Genomes Diseases and Diversity
1/203
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
204 Terms
what causes cancer
mutations that alter the function or regulation of genes involved in affecting mitotic/apoptotic rates
what are cell numbers the product of
rates of cell division-mitosis- and cell death-apoptosis
is cancer a single disease
NO it is a diverse group of conditions sharing in an increase of cell numbers within a particular tissue
malignant versus benign
malignant=invasive and spreads to other tissues
benign=non life threatening and does not spread to other tissues
where is cancer most common
in epithelial tissues known as carcinomas and is about 80% of cancer
epithelial tissues
sheets of cells forming the upper layers of skin and lining the walls of cavities and tubes within organs
sarcoma
arising from connective tissue (bone or cartiledge)
why are epithelial cells the most commonly subject to cancer
highest exposure to carcinogens
prone to damage due to environmental exposure so they have a high division rate

why do cancers arise more frequently in tissues with high division
with higher mitotic rates comes fewer barriers to cell division and more frequent cell division increasing the likelihood of mutation
do all cells divide at the same rate
no→muscle and brain tissue divide slowly
p53
tumour suppressor gene and is considered a critical domain that forms a tetramer
loss of function mutation
reduction of function of protein encoded by a gene
hypomorhpic is partial loss
null is total loss
tumour suppressor
gain of function mutation
activity of resulting protein is increased
Ras
dominant interfering
protein opposes wild type protein function typically by binding to it or its target blocking the function
P53 mutation
explain why p53 mutations are so bad
if even a single subunit of tertramer is mutated then there is a complete function loss and mutant p53 that still binds to the tetramer is a dominant interfering mutation
what do carcinogens cause
cancer/ are mutagenic agents
two options for cells with dna damage
apoptosis
senescence(placed in a non replicative state)
how do viruses invoke cancer
insertion into the genome of their hosts
viral gene may carry a gene that enables the host cell to evade normal controls
viruses may integrate their genomes close to a host gene that regulates cell division leading to aberrant gene expression
how often do human cells divide
a rate of 10^7 per second
how do cancer cells typically arise
from a single cell eventually forming a tumor population
was debated due to many tumours appearing to be polyclonal upon diagnsois however most tumours are monoclonal meaning they comes from a single precurors cell
how did they determine monoclonality of tumours
2 ways
B-call tumors create single antibodies for specific antigens and therefore they would produce many different antibodies if they arose from multiple, individually transformed cellls
they producde a single antigen
and from chromosomal lesion analysis which had all cells of the same tumour presenting with the same legions
Philadelphia(Ph) chromosomes found in chronic myeloid leukamia and results in the translocation of BCR gene and its promoter upstream of the Abl gene to create new fusion gene (Bcr-Abl) with the protein product creating an increase in kinase acitivty
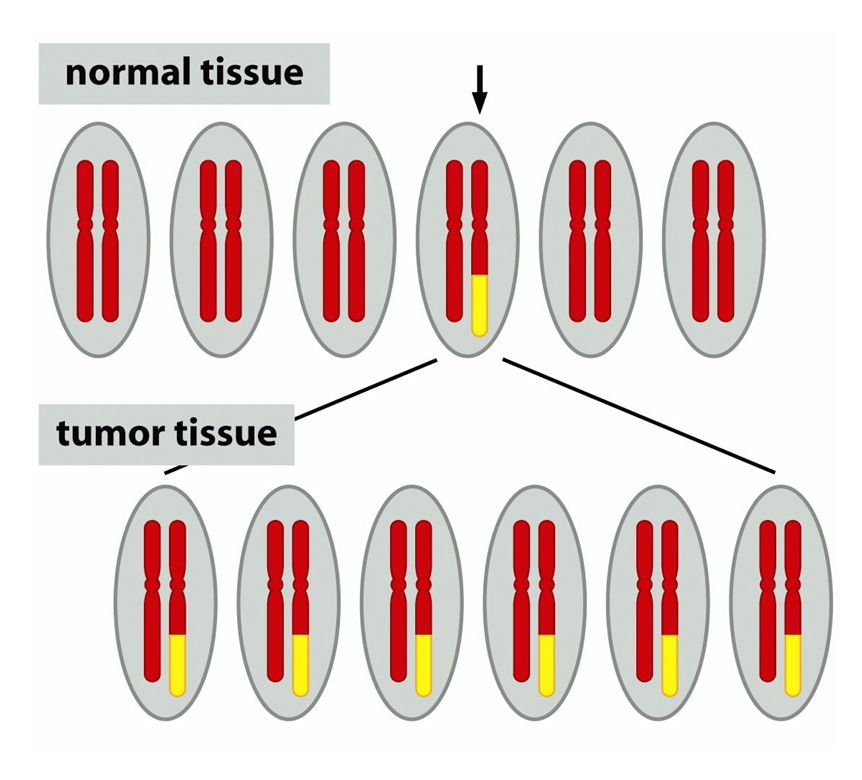
how many independent mutations generally required for cancer
6-8→cancer isnt caused by a single mutation
multicellular organisms cannot be successfull if cells dont work together to ensure success as a whole
chromothripsis
large scale chromosomal breakage producing multiple mutations similtanesouly
oncogene
👉 Proto-oncogene = normal "go" signal
👉 Oncogene = stuck accelerator leading to cancer
An oncogene is a gene that, when mutated or overexpressed, can turn a normal cell into a cancerous one by promoting uncontrolled growth and division.
usually a gain of function mutation
tumour suppressor gene
normally opposes cancer and generally results in a loss of function of dominant interfering mutation
what are the four barriers to transformation
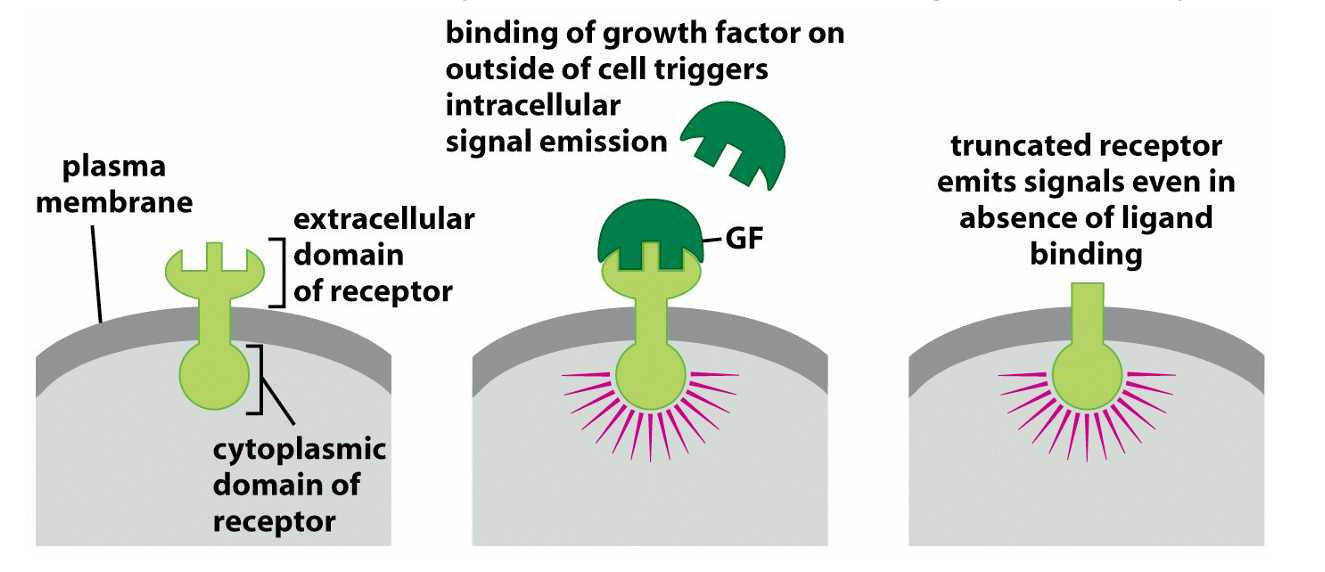
requirement for growth factors: they enable division and are provided by other cells via paracrine signalling→cancers msut find a way to increase the supply and do this by either making it themselves are mutating the signalling molecule downstream to appear that they are always switched on
Tumour suppression genes act as a break on proliferation: P53 trasncription factor collaborates with other proteins(ATM, ATR, CHk1 and 2) involved in DNA damage detection and repair; when activated it blocks entry in mitosis to allow dna repair to be carried out
extensive DNA damage results in apoptosis via Noxa, Bax, Puma since it is safer to destroy then repair
requirement for O2 and nutrients→ this is regulated by blood vessel proximity so they require new blood vessels via neovascularisation to grow beyond 1cm³
new blood vessels are produced via angiogenesis and this is linked to production of vascular endothelial growth factor by the tumour
the immune system so evasion of cytotoxic T cells and natural killer cells
how common is mutant p53 gene
half of all cancers
what are the six characteristics of transformed cells
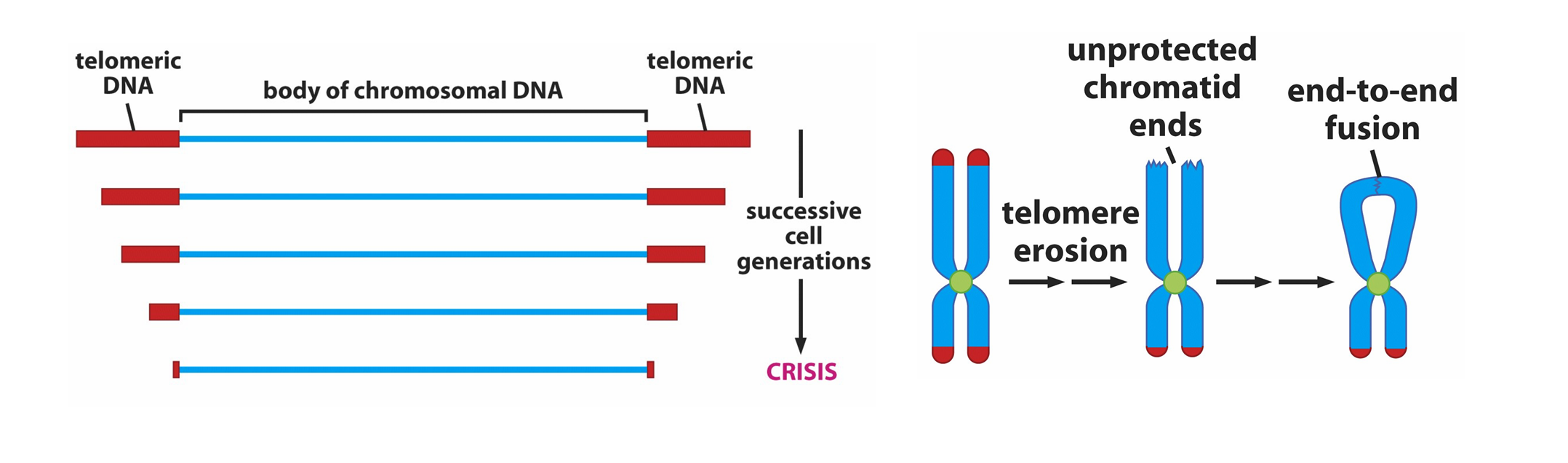
ability to grow in lab cultures(in vitro) for long periods of time; in exposure to serum growth factor non transformed cells only can go through about 25 cell division(Hay Flicks limit) due to telomere shortening→cells that are transformed express telomerase which repairs telomeres and is usually only found in stem and embryonic cells but many cancers experience reactivation of the gene
reduced requirement for growth factors via ability to make their own GF via autocrine growth or by increasing growth factor receptors or mutating gene functioning in GF receptor pathways such that the signaling cascade is permanently switched on as with Ras and Braf
anchorage independence: most cells minus red blood cells wont grow unless attached to solid support and normal cell in vitro will stop dividing once a monolayer of cells is formed whereas transformed cells will continue dividing on top of one another
altered morphology: normal cells adopt a fully flattened confirmation in culture whereas transformed cells become more rounded or adopt a spindle morphology which relates to cytoskeleton changes of disorganization and flexibility
loss of contact inhibition: normal cells cease to divide when in contact with other cells on all sides
ability to form tumours in immunocompromised mice which are those that lack T Cells by blocking thymus development which is where T cells develop
How do transformed cells often portray their presence in conjugation with healthy cells
by forming foci where cells pile up into 3-D aggregates
transformed versus healthy cells in culture
normal cells adopt a fully flattened conformation whereas transformed cells become more rounded or adopt a spindle morphology relating to cytoskeleton changes in disregulation
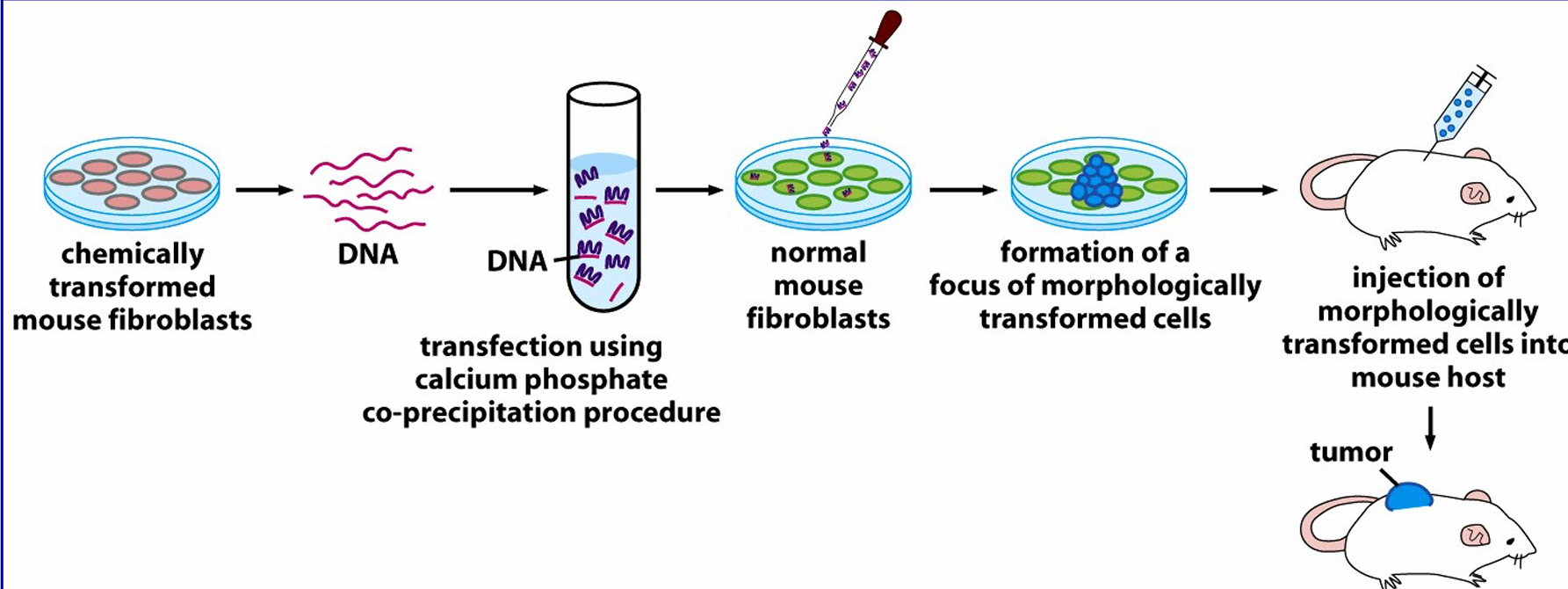
describe the process of transfection
it introduces individual genes into cells
involves formation of a complex between DNA and calcium salt which precipiates the DDNA which when added to cells is taken up via pinocytosis and escapes into the cell cytosol where it can enter the nucleas and become expressed
individual mutations may produce features of transformed cells without full transformation
oncogenes
greek for bulk or mass and generally are genes promoting cell division and in mutant forms act at an advanced rate
driver versus passenger mutations
passenger: most mutations in a tumour are irrelevant
driver= drive cancer development such as Ras and B Raf
without a driver passengers are not going anywhere however without passengers the drive can still get to the destination
how was it determined that cancer was a disease of the genes
Francis Peyton Rous viruses can cause cancer.
Rous investigated a chicken with a malignant tumor. He extracted material from the tumor, filtered it to remove cells, and injected it into healthy chickens. Remarkably, the recipient chickens developed similar tumors, demonstrating that a virus—later named the Rous sarcoma virus (RSV)—was responsible for transmitting cancer
Bishop isolated the RSV gene and termed is Src for sarcomes and quickly realized that the gene was present in genomes of all cells
V-Src is a hijacked hene that become part of the viral genome through selective advantage upon host entry
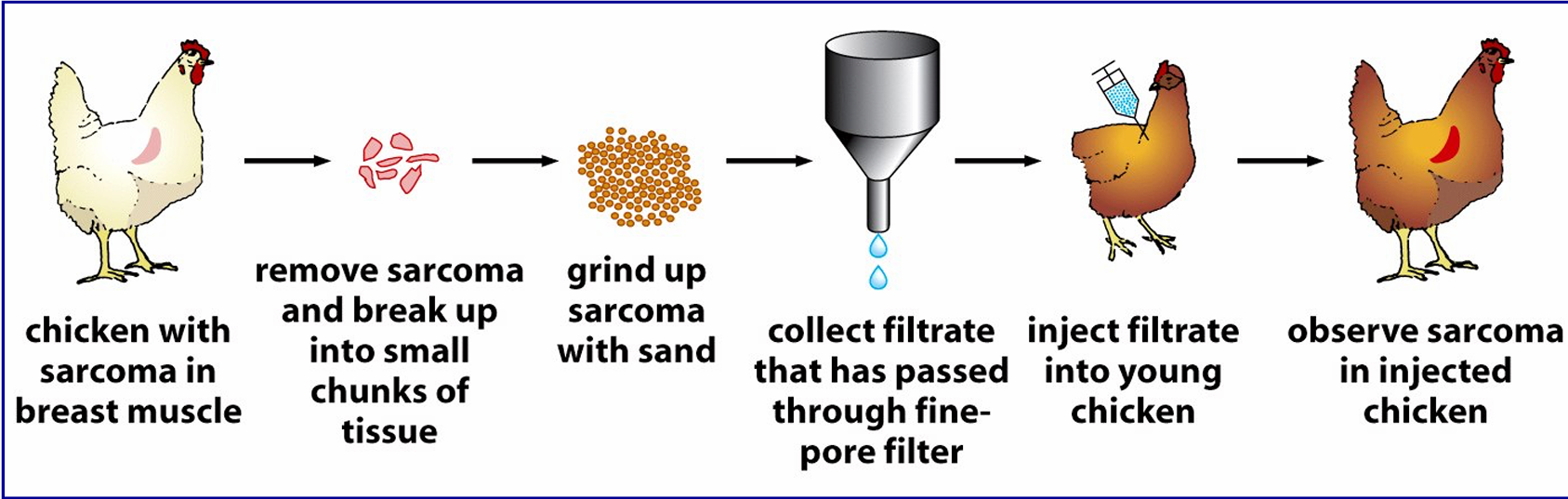
what was the first oncogene discovered
Src
how do growth factors promote cell divison
by turning on signaling cascades switching new genes required for replication
describe epidermal growth factor recpetor(erB) in cancer
it is upregulated in stomach, brain and breast cancer and makes cells hyper responsive to ambient levels of GF that wouldnt normally stimulate cell division
roles of ras and braf
🧬 RAS
RAS (like KRAS, HRAS, NRAS) is a small GTPase.
It acts like an on/off switch for signaling pathways that control cell growth, differentiation, and survival.
When a growth factor binds to a receptor (like EGFR), RAS gets activated (binds GTP) and sends signals downstream.
If RAS is mutated (gain-of-function), it stays "ON" all the time → leading to constant cell division and cancer.
🧬 BRAF
BRAF is a serine/threonine kinase that is part of the MAPK/ERK pathway — it acts downstream of RAS.
When RAS is activated, it activates BRAF, which then activates MEK → ERK → genes that cause cell growth and survival.
BRAF mutations (like the famous V600E mutation) make BRAF constantly active, even without RAS signals, promoting uncontrolled cell growth.
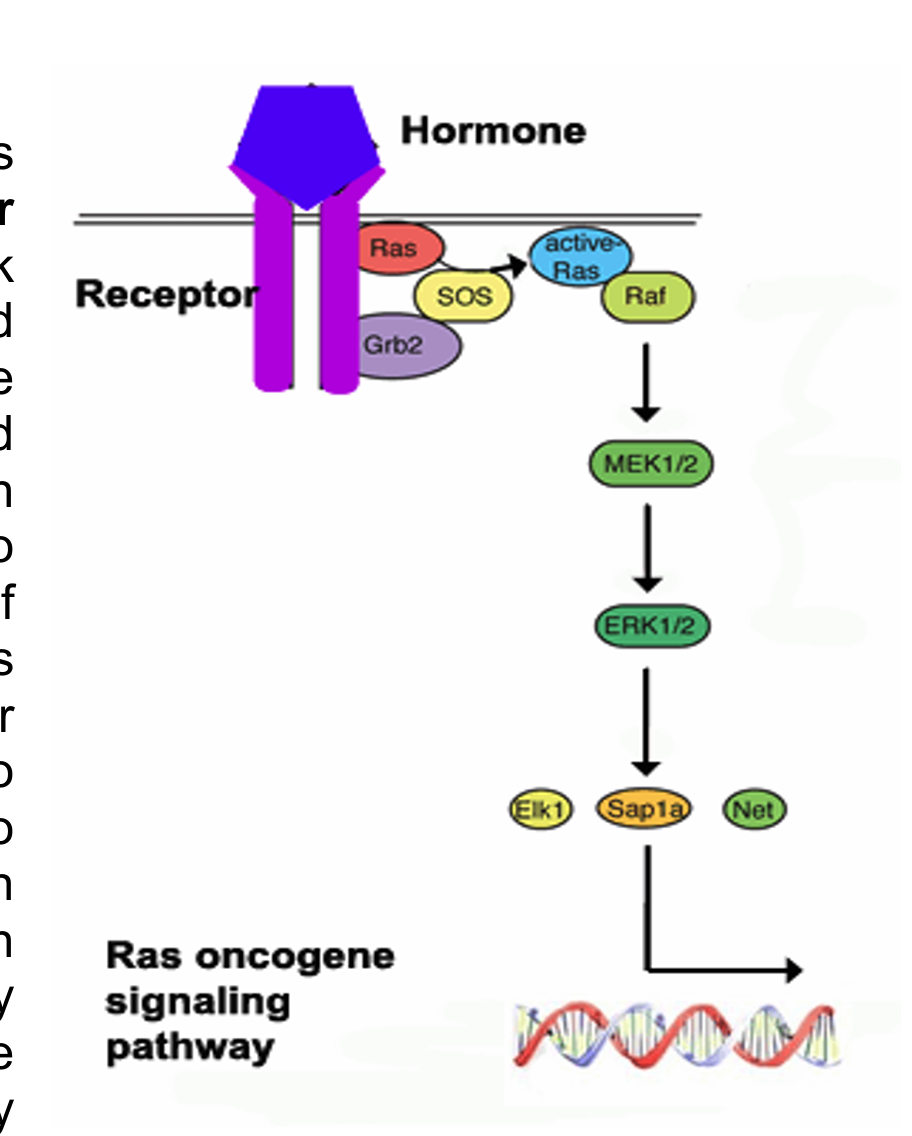
how does ras GTP drive raf activation
it recruits it to the plasma membrane where it can then be phosphorylated by membrane-associated kinase
Ras cannot bind to Raf unless Ras is GTP bound and in binding a GTP molecules it needs help from a protein called GDP/GTP exchange factor that assists Raf in letting go of GDP in exchange for GTP
Sos
function of Sos(son of sevenless)
helps Ras bind GTP; acts as a GDP/GTP exchange factor'
Sos also doesnt live at the plasma membrane so gets there via the growth factor receptor binding protein 2 which has a domain SH3 that Sos bind to
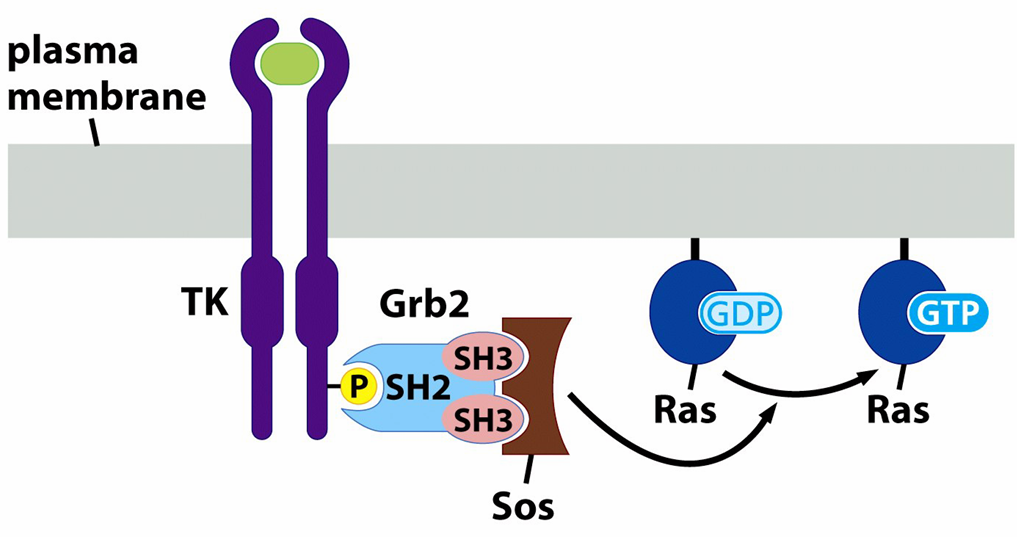
what does ligand induced activation of growth factor receptor cause
it creates binding sites for GRB2 and Sos and induced Ras activation through Sos-mediated stimulation of GTP exchange on Ras where it can then recruit Raf to the membrane where it becomes phosphorylated and further amplifies the signal by activating downstream kinase MEK and ERK
what do Ras mutations cause and what percent of cancers have Ras mutations and where are they found
, 30%, an increase in GTP affinity. they occur at positins 12 and 13 of Ras proteins around the edges of the GTP binding loop(P-loop)
gain of function mutations
what enables mutant Ras to transform cells more efficiently
if p53 is also mutated
what is the most difficult to treat cancer form and how does it arise
malignant melanoma due to chemotherapeutic drug resistance
it arises due to pigment-producing cells(melanocytes) becoming transformed due to chromosomal lesions frequently involving B-Raf kinase
most arise in the exposed areas of the skin due to sunlight
one of the few cancers that incidence does not correlate with age
what is the drug used to treat malignant melanomas
dacarbazine which elicits a response in 15-25% of melanoma patients but complete response are only at 5%
How was Raf discovered
in 1983 as a viral oncogene designated V-Raf(rapidly accelerated fibrosarcoma) that was isolated from 3611-MSV a retrovirus that induces fibrosarcoma in mice
what are Raf family members
serine/threonine kinases that are regulated via extracellular cues and mutations in B-raf kinase are found in about 70% of melanomas
what do Braf mutations predominantly alter
a single AA residue with 80% of mutations found at position 600 of Braf kinase where a valine is altered to a glutamic acid (V600E) producing a 500 times more active kinase than wild type and increases MEK and ERK levels
why are additional mutations on top of Braf required
Braf mutations V600E are found in benign moles and mutations in CDKN2a encoding for ARF and P16 proteins cooperate with Raf to transform cells
name two Raf inhibitors
they represent cancer chemotherapies
sorafenib= C-Raf inhibitor for renal cancer
Zelboraf=inhibits V600E B-Raf
how does p53 stop the cell cycle
via p21 or apoptosis through Noxa, Puma, Bax
how was p53 discovered
normal p53 functions to suppress transformation and contributes to apoptosis and usually as a tetramer and mutant P53 joins the tetramer distrupting its functions
single p53 mutant molecules could disrupt the functions of the tetramer such that only 1/16 of p53 in cell carrying dominant negative p53 allele would be functional
half life of p53
short at about 20 minutes and its stability is controlled through Mdm2 which binds to p53 and targets the protein for degradation via ubiquitin proteasome protein destruction pathway
Mdm2 expression is controlled by p53 which is interesting since it controls the synthesis of its own inhibitor
what is p53
a transcription factor
how is mdm2 degraded
in response to cell damage
in healthy cells Mdm2 has the upper hand to P53 and the half life of p53 is enhanced by DNA damage which results in its degradation being inhibited, allowing for p53 stabilization and activation in stress response.
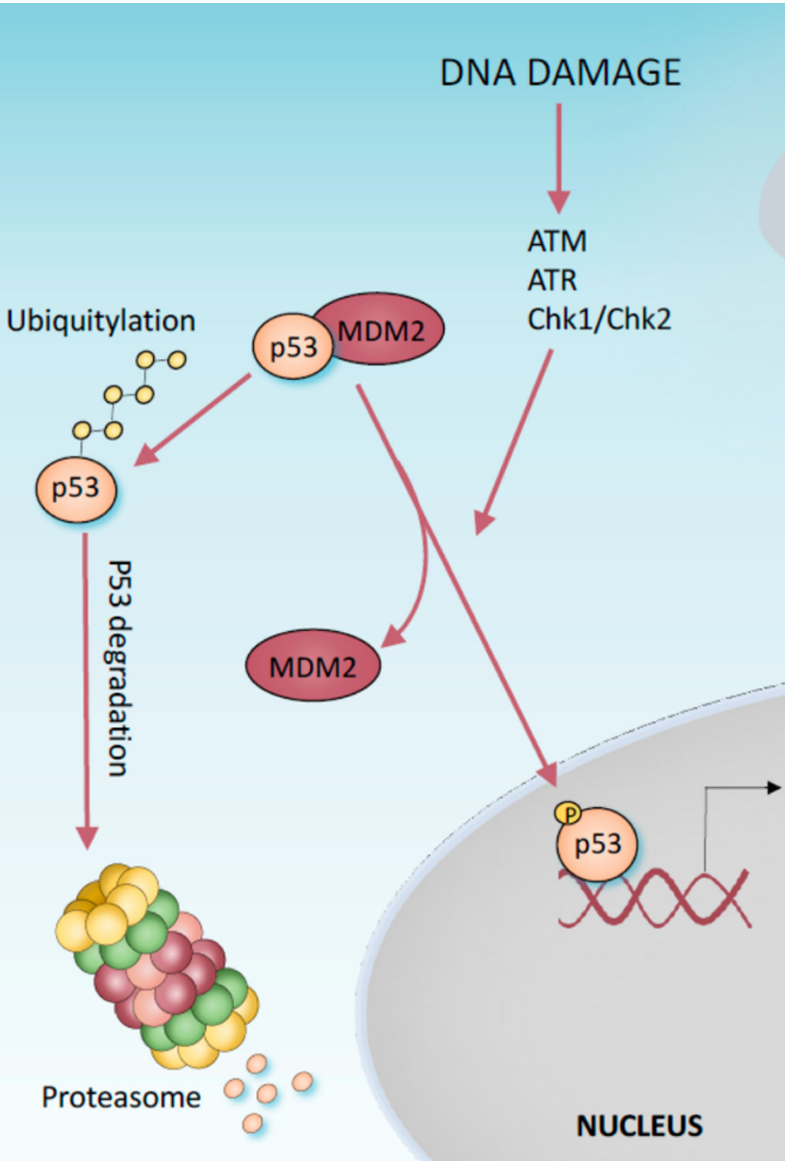
what happens when p53 is stabilized
p21 is rapidly expressed halting the cell cycle by inhibiting certain kinases crucial for cell division like cyclin dependent kinases
different between high versus low levels of DNA damage
high=apoptosis
low=limiting divison
where are P53 mutations most commonly found
in the DNA binding domain and they are dominant interfering by neutralizing protein function of wild type p53 in the same tetramer
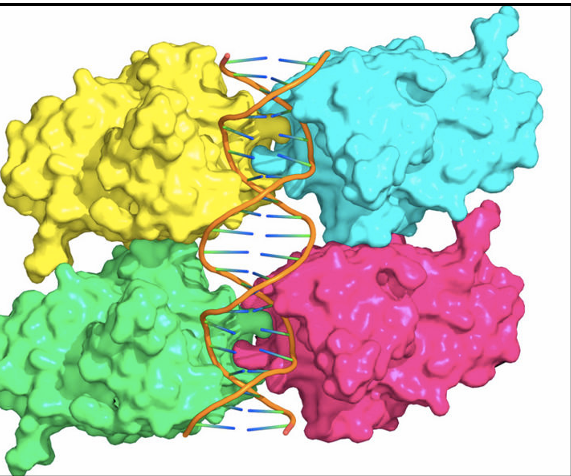
what is a hallmark of cancer
elevated P53
Li Fraumeni Syndrome
inheriting p53 mutation and 60% get cancer by 22
what are actionable cancer genes
KRAS, EGFR, BRCA 1 and 2
what is the result of most mutagenic events
little or no functional consequence but certain mutations can dedispose acquistion of further mutation
what is a candidate oncogene
mutation shared among different tumours
how is cancer mainly diagnosed
through histology
what mutations are more common in people who have already recieved cancer treatment previously
ligan binding domain mutations in the gene encoding the oestrogen receptor(ESR1) in metastatic breast cancer
metastatic
cancer spreading from original location to other locations in the body
microbiome
entire microbial ecosystem within a host including the microorganisms, their genomes, and surrounding environmental conditions
can be acquired vertically, horizontally, and environmentally
germ theory of communicable diseases and its ramifcations
a specific microbial pathogen causes a discrete communicable human disease
1885 Louis Pasteur speculated that life of an animal host is not possible without its resident microbes but commitment to germ theory led to backlash at the idea of positive microbes
how many virus particles per individuals
10^13
what is included in the virome
viruses that can infect human cells, other microorganisms, and bacteriophages
what influences microbiome
diet, breast milk versus formula, medications such as antidepressants, host genetics, geography, and age
significance of gut mycobiome
fungi, small but important
Candida and Aspergillus influence the gut bacterial microbiome
what is the term for the gut microbiota
a microbial organ within a host organ since the metabolic activities resemble those of an organ and it has roles in metabolism and immunity
what microorganism in the gut are responsible for methane production
archae
is fungal abundance on the skin high or low
it is low even on the feet where its diversity is high
what dominates the vaginal microbiota
lactobacillus
offers more protection compared to a diverse microbiota
where is the primary source for the lung microbiome
upper respiratory and it is dominated by staphylococcus
what is the underworlds project
human health cencus by sampling urban gut at multiple locations by sewage sampling
how does the soil contribute to human health and what is the one health concept
both directly and indirectly through the plants and animals we consume
humans are inseparably linked health of other ecosystem compenents
holobiont
a complex and interconnected system of organisms living in close association with each other often involves assemblages of smaller organisms known as the symbiome in a larger host
what does the early life microbiome development do and what is the difference between c section verus vaginal delivery
trains the immune system
C section birth is dominated with staphylococcus and Vaginal delivery is lactobacillus
is there an increase or decrease in microbiome diversity in urban environments
a decrease
how do microbiome studies characterize presence of bacteria in a sample
by sequencing a fragment of 16S ribosomal RNA encoding gene however the resolution is limited
shotgun metagenomics versus shotgun metatranscriptonomics
metagenomics: DNA sequencing cannot distinguish between live or dead bacteria
transcirptongenomics= real time functional programming
problem with donating feacal samples
difficult to safe gaurd privacy of donor due to things such as gender being able to be deduced through gene sequencing
microbiota versus microbiome
living organisms in a defined or specific environment
microbiome encompasses the genomesof those organisms
where are obligate anaerobic bacteria found
they dominate the gut microbiome
bacteriodetes and Clostridia
what role do gut microbiota play
they produce short chain fatty acids by playing a crucial fermentation role with the by products of butyrate and acetate
SCFAs
short chain fatty acids: microbial derived metabolite which has a pyruvate precursor
profile depends on the fibre fermentation of different bacteria and the bacterial metablism of amino acids derived from protein
used as an energy source by colonocytes which supports the maintenance and growth of intestinal epithelial cells
can improve integrity, glucose and lipid metabolism, and the immune response
what is promoted by a high fibre diet
lower gut Ph
virome
all bacteriophage, other viruses and endogenous viral elements that have integarted into the host genome
is characterizing the human virome easy or hard
difficult due to absence of conserved gene region such as bacterial 16S and an incomplete viral genome library
Dysbiosis
imbalance of microbial community composition generally between beneficial such as lactobacillus and harmful Ecoli
‘Factors influencing dysbiosis include loss of symbionts, outgrowth of pathobionts and opportunistic bacteria
overall disruption of ecological links
does dysbiosis cause disease
a tricky discussion topic because it is hard to determine whether or not it is the result or cause of disease and purely observational data is not helpful if action is implied
what is loss of gut microbial diversity linked to
obesity and autoimmine conidtions generally due to antibiotic use, sterile water, processed foods, C section
BLOSSUM versus VANISH
blossum taxa are those that are selected for in urbanized societies and VANISH are volatile in industrialized societies taxa
what do traditional populations have more of versus ubranized
carbohydrate active enzymes
Cazymes
IBD
including Crohns and ulcerative colitis is a chronic inflammation of the intestine
decrease in SCFA producing organisms such as Clostridium spp
describe the colonic epithelium
hypotoxic, but intestinal inflammatin or antibiotic use can increase epithelial oxygenation and distrupt anaerobisis leading the dysbiotic expression of proteobacteria
gut microbiota transplantation in mice
implies a causal relationship between gut microbiome and obesity development since gut derived SCFA interventions combat obesity and metabolic disorders