10.1 Radicals
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
77 Terms
What are the two main ways a covalent bond can break?
Heterolytic cleavage (forms ions) and homolytic cleavage (forms radicals)
What happens in heterolytic bond cleavage?
Both bonding electrons go to one atom, forming a cation and an anion
What happens in homolytic bond cleavage?
Each atoms gets one electron from the bond, forming two radicals
What is a radical?
A species with an unpaired electron, often highly reactive
In what type of bonds does heterolytic cleavage usually occur?
Polar covalent bonds, often under (Δ) or light (hv)
Example of a heterolytic cleavage?
H-Cl → H⁺ + Cl⁻
What types of reactions use heterolytic bond cleavage?
Ionic mechanisms such as SN1, SN2, E1, and E2 reactions
What types of reactions use homolytic bond cleavage?
Radical reactions like halogenation of alkanes
“Hetero” means what, and how does it help you remember?
“Different”- electrons go to a different atom and forms ions
“Homo” means what, and how does it help you remember?
“Same”- electrons are split equally, forming radicals
Which cleavage type produces species with opposite charges?
Heterolytic cleavage
Which cleavage type produces species with unpaired electrons?
Homolytic cleavage
Why are radicals highly reactive?
They seek to pair their unpaired electrons by forming new bonds
What type of bond cleavage forms ions?
Heterolytic bond cleavage
What type of bond cleavage forms radicals?
Homolytic bond cleavage
In ionic reactions, how many electrons move in a curved arrow?
Two electrons (double-barbed arrow)
What is another name for a single-barbed arrow?
A fishhook arrow
What does a double-barbed arrow represent?
The movement of two electrons in an ionic process.
What does a single-barbed (fishhook) arrow represent?
The movement of one electron in a radical process.
Which type of arrow is used exclusively in radical mechanisms?
Fishhook (single-barbed) arrows.
Which type of mechanisms use double-barbed arrows?
Ionic mechanisms (like SN1 or SN2).
What do radicals contain that make them reactive?
An unpaired electron.
What is the hybridization and geometry of a carbocation?
sp² hybridized, trigonal planar.
How many nonbonding electrons does a carbocation have?
None (0).
What is the hybridization and geometry of a carbanion?
sp³ hybridized, trigonal pyramidal
How many nonbonding electrons does a carbanion have?
Two (a lone pair)
What is the hybridization and geometry of a carbon radical?
Between sp² and sp³, usually nearly trigonal planar.
How many nonbonding electrons does a carbon radical have?
One (unpaired electron)
Why is a carbanion pyramidal but a carbocation planar?
The carbanion’s lone pair repels bonding pairs, bending the structure; the carbocation has no lone pairs, so it stays flat.
Why is a radical almost planar?
It has only one unpaired electron, causing minimal repulsion and allowing a nearly flat shape
What is “inversion” in carbon radicals?
A rapid flipping of the shallow pyramidal shape through a planar form (very low energy barrier)
Which has the highest barrier to inversion: carbocation, radical, or carbanion?
Carbanion (because of the lone pair’s stronger repulsion)

What is the geometry of a carbon radical?
Trigonal planar (sp²-like).
What does the unpaired electron in a radical occupy?
A p orbital perpendicular to the plane of the σ bonds.
What is the order of stability for carbon radicals?
Tertiary > Secondary > Primary > Methyl.
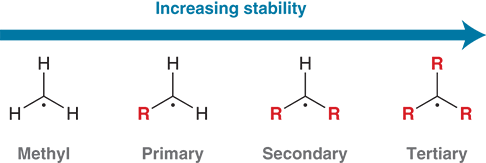
What is the main factor that stabilizes carbon radicals?
Hyperconjugation from neighboring alkyl groups.
What is hyperconjugation?
Delocalization of electron density from adjacent C–H σ bonds into the half-filled p orbital of the radical
How does the number of alkyl groups affect radical stability?
More alkyl groups → more hyperconjugation → higher stability
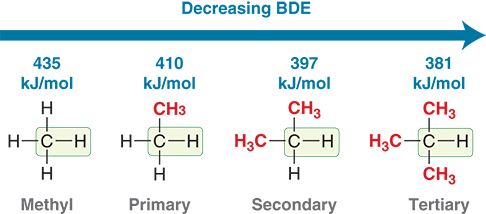
What is the relationship between radical stability and bond dissociation energy (BDE)?
Lower BDE corresponds to higher radical stability.
Which C–H bond is easiest to break homolytically (which bond requires more energy to break): primary, secondary, or tertiary?
Tertiary C–H bond (forms the most stable radical).
How much more stable is a tertiary radical compared to a secondary radical?
About 16 kJ/mol more stable.
Why can radicals be treated as planar in mechanisms?
Because the unpaired electron resides in a p orbital, allowing overlap with neighboring bonds for hyperconjugation.
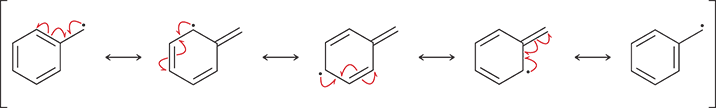
When does a radical show resonance?
When the unpaired electron is adjacent to a π bond (in an allylic position).
How many fishhook arrows are used to draw the resonance of an allylic radical?
Three fishhook arrows.
What does each fishhook arrow represent?
The movement of a single electron.
Describe the 3-arrow movement in an allylic radical.
One electron from the π bond moves toward the radical, one moves to form a new π bond, and the radical’s unpaired electron moves toward the π system.
Give the resonance forms of CH₂=CH–CH₂•
CH₂=CH–CH₂• ↔ •CH₂–CH=CH₂
What happens to the unpaired electron in resonance?
It becomes delocalized across two or more carbons.
How does delocalization affect stability?
It increases stability by spreading out electron density.
Why are allylic radicals more stable than tertiary radicals?
Because resonance delocalization provides additional stabilization beyond hyperconjugation.
What type of arrow is used to show single-electron movement?
A single-barbed arrow, also called a fishhook arrow
How is resonance for radicals different from resonance for ions?
Radical resonance uses fishhook (single-barbed) arrows, while ionic resonance uses double-barbed arrows for pairwise electron movement.
What is a benzylic radical?
A radical in which the unpaired electron is on a carbon adjacent to a benzene ring
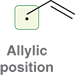
What is an allylic radical?
A radical in which the unpaired electron is on a carbon adjacent to a C=C double bond.
Why are benzylic radicals especially stable?
The unpaired electron can delocalize throughout the aromatic π system by resonance.
How many resonance structures can a benzylic radical have?
Several (the unpaired electron can be delocalized across multiple ring carbons)
What experimental evidence supports radical stability trends?
Bond dissociation energy (BDE) values — lower BDE means a more stable radical forms.
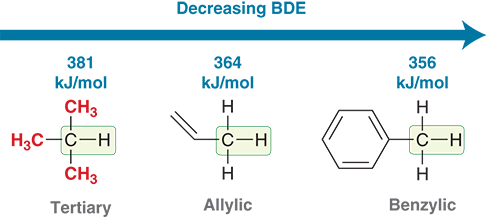
Which has a lower C–H bond dissociation energy: benzylic or tertiary?
Benzylic (because it forms a resonance-stabilized radical)
Which radicals are resonance-stabilized?
Allylic and benzylic radicals.
What is the stability order of radicals (most to least)?
Benzylic ≈ Allylic > Tertiary > Secondary > Primary > Methyl.
Why is a benzylic C–H bond easier to break than a tertiary C–H bond?
Breaking it forms a resonance-stabilized benzylic radical
What is the key concept linking resonance and radical stability?
The more delocalized the unpaired electron, the more stable the radical.
What is an allylic radical?
A radical on a carbon adjacent to a C=C double bond
What is a vinylic radical?
A radical located directly on a carbon of a C=C double bond.
Which radical type is stabilized by resonance?
Allylic (and benzylic) radicals.
Does a vinylic radical exhibit resonance?
No — the unpaired electron cannot delocalize into the π bond.
Which is more stable: allylic or vinylic radical?
Allylic — because of resonance stabilization.
How does the stability of a vinylic radical compare to a methyl radical?
The vinylic radical is about 30 kJ/mol less stable (higher in energy).
Which requires more energy to form: a methyl radical or a vinylic radical?
A vinylic radical — its C–H bond has a higher BDE.
What is the overall radical stability order?
Benzylic ≈ Allylic > Tertiary > Secondary > Primary > Methyl > Vinylic.
Why are vinylic radicals so unstable?
The unpaired electron resides in an sp² orbital that can’t overlap with the π system.
How can you remember the difference between allylic and vinylic?
Allylic = Adjacent to a double bond (resonance); Vinylic = directly on the double bond (no resonance)