A Level Chemistry - Bonding
5.0(1)
Card Sorting
1/97
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
1
New cards
Metallic bonding
The attraction between delocalised outer shell electrons and positive metal ions in a lattice
2
New cards
Giant
Endlessly reprating
3
New cards
Delocalised
Not associated with a particular atom
4
New cards
What does the number of delocalised electrons depend on?
How many electrons are lost from each metal ion
5
New cards
Metallic bonding of sodium
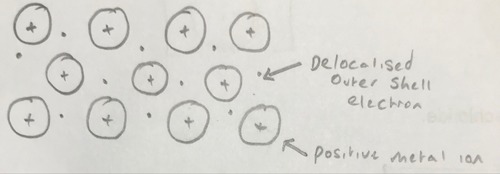
6
New cards
Metallic bonding of magnesium
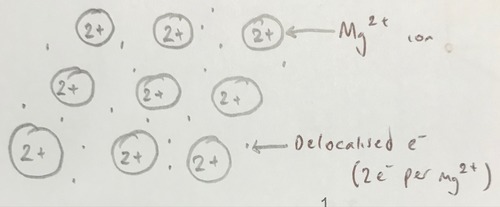
7
New cards
Ionic bonding
The electrostatic attraction between oppositely charged ions in a lattice
8
New cards
Diagram of a giant ionic lattice
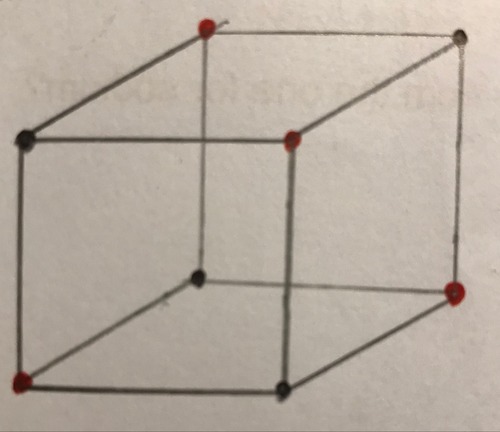
9
New cards
Boron bonding
Don't for ions (but will form covalent compounds)
10
New cards
Carbon and silicon bonding
Don't form ions
11
New cards
Tin bonding
Sn4+ is most stable but can form Sn2+
12
New cards
Lead bonding
Pb2+ is most stable but can form Pb4+
13
New cards
Transition metal bonding
Form more than one stable ion
14
New cards
Ammonium
NH4 +
15
New cards
Hydroxide
OH -
16
New cards
Nitrate (III)
NO2 -
17
New cards
Nitrate (V)
NO3 -
18
New cards
Cyanide
CN -
19
New cards
Hydrogen carbonate
HCO3 -
20
New cards
Hydrogen sulfate
HSO4 -
21
New cards
Do hydrogen phosphate
H2PO4 -
22
New cards
Carbonate
CO3 2-
23
New cards
Sulfate (IV)
SO3 2-
24
New cards
Sulfate (VI)
SO4 2-
25
New cards
Hydrogen phosphate
HPO4 2-
26
New cards
Phosphate (V)
PO4 3-
27
New cards
Covalent bond
A shared pair of electrons
28
New cards
Lone pair
A pair of electrons which is not bonded
29
New cards
Non-octet molecules
Molecules where the central atom doesn't have a noble gas electron arrangement eg BF3, SF6
30
New cards
Coordinate bond
A covalent bond in which both electrons of the shared pair come from the same atom
31
New cards
How do coordinate bonds form?
The atom that donates electrons has a lone pair. The atom that accepts the electron pair doesn't have a full outer shell (it is electron deficient) and can fit a lone pair
32
New cards
How are coordinate bonds shown?
Using an arrow pointing from the the atom donating the electron
33
New cards
What is a molecules shape based on?
The total number of electron pairs around the central atom
The number of bonding pairs of electrons
The number of lone pairs of electrons
The number of bonding pairs of electrons
The number of lone pairs of electrons
34
New cards
Electron Pair Repulsion Theory
Pairs of electrons repel each other so that they as far apart as possible
Lone pairs are more compact so repel more
The molecule takes up a shape which minimises repulsion
Lone pairs reduce bond angles by 2.5° each
Lone pairs are more compact so repel more
The molecule takes up a shape which minimises repulsion
Lone pairs reduce bond angles by 2.5° each
35
New cards
Bond on the plane of the paper
Straight line

36
New cards
Bond coming out of the paper
A wedge

37
New cards
Bond going into the paper
Dotted line
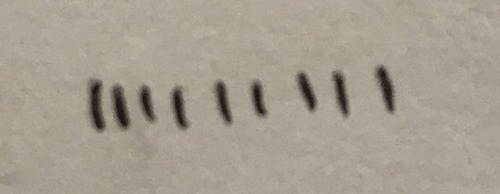
38
New cards
2 bond pairs 0 lone pairs
Linear - 180°

39
New cards
3 bond pairs 0 lone pairs
Trigonal planar - 120°
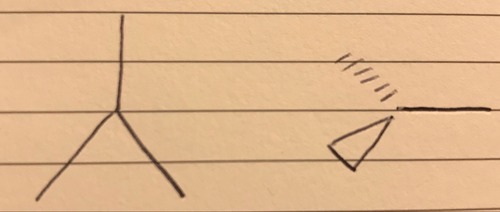
40
New cards
4 bond pairs 0 lone pairs
Tetrahedral - 109.5°
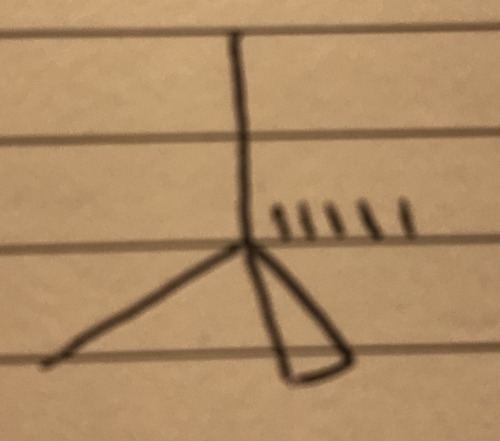
41
New cards
5 bond pairs 0 lone pairs
Trigonal Bipyrimidal - 90° and 120°
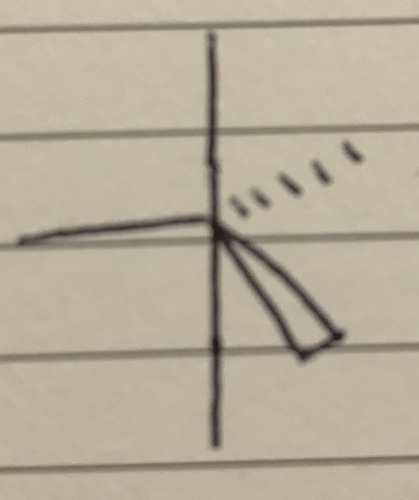
42
New cards
6 bond pairs 0 lone pairs
Octahedral - 90° (and 180°)
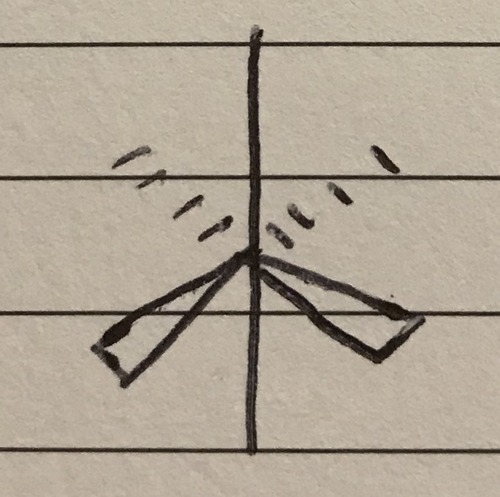
43
New cards
2 double bonds 0 lone pairs
Linear - 180°

44
New cards
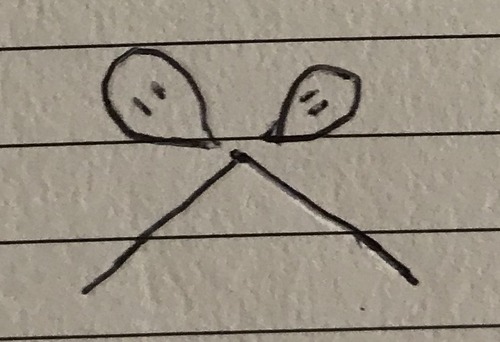
3 double bonds 0 lone pairs
Trigonal planar - 120°
45
New cards
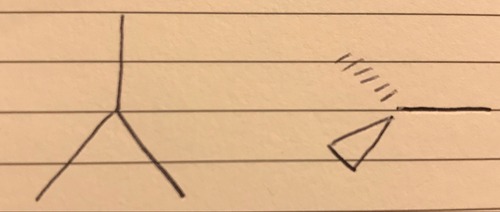
2 bond pairs 1 lone pair
V-shaped/bent - 117.5°
46
New cards
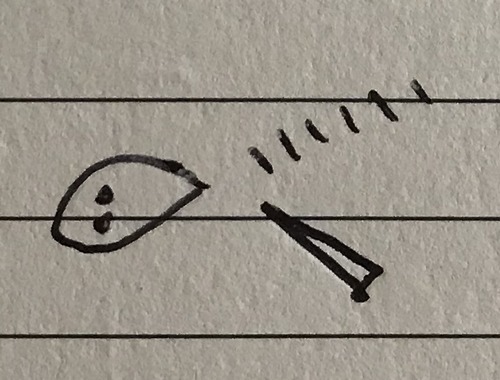
3 bond pairs 1 lone pair
Trigonal pyramidal - 107°
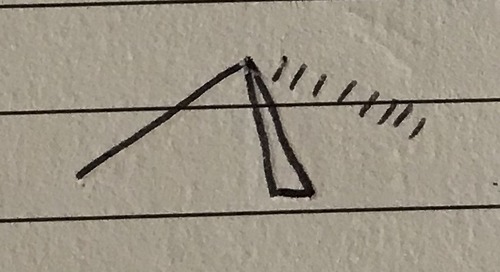
47
New cards
2 bond pairs 2 lone pairs
V-shaped/bent - 104.5°
48
New cards
3 bond pairs 2 lone pairs
Trigonal planar - 120°
49
New cards
4 bond pairs 2 lone pairs
Square planar - 90°
50
New cards
How to explain molecule shape
Number of lone pairs and bond pairs and where. Electron pairs repel. Lone pairs repel more. State the bond angle and why
51
New cards
Define electronegativity
The power of an atom to attract the pair of electrons in a covalent bond
52
New cards
Trend of electronegativity across a period
Increases as nuclear charge (proton number) increases
53
New cards
Trend of electronegativity down a group
Decreases as the number of shells increases so atomic radius and shielding increases
54
New cards
What is the most electronegative element?
Fluorine
55
New cards
What makes a bond polar?
When there is an uneven distribution of electrons
56
New cards
What makes a non-polar covalent bond?
No or very small difference in electronegativity. The electron pair is shared equally with no dipole in the bond
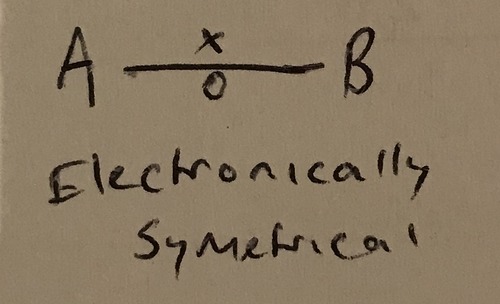
57
New cards
Non polar covalent bond examples
H2, Cl2, O2, NaCl, CH
58
New cards
What makes a polar covalent bond?
A difference in electronegativity. The electron pair is closer to the more electronegative atom, making it slightly negative
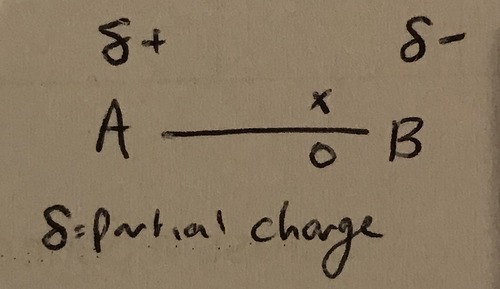
59
New cards
Polar covalent bond examples
HCl
CCl
BeCl
CCl
BeCl
60
New cards
What makes an ionic bond?
A large difference in electronegativity. Electrons are pulled so far towards the electronegative atom that the less electronegative atom loses the electron
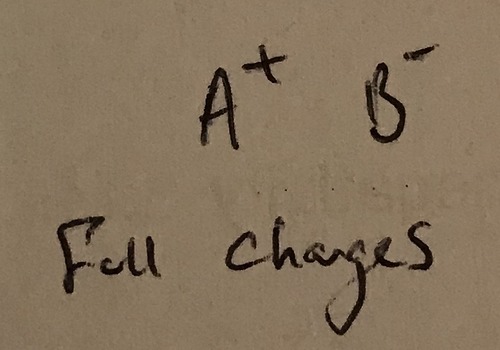
61
New cards
Permanent dipole
A small charge difference across a bond resulting from a difference in electronegativities of the bonded atoms
62
New cards
What can happen if there are multiple dipoles in a molecule?
If they make the molecule electronically symmetrical eg CO2, the dipoles cancel out
63
New cards
Define intermolecular forces
The forces of attraction between molecules
64
New cards
What are van der Waals' forces?
These are the weakest intermolecular forces which occur between all substances between molecules of atoms
65
New cards
Instantaneous dipole
A constantly forming a disappearing dipole caused by the movement of electrons which unbalanced the charge distribution within the molecule
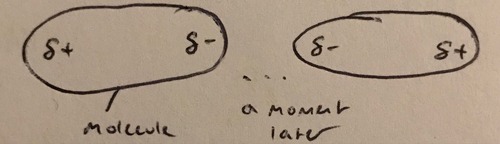
66
New cards
Why do non polar substances have low boiling points?
They only have van der Waals forces between molecules which are very weak
67
New cards
What determines the strength of van der Waals forces?
Bigger molecules have more electrons so the induced dipoles are large resulting in stronger forces
68
New cards
What causes van der Waals forces?
The random movement of electrons causes an instantaneous dipole across the molecule which indices a dipole in neighbouring molecules resulting in weak forces of attraction
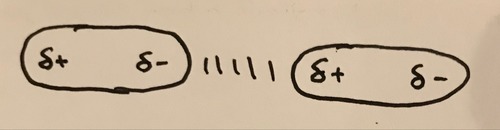
69
New cards
Which substances only have van der Waals forces?
Simple molecules and simple atomic substances
70
New cards
What are permanent dipole-dipole forces?
These occur between molecules which have a permanent dipole in addition to van der Waals forces. The negative pole of one molecule attracts another's positive pole. These are generally stronger than van der Waals forces
71
New cards
What are hydrogen bonds?
These are the strongest intermolecular forces which occur between molecules which contain a hydrogen atom bonded to either F, O or N. The bond forms between the slightly positive H atom in one molecule and a lone pair in the other
72
New cards
Properties of hydrogen bonds
Higher boiling point than expected due to the strength of the hydrogen bonds
Substances which can hydrogen bond tend to dissolve in water because they form hydrogen bond with water
Substances which can hydrogen bond tend to dissolve in water because they form hydrogen bond with water
73
New cards
What must be broken to melt or boil an ionic, metallic or macromolecular structure?
The bonds
74
New cards
What must be broken to melt or boil a simple molecular or atomic substance?
The intermolecular forces
75
New cards
What is needed to conduct electricity?
Charged particles which are free to move such as delocalised electrons or ions
76
New cards
What is the solubility rule?
Substances can dissolve if dilute and solvent molecules attract one another - like dissolves like. Ionic and polar substances dissolve in polar solvent sand non polar substances dissolve in non polar solvents
77
New cards
Structure of ionic compounds
A giant ionic lattice structure where negative and positive ions alternate so each ion is surrounded completely by oppositely charged ion held together by ionic bonds
78
New cards
Why do ionic compounds have high melting points?
The ions are held together by many strong ionic bonds that need a lot of energy to break
79
New cards
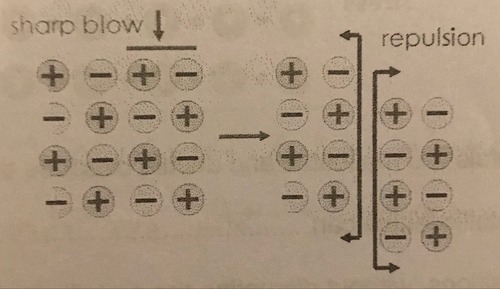
Why are ionic compounds brittle?
If enough force is applied, the layers can slide over each other. Like charges will move next to each other, causing repulsion which breaks down the lattice structure
80
New cards
Why do ionic compounds only conduct electricity when molten or aqueous?
The ions are only free to move in theses states so can carry current
81
New cards
Why do metals have high melting and boiling points?
Metallic bonds are generally strong so large amounts of energy is needed to break them
82
New cards
Why can metals conduct electricity?
The delocalised electrons can move through the structure and carry the current
83
New cards
Why are metals strong?
Metallic bonds are strong and extend through the giant metallic lattice structure
84
New cards
What does metallic bond strength depend on?
The size and charge of the metal ion (it's charge density). Smaller and more highly charged ions form stronger bonds
85
New cards
Malleable
Can be hammered or pressed into shape without breaking or cracking
86
New cards
Ductile
Can be drawn into thin wires
87
New cards
Why are metals ductile and malleable?
The layers of ions in the giant metallic lattice can slide over each other into new positions without disrupting the metallic bond
88
New cards
Types of covalent substances
Simple molecular and macromolecular
89
New cards
Why do simple covalent molecules have low melting points?
The forces of attraction between molecules are weak. Not much energy is needed to break the intermolecular forces
90
New cards
Why can't simple covalent molecules conduct electricity?
They don't contain ions or delocalised electrons so can't carry a charge
91
New cards
Solubility rule of simple covalent molecules
Generally insoluble in water unless they can form hydrogen bonds with water or react with it
92
New cards
Structure of diamond
A giant arrangement of carbon atoms bonded to four other carbon atoms
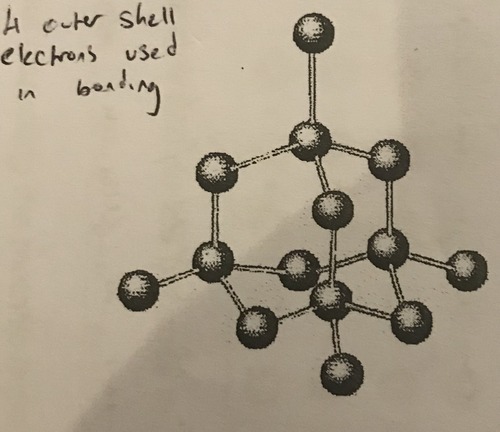
93
New cards
Structure of graphite
A giant arrangement of carbon atoms bonded to three other carbon atoms in layers
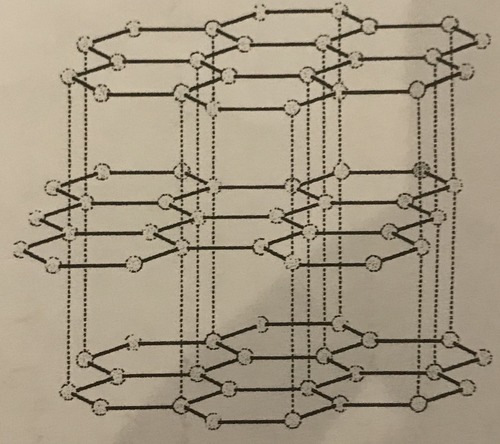
94
New cards
Structure of diamond
A giant arrangement of carbon atoms bonded to four other carbon atoms
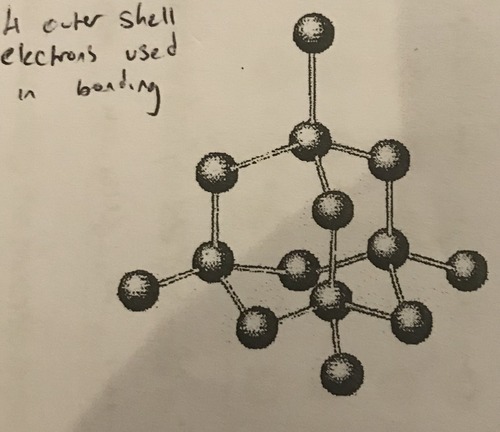
95
New cards
Why do diamond, graphite and graphene have high melting points?
Many covalent bonds between all the atoms which take lots of energy to break
96
New cards
Structure of graphene
A single sheet of graphite
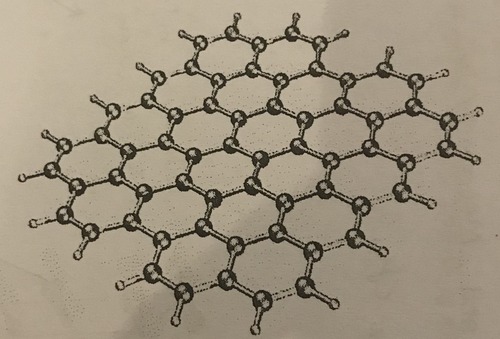
97
New cards
Why do graphite and graphene conduct electricity?
One electron per carbon isn't involved in bonding and is delocalised along the layer allowing it to carry a current
98
New cards
Why are diamond, graphite and graphene insoluble in water?
The covalent bonds are very strong and the lattice does not break up when any solvent is added