Redox and electrode potentials
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
Define reduction and oxidation
Reduction- gain of electrons and a decrease in oxidation number
Oxidation- loss of electrons and a increase in oxidation number
In a redox reaction there is always a reducing agent and a oxidising agent, define each one
Oxidising agent- takes electrons from the species being oxidised, whilst being reduced itself.
Reducing agent- Adds electrons to the species being reduced, whilst being oxidised itself.
What side are the electrons on of the equation in oxidation and reduction
Oxidation- right hand side
Reduction- left hand side
Writing a redox equation from half equations
1. Balance the electrons
2.Add and cancel the electrons
3.Finally cancel any species that are on both sides of the equation
Example of writing redox equation from half equations
reduction: H2O2 + 2e- > 2OH-
oxidation: Cr3+ + 8OH- > CrO42- + 4H2O + 3e-
1. Balance the electrons:
3H2O2 + 6e- > 6OH- (x3)
2Cr3+ + 16OH0 > 2CrO42- + 8H2O + 6e- (x2)
2.Add and cancel the electrons
3H2O2 + 2Cr3+ + 16OH- > 2CRO42- + 6OH- + 8H2O
3.Cancel species on both sides of the equation
3H2O2 + 2Cr3+ + 10OH- > 2CRO42- + 8H2O
Writing a redox equation from oxidation numbers
1. Overall increase in oxidation number=overall decrease in oxidation number
2.Assign oxidation numbers to identify the atoms that change their oxidation number
3.Balance the species that contain the elements which change there oxidation number to match the increase in oxidation number to the decrease in oxidation number
4.Balance any remaining atoms
Example of writing redox equation from oxidation numbers, Sulfur reacts with concentrated HNO3 to form H2SO4, NO2 AND H2O. Construct the overall redox equation
S + HNO3 > H2SO4 + NO2 + H2O
Sulfur goes from 0 to +6, oxidation no change = +6
N goes from +5 to +4, oxidation no change = -1
To match increase of +6 of sulfur, you need a total decrease of -6 from nitrogen so multiply anything containing nitrogen by 6.
S + 6HNO3 > H2SO4 + 6NO2 + H2O
Balance any remaining atoms
S + 6HNO3 > H2SO4 + 6NO2 + 2H2O
Predicting products of redox reactions
-In aqueous redox reactions, H2O is likely formed.
-Other likely products are H+ and OH-
-Ensure both sides are balanced by charge
Example of predicting products of redox reactions
MnO4- + H+ > Mn2+
1.Assign oxidaition number and the changes in oxidation number.
Mn goes from +7 to +2, overall oxidation change= -5
2.Balance the electrons
MnO4- + H+ + 5e- > Mn2+
3.Balance any remaining atoms and predict further species
MnO4- + 8H+ + 5e- > Mn2+ + 4H2O
Redox titration using Manganate (VII)
Reagent: Potassium Manganate (VII) under acidic conditions (KMnO4 (aq) )
Reduction of MnO4- to Mn2+ , change in oxidation number from +7 to +2
Equation for manganate titrations using fe2+/fe3+
Reduction: MnO4- + 8H+ + 5e- > Mn2+ + 4H2O
Oxidation: Fe2+ > Fe3+ + e-
Overall: MnO4- + 8H+ + 5Fe2+ > Mn2+ + 5Fe3+ + 4H2O
Practical procedure of KMnO4 redox titration under acidic conditions
1. A standard solution of KMNO4 is added to the burette, this is dark purple in colour
2.Add the reducing agent to a conical flask, an excess of dilute H2SO4 (aq) is added to provide the H+ ions required for the reduction of MnO4-. An indicator is not needed as the reaction is self indicating
3.During the titration the Manganate solution reacts and is decolourised as it is being added. The endpoint is judged by the permanent pink colour, indiciating an excess of MnO4- ions.
4.Repeat until concordant titres are obtained
What if KI was not in excess, what would happen to the percentage mass of copper
It would be lower due to a smaller titre
KMnO4 is a deep purple colour, where is the meniscus read from
Readings are read from the top of the meniscus.
Burette measures by difference so no effect
What are manganate (VII) / MnO4- titrations used for
Analysis of different reducing agents, key ones are:
-Fe2+ (aq) , Iron (II) Ions
-(COOH)2 (aq), ethanedioic acid
Same principles can be used for other reducing agents provided they reduce MnO4- to Mn2+
Calculation for calculating the percentage purity of an Iron (II) Compound
MnO4- + 5Fe2+ + 8H+ > 5Fe3+ + Mn2+ + 4H2O
1.Calculate moles of MnO4- that reacted
2.Determine moles of Fe2+ reacted as ratio is 1:5, multiply moles of MnO4- by 5.
3.Scale up to find moles of Fe2+ in standard solution by x10.
4.Find mass of iron compound
5.Percentage purity = mass of iron compound/ mass of impure sample x100
Determentation of formula
Hydrated ethanedioic acid has the formula (COOH)2.XH2O, value of x can be determined by reacting the solution with acidified MnO4- ions
2MnO4- + 6H+ + 5(COOH)2 > 2Mn2+ +10CO2 + 8H2O
1.Calculate moles of MnO4- reacted
2.Determine moles of (COOH)2 reacted by multiplying it by 2.5
3.Calculate molar mass of (COOH)2. xH2O using the given mass and the moles
4.Take away Mr of (COOH)2 from Mr and divide this by 18 to calculate x
Non familiar redox titrations
Manganate (VII) titrations can be used for analysis of many different reducing agents.
KMnO4 can be replaced with other oxidising agents such as H+/Cr2O7-
Iodine thiosulfate titrations
-Thiosulfate, S2O3^2- ions are oxidised .
-Iodine, I2, is reduced.

What are iodine/thiosulfate titrations used for?
-ClO- content in household bleach
-Cu2+ content in copper(II) compounds
-Cu content in copper alloys
Obtains analysis of different oxidising agents
Procedure of iodine/thiosulfate titration
1.Add a standard solution of Na2S2O3 to a burette.
2.Prepare a standard solution of the oxidising agent to be analysed and add to a conical flask. Then add an excess of potassium iodide, KI to the conical flask and HCl to provide the H+ ions for the reaction. The oxidising agents reacts with the iodide ions to produce iodine turning the solution a yellow-brown colour
3.Titrate this solution with Na2SO3, iodine is reduced back to I- ions and the brown colour fades gradually.
4.When the endpoint is approached and the iodine colour fades to a pale straw colour, add starch indicator endpoint is when the blue-black colour changes to colourless
Calculating concentration of ClO- ions in bleach
ClO- + 2H+ + 2I- > Cl- + I2 + H2O
2S2O32- + I2> S4O62- + 2I-
1mol ClO- = 2 mol S2O32-
1.Calculate moles of S2O32- reacted
2.Determine moles of ClO- reacted by dividing by 2
3.Determine moles of ClO- in standard solution by x10.
4.if 250cm3 bleach was prepared using 10cm3 bleach.
10cm3 bleach = x moles of ClO-
1cm3 bleach = x/10 moles of ClO- = concentration of ClO-
Analysis of copper
-copper(ii) salts produced by cu2+ ions produced by dissolving compound in water, insoluble copper compounds can be reacted with acids
-copper alloys eg brass, alloy is reacted and dissolved in CONCENTRATED NITRIC ACID, HNO3 followed by neutralisation to form Cu2+ ions
Copper ions and thiosulfate ions equations
2Cu2+ + 4I- > 2CuI (s) + I2
-White precipitate of copper iodide forms and brown solution of I2 forms.
Iodine in mixture is titrated with Na2S2O3
2S2O32- + I2 > S4O62- + 2I-
1 mole of Cu2+ = 1 mole of S2O32-
Standard practical procedure for analysis of brass
1.sample of brass reacted and dissolved in concentrated nitric acid forming solution of cu2+ and zn2+ ions, solution is then neutralised
2.excess KI is added, Cu2+ react with I- to form I2
3.I2 is titrated with S2O32-
Calculation for analysis of brass
1.Calculate mole of S2O3- reacted
2.Determine moles of Cu2+
3.Work out mass of Cu2+ by multiplying moles by 63.5
4.Take this mass away from overall mass of brass
5.percentage composition of cu/zn
How to obtain a more accurate value of Cu2+ content in brass
-Use a larger mass of the copper ore
-Repeat titrations calculate a mean from concordant results
-Larger concentration of S2O32- to increase the titre volume and reduce percentage error
-Use 3 decimal place balance to reduce the percentage error
Define volatic cell
Converts chemical energy into electrical energy
Define half cell
Contains the chemical species present in a redox half equation.
-Volatic cell is made by connecting two different half cells
-Chemicals in two half cells must be kept apart
-if allowed to mix, electrons flow in uncontrolled way and heat energy is released rather than electrical energy
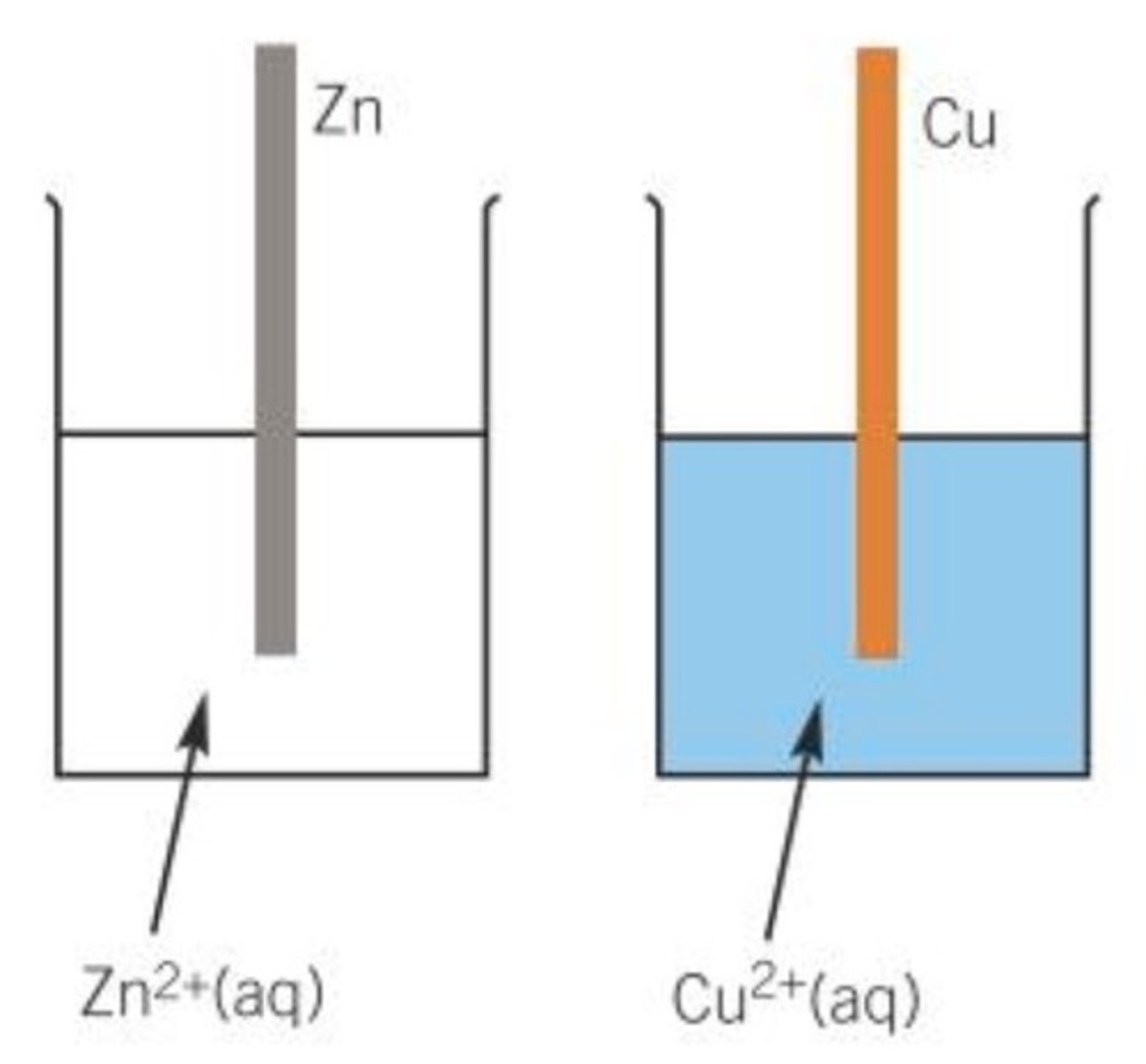
Metal/metal ion half cells
-Metal rod dipped into its solution of its aqueous metal ion.
Represented using a vertical line as a phase boundary between aqueous solution and the metal, eg:
Zn2+ (aq) l Zn (s)
Zn2+ (s) + 2e- > Zn (s), forward reaction is reduction, reverse reaction is oxidation
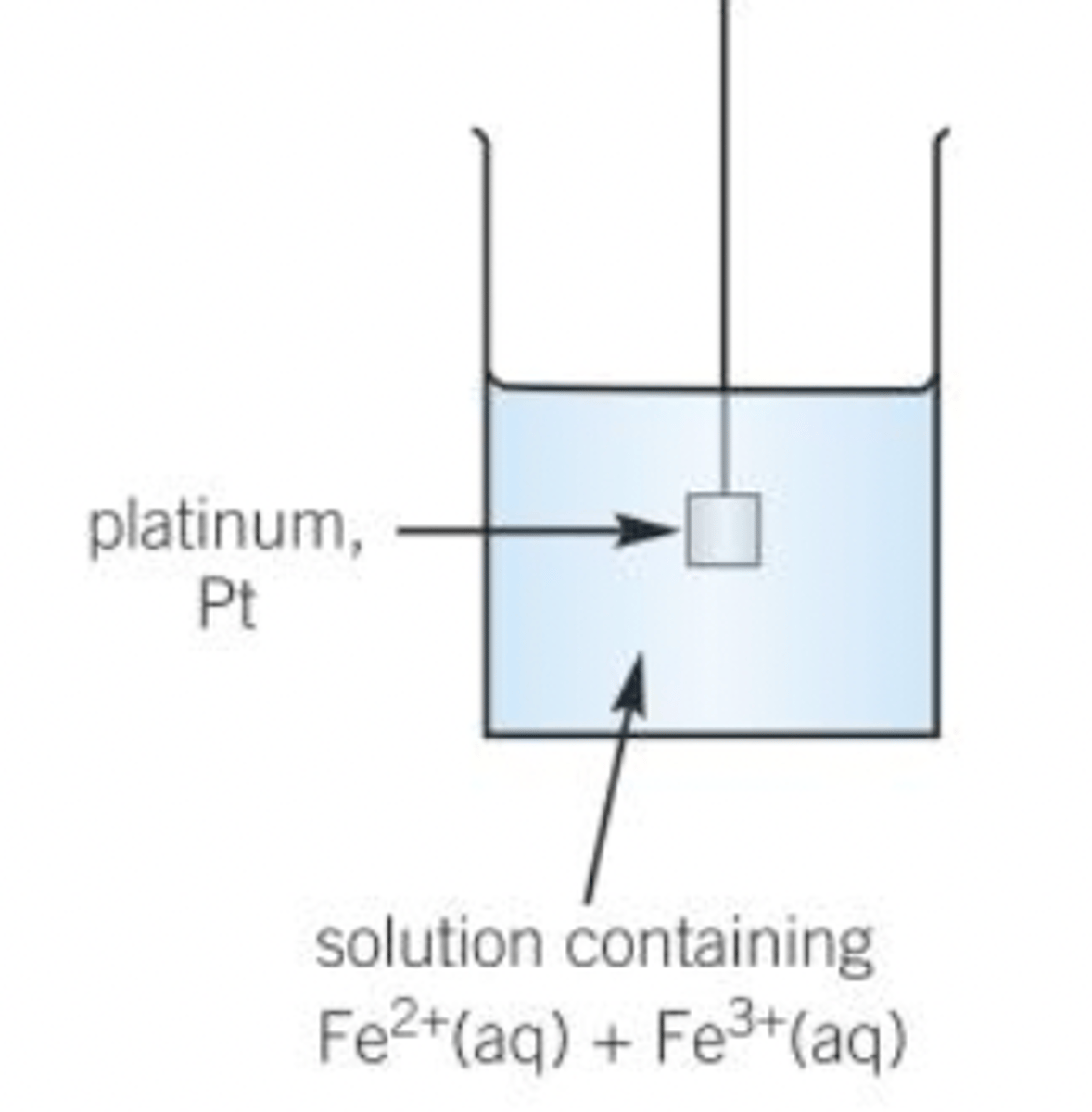
Ion/Ion half cells
-Inert metal electrode made of platinum, Pt dipped into an aqueous solution containing ions of the same elements in different oxidation states
eg Fe2+(aq) I Fe3+ (aq)
In a volatic cell with two metal/metal ion half cells...
The more reactive metal loses electrons and is oxidised, this is the NEGATIVE electrode.
The less reactive metal gains electrons and is reduced, this is the POSITIVE electrode
What is the tendency to be reduced and gain electrons measured as
The standard electrode potential
Define standard electrode potential
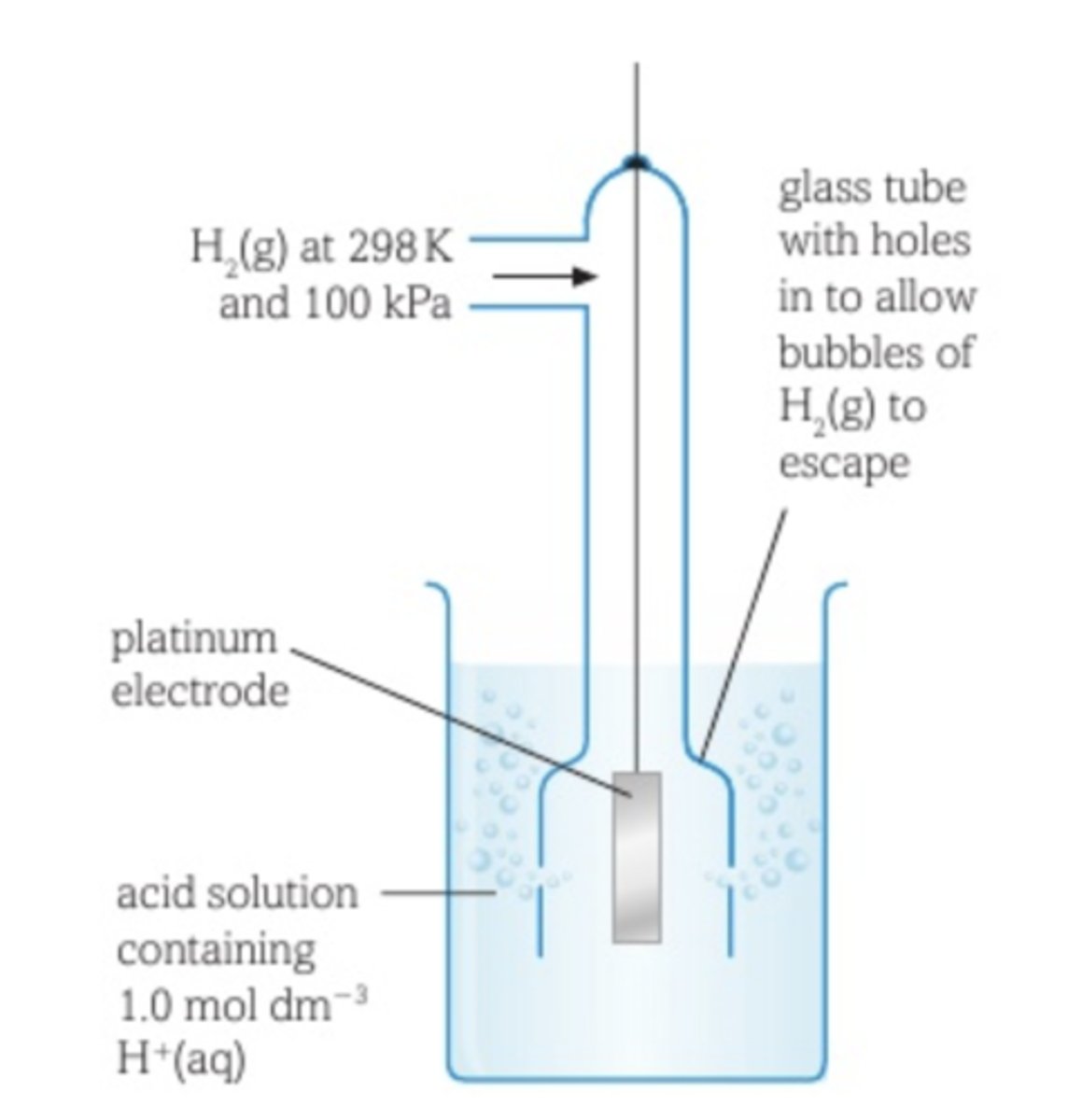
The e.m.f of a half cell connected to a standard hydrogen half cell under standard conditions of 298K, solutions of concentrations 1moldm-3 and a pressure of 100KPa.
Hydrogen half cell is used as a reference when measuring standard electrode potential, what do they look like
Half cell containing hydrogen gas, H2 and H+ solution
What does the sign of the standard electrode potential show
Sign of the half cell connected to the standard hydrogen half cell, as the hydrogen half cell has a standard electrode potential of 0V. Shows relative tendency to gain electrons and be reduced.
Measuring standard electrode potential
To measure standard electrode potential, half cell is connected to a standard hydrogen half cell:
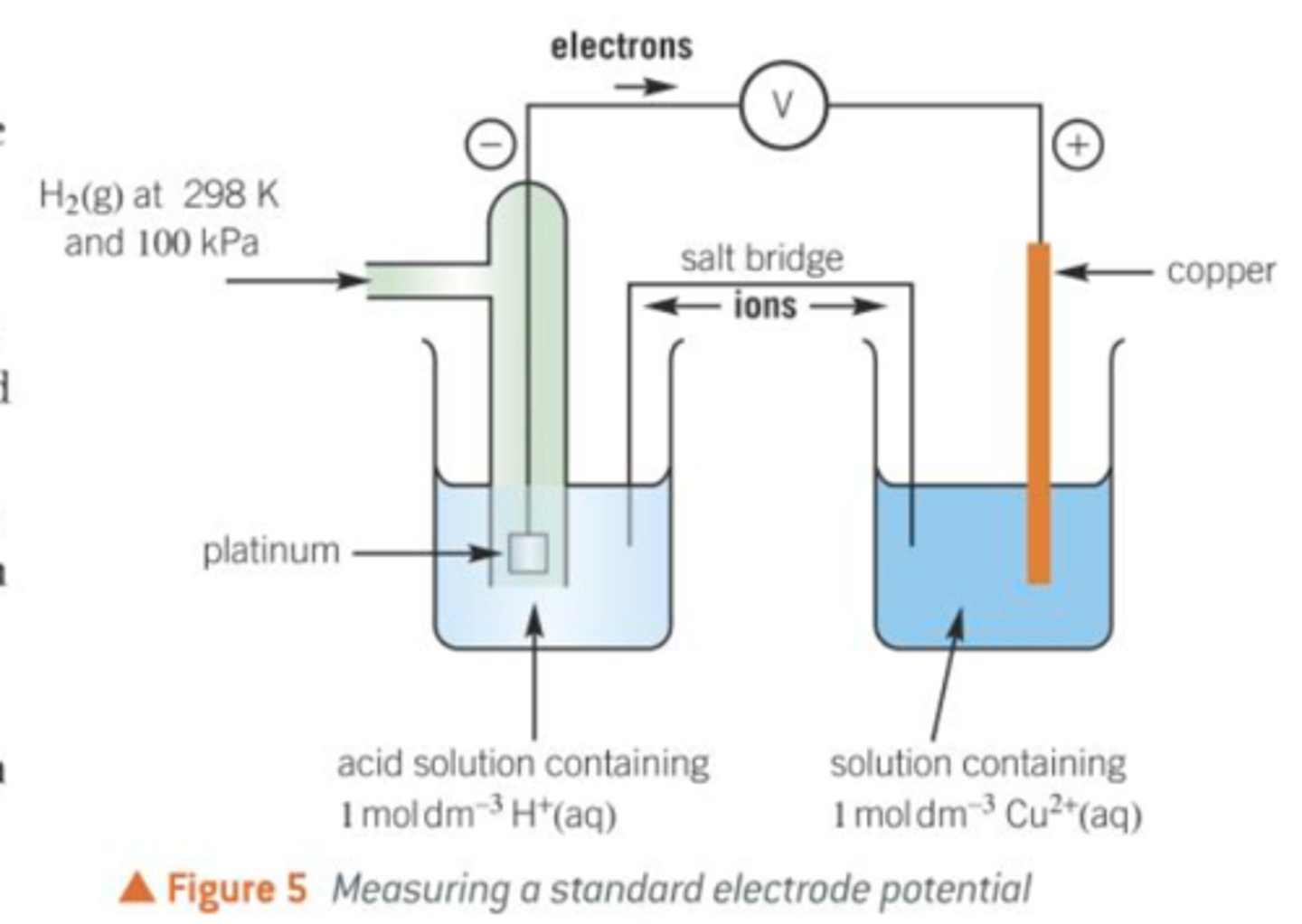
Salt bridge
Connects two solutions to allow movement of ions
-Contains a concentrated electrolyte, that does not react with either solution usually a strip of filter paper soaked in KNO3.
Wire
Connects two electrodes to allow movement of electrons
In a table of standard electrode potentials..
Forward reaction is always reduction.
- To work out oxidation reaction, reverse the equation.
The more negative the standard electrode potential...
-greater the tendency to lose electrons and undergo oxidation
The more positive the standard electrode potential....
-greater the tendency to gain electrons and undergo reduction
Conclusions from e values
Metals tend to have negative e values and lose electrons, non metals tend to have positive e values and gain electrons.
-More negative the e value, greater tendency to be oxidised and lose electrons
-More positive the e value, greater tendency to be reduced and gain electrons
What is the flow of electrons in a volatic cell
Electrons flow from the more negative half cell to the less negative half cell.
E cell equation
Ecell = E(positive electrode) - E(negative electrode)
Standard electrode potentials reducing and oxidising agents
Oxidising agent takes electrons away from species being oxidised, so they are reduced and on the left.
Reducing agents adds electrons to the species being reduced, so they are oxidised and on the right.
Strongest oxidising agent is at the bottom on the left. (MOST POSITIVE E VALUE)
Strongest reducing agent is at the top on the right (MOST NEGATIVE E VALUE)
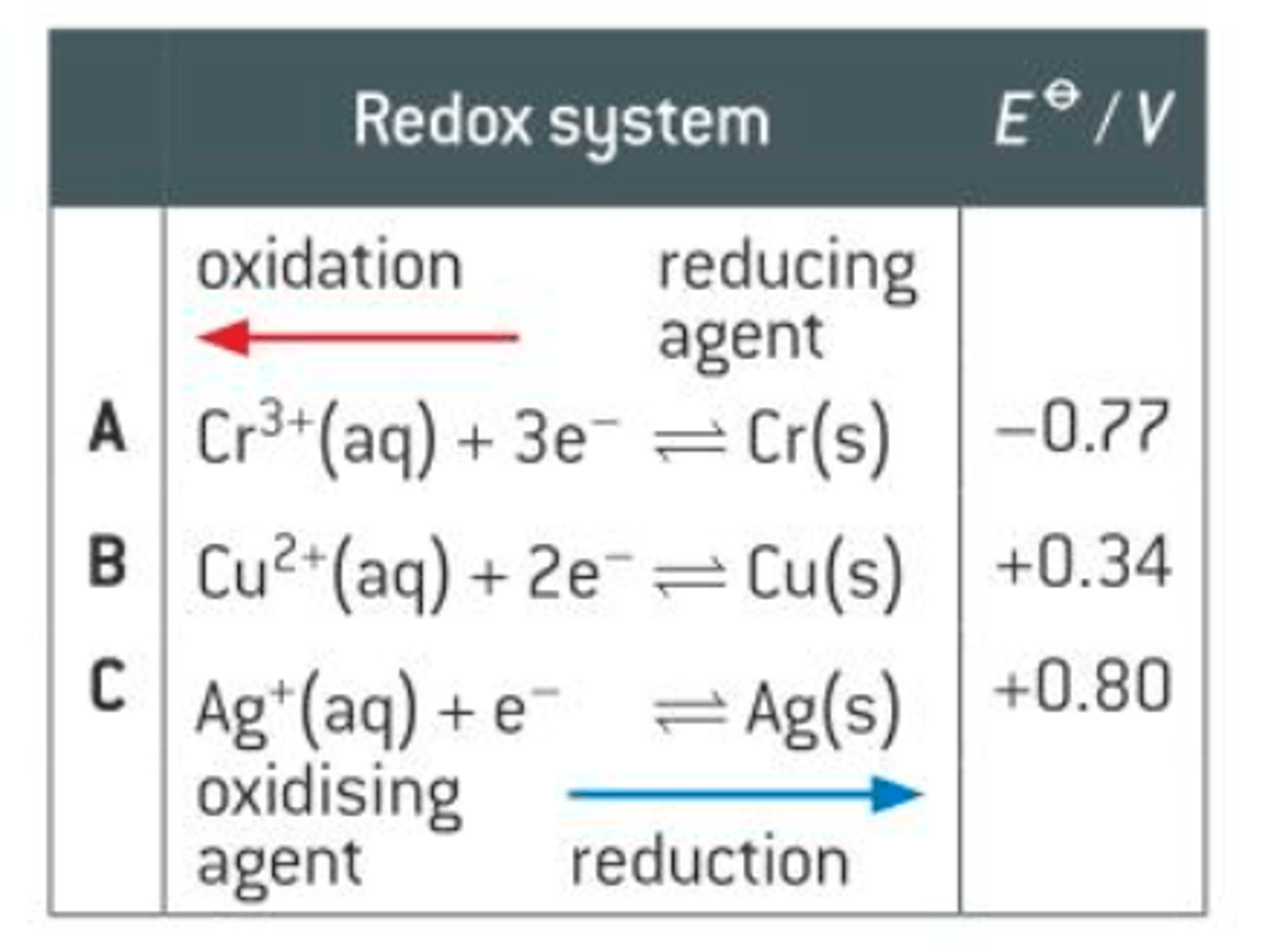
Predicting reaction feasability
Redox system with more negative e value is oxidised and lose electrons, position of equilbirium shifts left
Redox system with more positive e value is reduced and gains electrons, position of equilbirium shifts right
Write the overall equation
All feasible reactions have a positive Ecell value.
Limitations of standard electrode potentials
-reactions have very large activation energies, resulting in a slow rate of reaction
-Non standard conditions
-Only apply to aqueous equilbiria, many reactions are not aqueous
Why is concentration a limitation of standard electrode potentials
Zn2+ + 2e- > Zn
conc of Zn2+ > 1moldm-3, position of eq shifts right removing electrons so e becomes less negative
conc of Zn2+ < 1moldm-3, position of eq shifts left adding electrons so e becomes more negative
any change to conc affects electrode potential
Requirement for modern day cells and batteries
Two electrodes to have two different electrode potentials
Primary cells
-Non rechargeable, one time use
-Electrical energy produced by oxidation and reduction at electrodes
-Used for low current, long storage devices such as clocks
-Modern day based on alkaline Zn/MnO2 with an alkaline KOH electrolyte
Secondary cells
-Rechargeable, cell reaction producing electrical energy can be reversed
-Chemicals in cells are regenerated and used again
eg: lead-acid batteries in car batteries
lithium-ion and lithium-ion polymer cells in appliances
Define fuel cell
Fuel cell uses the energy from the reaction of a fuel with oxygen to produce electrical energy
Features of a fuel cell
-Require continuous supply of fuel and oxygen
-Operate continuously as long as fuel and oxygen are added
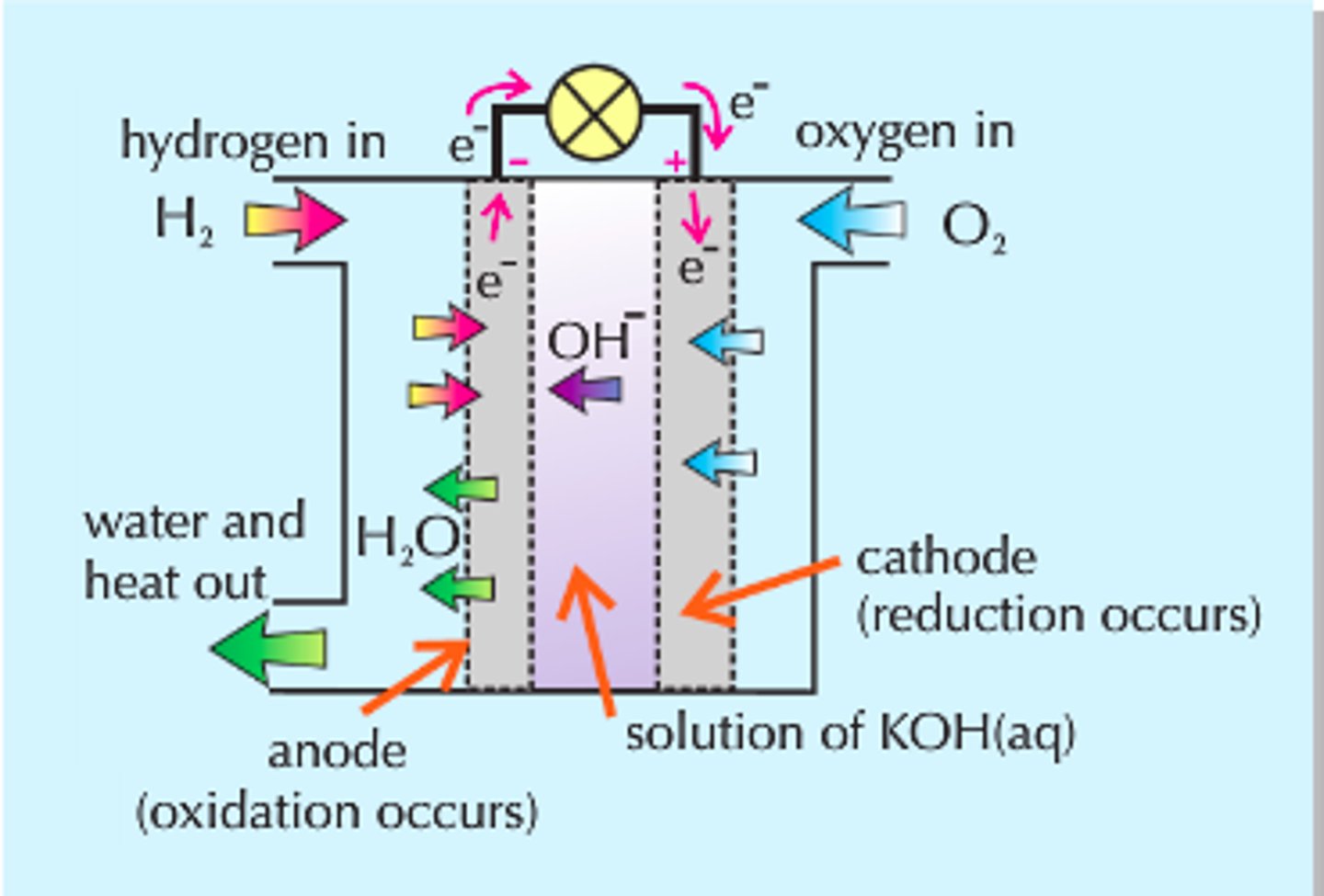
How does a fuel cell work
Fuel and oxygen flow into fuel cell, products flow out. Electrolytes remain in the cell.
Fuel cells do not need to be recharged
Hydrogen fuel cells
Electrolyte can be acidic or alkaline
H2(g) + 1/2 O2 (g) > H2O (l)
cell voltages are both 1.23 V
Advantages and disadvantages of fuel cells
Advantages:
-Efficent as more energy is converted into electrical energy, combustion wastes alot of energy as thermal energy.
-Don't need to be recharged, just need a constant supply of oxygen and fuel
-Only waste product is water, no CO2 emitted like a combustion engine
Disadvantages:
-Hydrogen is highly flammiable
-Expensive to store and transport hydrogne
-Energy is required to make hydrogen and oxygen in the first place
Hydrogen fuel cells
oxidation:
Acidic: H2>2H+ + 2e-
Alkaline: H2 + 2OH- > 2H2O + 2e-
reduction:
alkaline: 1/2o2 + h2o + 2e- > 2oh-
acidic: 1/2o2 + 2h+ + 2e- > h2
overall:
1/2o2 + 2h+ > h2o , cell voltage is 1.23V