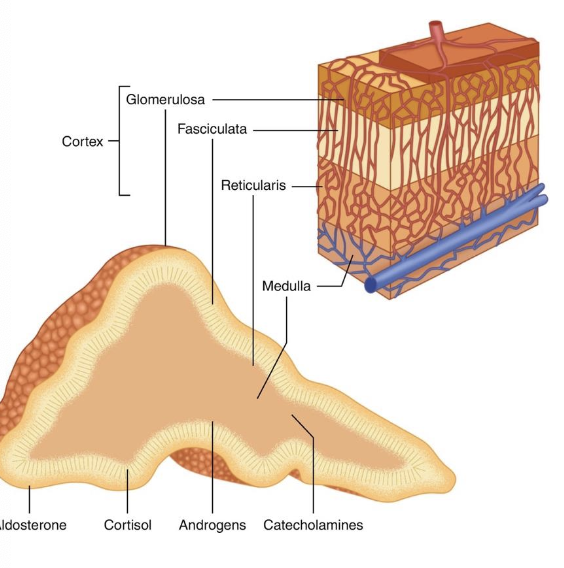
what are the most common adrenal tumor types in dogs?
-41% adrenocortical tumors (3/4 adenoma, 1/4 adenocarcinoma)
-32% pheochromocytomas (tumor of adrenal medulla)
-27% of adrenal tumors are metastatic lesion
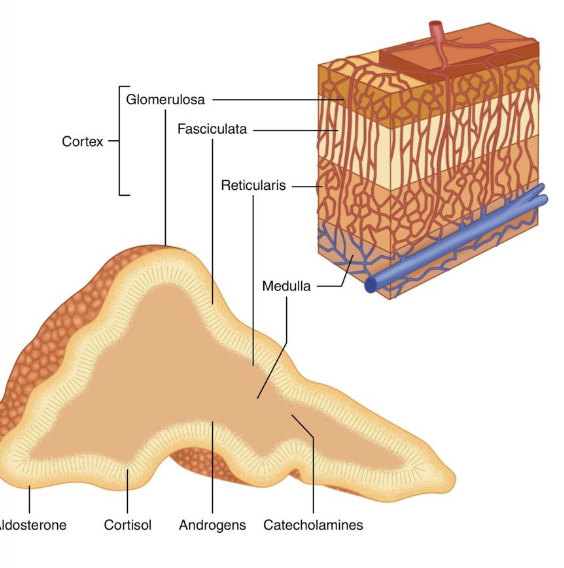
what are the most common adrenal tumor types in ctas?
-30% adrenocortical tumors (1/2 adenoma, 1/2 adenocarcinoma)
-10% pheochromocytomas (tumor of adrenal medulla)
-60% of adrenal tumors are metastatic lesion
what endocrinopathy are 15-20% of functional cortisol-secreting adrenal cortex tumors responsible for?
hyperadrenocorticism (cushing's)
pituitary-dependent adenomas account for 80-85% of tumors
what is the general size difference between adrenal adenomas and adrenal carcinomas?
adenomas are typically smaller
tumors over 2cm are more likely to be carcinomas
where do 20% of adrenocortical carcinomas invade?
into local vasculature
how common is adrenocortical carcinoma metastasis? what are the most common places?
about 50% of cortical carcinomas metastasize
liver and lungs most common
how can functional adrenocortical adenomas and carcinomas be distinguished?
by identification of mets
if there are no mets, there are no distinguishing clinical, biochem, or imaging findings to distinguish the two
what functional assessments are used when diagnosing adrenocortical tumors?
-observe symptoms consistent with cushing's syndrome
-serum biochem abnormalities
-ACTH stim test
-LDDST (better screening for adrenal dependent HAC)
-observe if contralateral adrenal is atrophied
what is the treatment of choice for adrenal-dependent hyperadrenocorticism (ADH)?
surgery (adrenalectomy)
what is the prognosis of ADH when treated with surgery?
perioperative mortality ranges from 19-28%, but long term prognosis is very good if survival to 4-weeks post-op
what is the median survival time for adrenocortical adenomas and carcinomas when treated with surgery?
adrenocortical adenoma MST: 230-778 days (7.6-25.9 months)
adrenocortical carcinoma MST: 688 days (22.9 months)
when surgery is not an option, what medical therapy is used to treat/manage adrenocortical tumors?
1. mitotane (used as a cytotoxic agent- need higher doses for PDH)
2. trilostane (not cytotoxic, but successfully used to manage ADH)
what is the median survival time of adrenocortical tumors treated with mitotane?
MST= 16 months
relapses common when used to treat PDH
what are pheochromocytomas?
neoplastic chromaffin cells in the adrenal medulla
(chromaffin cells are part of the sympathetic nervous system)
are all pheochromocytomas benign or malignant?
all are malignant
-25-40% have metastasis
-vascular invasion is common
what are the common sites of pheochromocytoma metastasis?
liver
spleen
lungs
local lymph nodes
what is the common signalment for pheochromocytomas?
older dogs, males overrepresented
what are the clinical signs of pheochromocytomas?
-may be episodic
-weakness/collapse
-panting, anxiety, restlessness
-exercise intolerance
-poor appetite, PU/PD, weight loss
what physical exam findings may be seen with pheochromocytomas?
PE findings may be normal
may also find tachypnea, panting, tachycardia, weakness, pallor, arrhythmias, hypertension
what pre-operative diagnositcs are necessary for pheochromocytomas?
-blood pressure
-imaging to look for local invasion, evidence of mets
- +/- catecholamine testing (plasma or urine normetanephrine)
what is the only definitive therapy for pheochromocytomas?
surgery
what is the prognosis of pheochromocytomas treated with surgery?
MST post surgery is 1 year, some dogs live 2-3 years
influenced by tumor size, presence of mets, and local invasion
what should you do if you find an incidental adrenal gland lesion?
-if adrenal nodule, mass or gland width >10mm noted on imaging (U/S): consider size, contralateral adrenal gland (atrophied, normal, similarly large), invasion, etc.
-consider functional assessment
-consider full AUS and thoracic rads to assess for potential metastatic disease
when is surgery considered if an incidental adrenal gland lesion is seen on ultrasound?
consider surgery if lesion is functional, locally invasive, or larger than 2.5cm
if incidental adrenal gland lesions found are smaller than 2cm, what should be done next?
if smaller than 2cm and non-functional, monitor closely with serial AUS (1, 2, 4, 6 months)
size and level of invasion can rapidly change
what are insulinomas?
pancreatic beta-cell tumors
are insulinomas common in dogs and cats?
uncommon tumor of dogs, rare in cats
what are the clinical signs of insulinomas?
signs generally associated with low blood glucose:
-weakness
-ataxia
-collapse
-behavior change
-seizure
-disorientation
what is the biologic behavior of insulinomas in dogs?
-generally malignant tumors in dogs
-50% have metastatic lesions (regional LNs and liver, rarely the lungs)
what is the typical signalment of insulinomas in dogs?
medium to large breed dogs
median age: 9-10 years
how can diagnosis of insulinomas be confirmed?
documenting hypoglycemia (BG under 60mg/dL) and concurrent normal or elevated serum insulin concentration
histopathology
is imaging helpful for diagnosing insulinomas?
-can be useful for surgical preparation
-thoracic and abdominal rads often unremarkable
-abdominal U/S can identify a mass 50% of time (better at ID'ing mets)
-abdominal CT more sensitive for ID'ing primary lesions, not specific for metastatic disease
what are the treatment options for insulinomas?
1. acute management of hypoglycemia
2. exploratory laparotomy
3. medical therapy
what does acute management of hypoglycemia in patients with insulinomas consist of?
IV dextrose (slow bolus) followed by CRI
give with caution as this can worsen unregulated insulin secretion
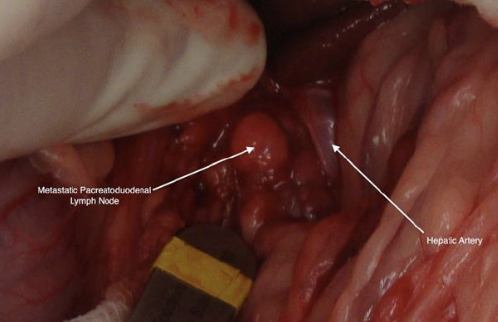
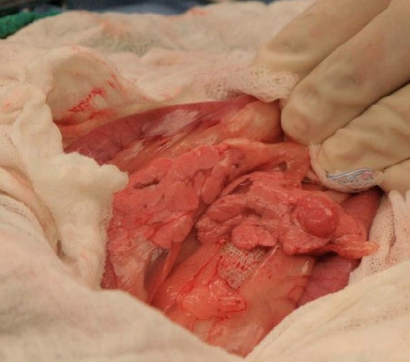
what should be performed during ex-lap for treatment of insulinomas?
-partial pancreatectomy
-resect suspected metastatic lesions when possible
what medical therapy is used for treatment/management of insulinomas?
-primarily used to control hypoglycemia
-cytotoxic agents (like streptozocin) have been used to kill pancreatic beta-cells
-dietary modification
-prednisone
what is the prognosis of insulinomas with and without surgery?
without surgery: good in short term, guarded-poor in long term
with surgery: MST 12-14 months