IB DP Physics: Unit -E
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
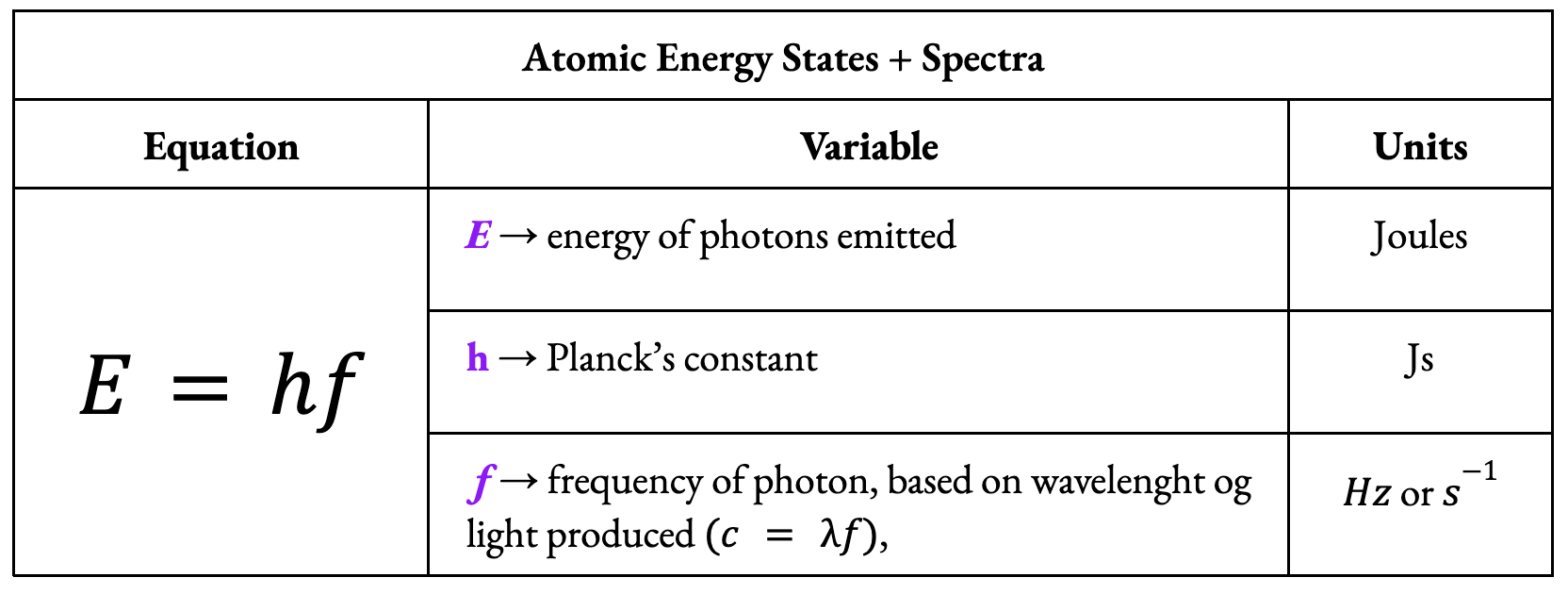
Energy of a photon: E = hf
Light energy is carried by a particle called a photon.
A photon with exact energy is absorbed, causing the electron to jump to a higher energy level in hydrogen.
When the atom de-excites, the electron jumps back down to a lower energy level, emitting a photon of energy equal to energetic difference of the
energy levels.
* When finding f, be sure E is in Joules, not eV.
Explain what is meant by the term absorption spectrum.
Absorption spectra are observed when white light passes through a cool, low-pressure gas.
• The cool gas absorbs specific wavelengths of light (its signature wavelengths) from the continuous spectrum.
• Black lines appear in the spectrum precisely where these wavelengths were absorbed by the gas.
An absorption spectrum occurs when electrons absorb energy and jump to higher energy levels. Arrows go up!!

What are the nucleons?
Proton (Z): a positively charged particle that adds mass to an atom.
Neutron (N): neutral particle that adds mass to an atom.
The total number of protons and neutrons is the nucleon number (A):
A = Z + N
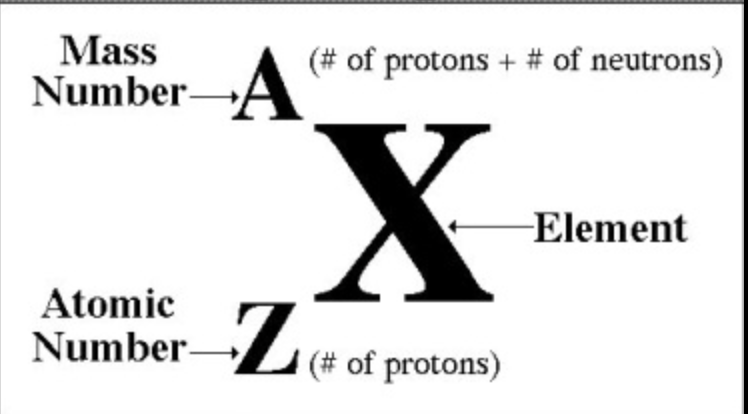
What is an Isotope?
Two or more atoms that have the same proton number but different nucleon numbers (as they have differing number of neutrons)
Identical Chemical properties but different physical properties.
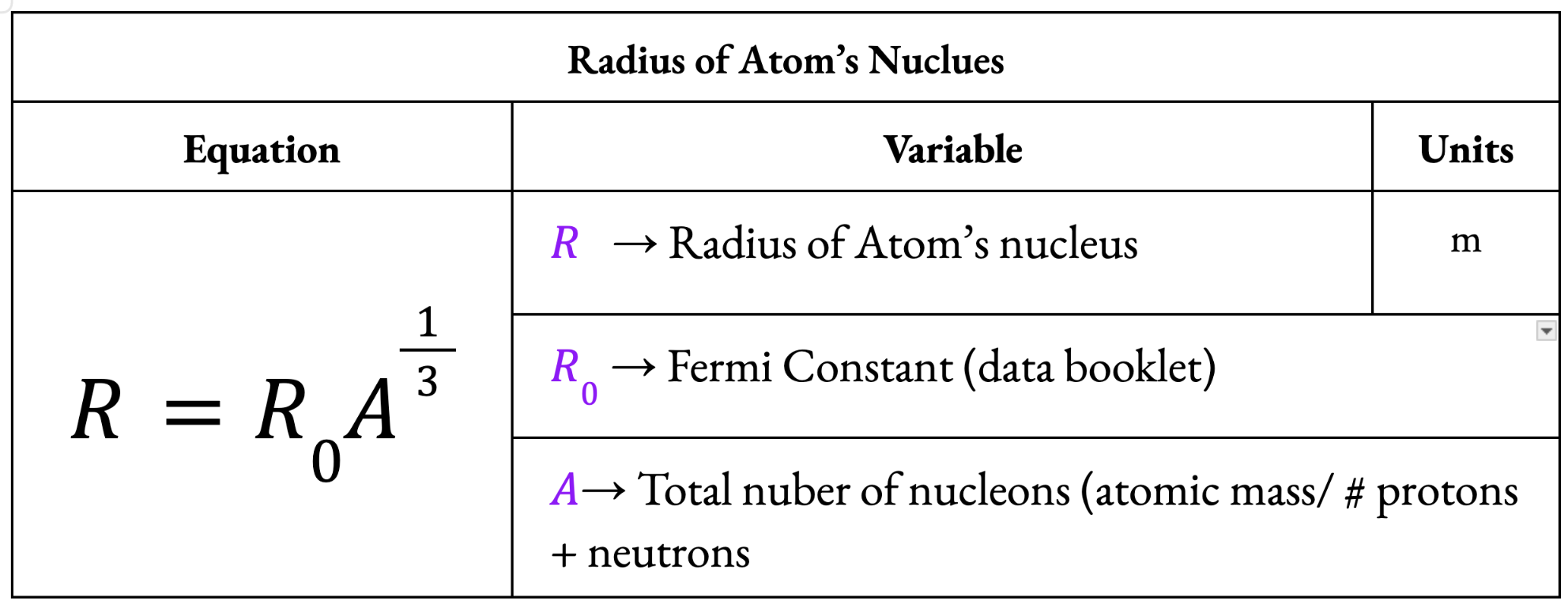
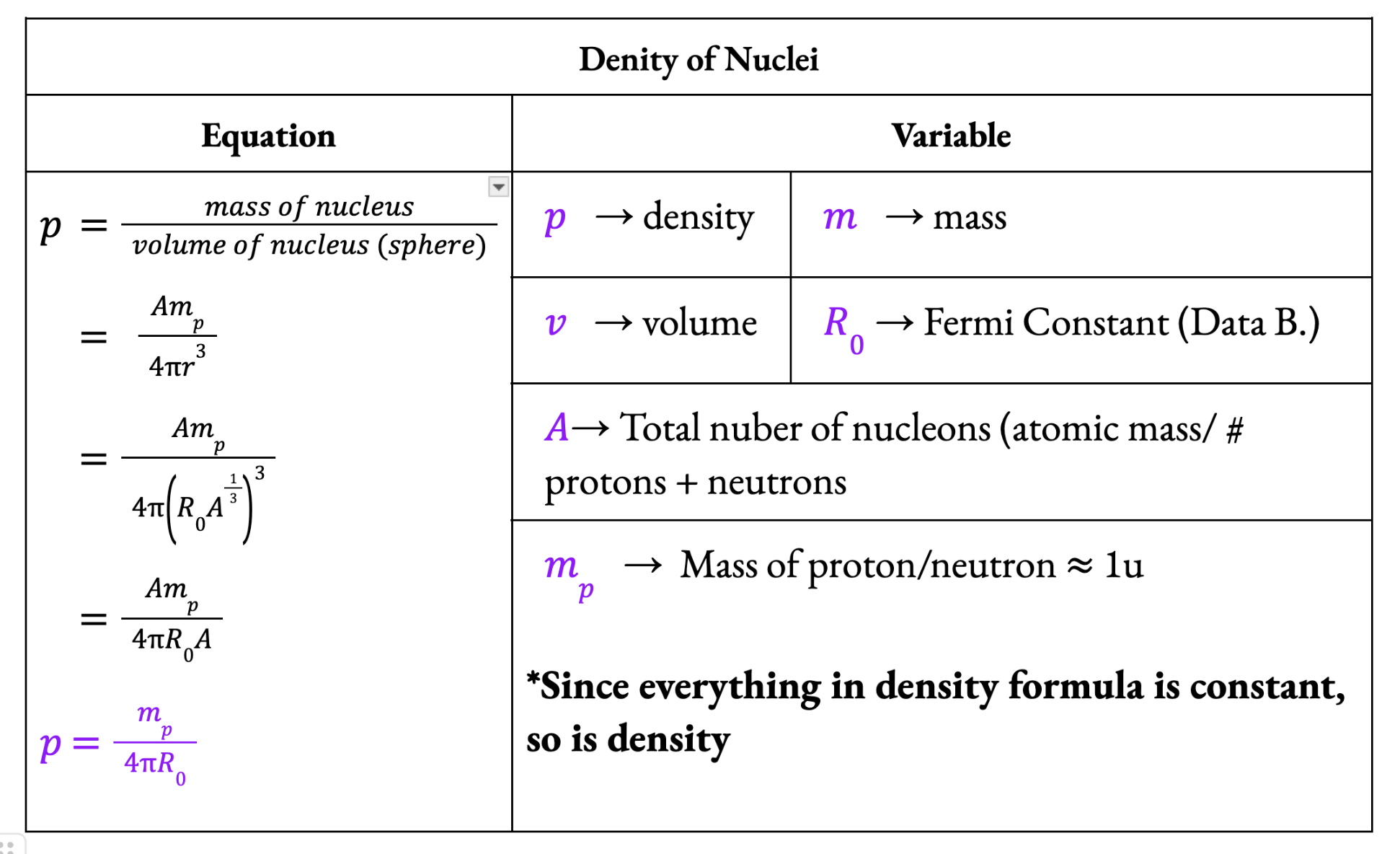
Fermi Radius (R0) = 1.20 × 10-15 (in data booklet)
The radius of a hydrogen nucleus, which contains only one proton
The Strong Force?
In a nucleus, due to the many positively charged protons, the Coulomb repulsion within the nucleus is enormous. To keep nuclei from spewing out protons, within the confines of the nuclei, the strong force overcomes the Coulomb Force.
The Strong Force:
Counters the Coulomb force to prevent nuclear decay and therefore must be very strong.
Is very short-range, since protons located far enough apart do, indeed, repel.
Thus, the strong nuclear force is an attractive force that acts between nucleons, holding the nucleus together.
1 u (unified atomic mass unit)
The standard unit of mass used to express atomic and molecular weights, defined as one twelfth of the mass of a carbon-12 atom.
What is meant by emission spectrum?
Spectrum of electromagnetic radiation emitted by a source, created as electrons move from higher to lower energy states, releasing photons at characteristic wavelengths.
Photons have energy equal to the energy lost by the electron as it transfers energy levels.
It appears as a discrete series of bright, colored lines on a dark background. Arrows go down!!
Geiger–Marsden–Rutherford Experiment
The Geiger-Marsden-Rutherford experiment involved firing alpha particles at thin gold foil.
Results:
Most alpha particles passed through the foil without deflection, or with small angles
Few were deflected at large angles, and a few even bounced backward.
This could only occur if a positively charged component existed within the atom to repel the alpha particles.
These surprising results led Rutherford to propose that:
Atoms contain a small, dense, positively charged nucleus at their center.
Electrons orbit this nucleus in mostly empty space.
Radius of an Atom’s Nucleus
Density of nuclei
All nuclei, although they have different radius, have the SAME DENSITY
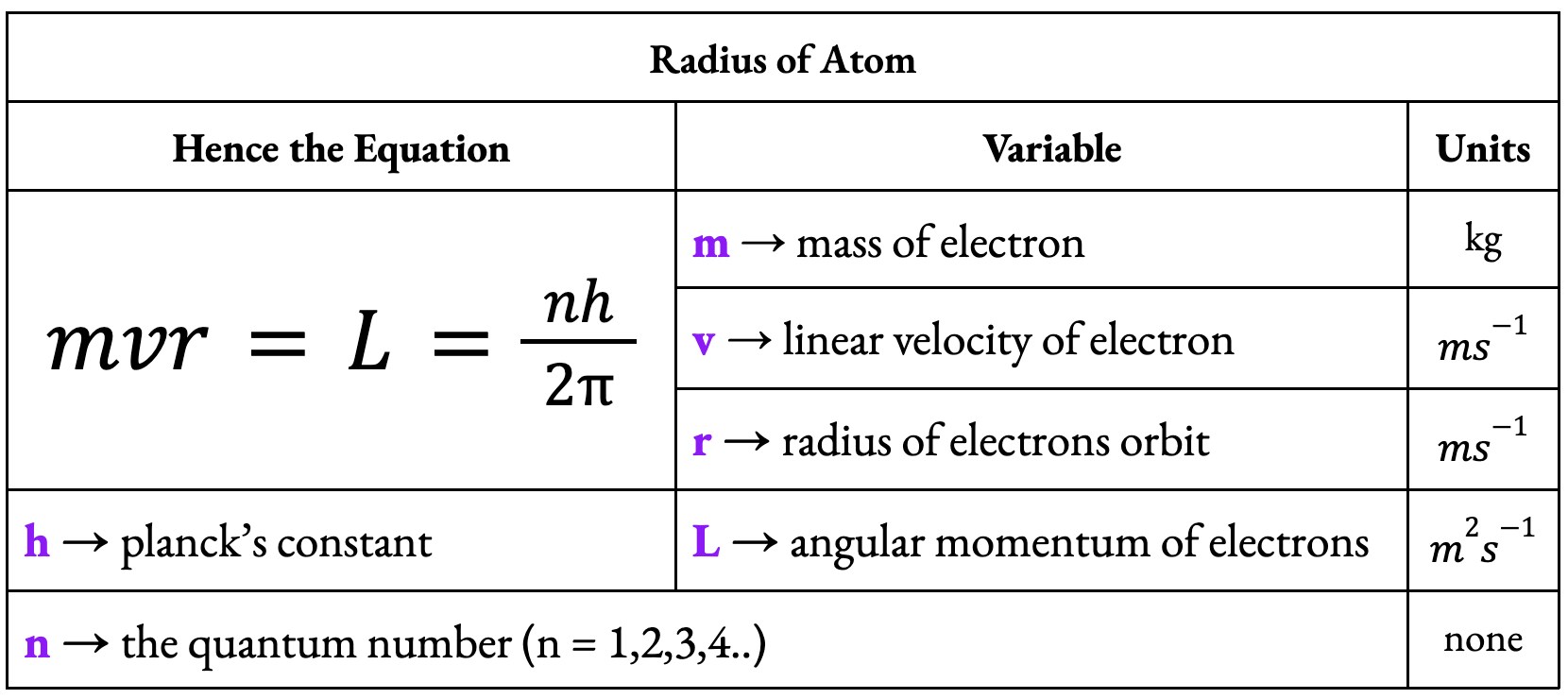
Bohr Model of the Hydrogen Atom (Idea #1)
Remember:
He thought that electrons orbited protons in Uniform Circular motion such that: Electric Force of electrons = Centripetal Force (net resultant force)
He thought that the angular momentum of this motion could be quantized
Angular momentum could only be an integral number of the basic quantity h/2pi
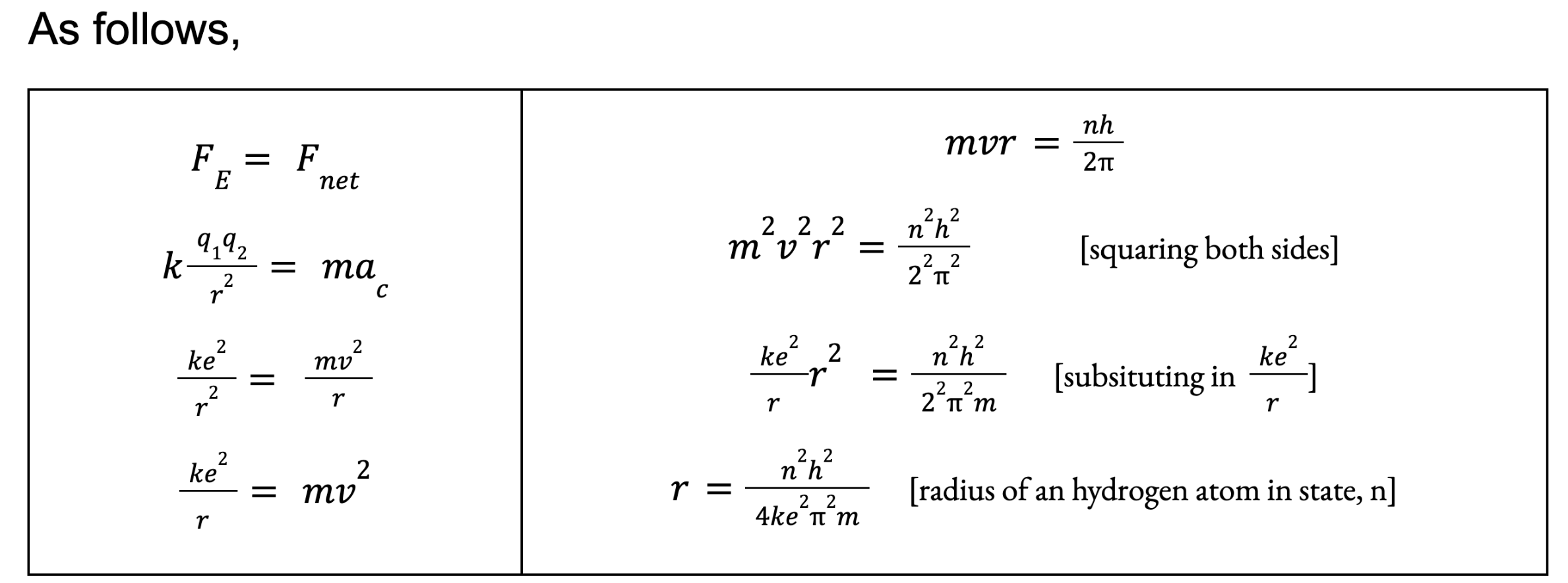
Bohr Model of the Hydrogen Atom (Idea #2)
Since he said Electric Force of electrons = Centripetal Force (net resultant force)
We could also derive a formula for the atomic radius of a hydrogen atom in a certain state, since the radius of the electron orbit determines this.
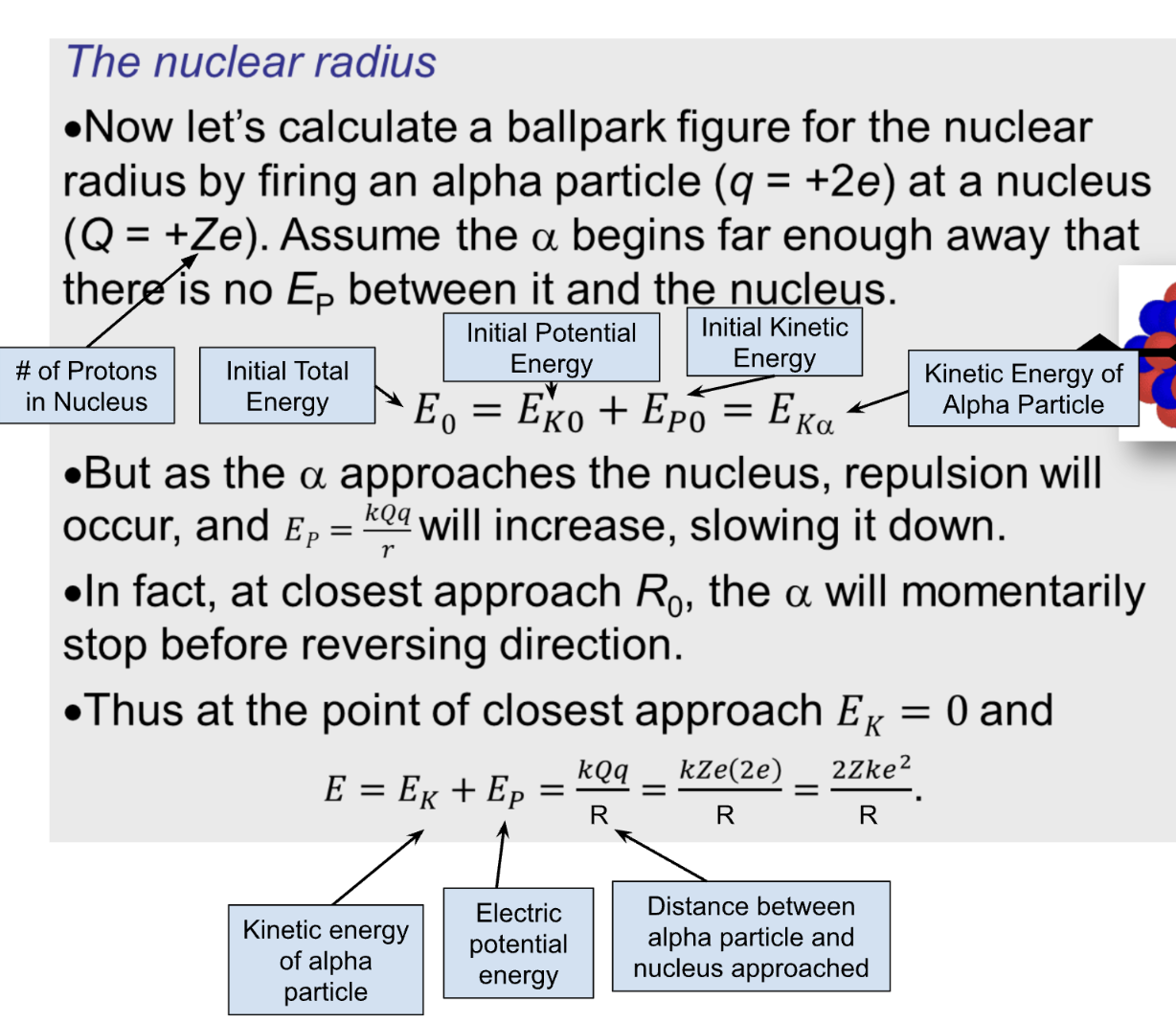
Method of Closest Approach
The predictions of Rutherford’s experiment are NOT true for alpha particles of intial energy greater than 28MeV
initial kinetic energy is inversely proportional to closest approach distance
Energy Value of Hydrogen Energy levels (Bohr’s Model)
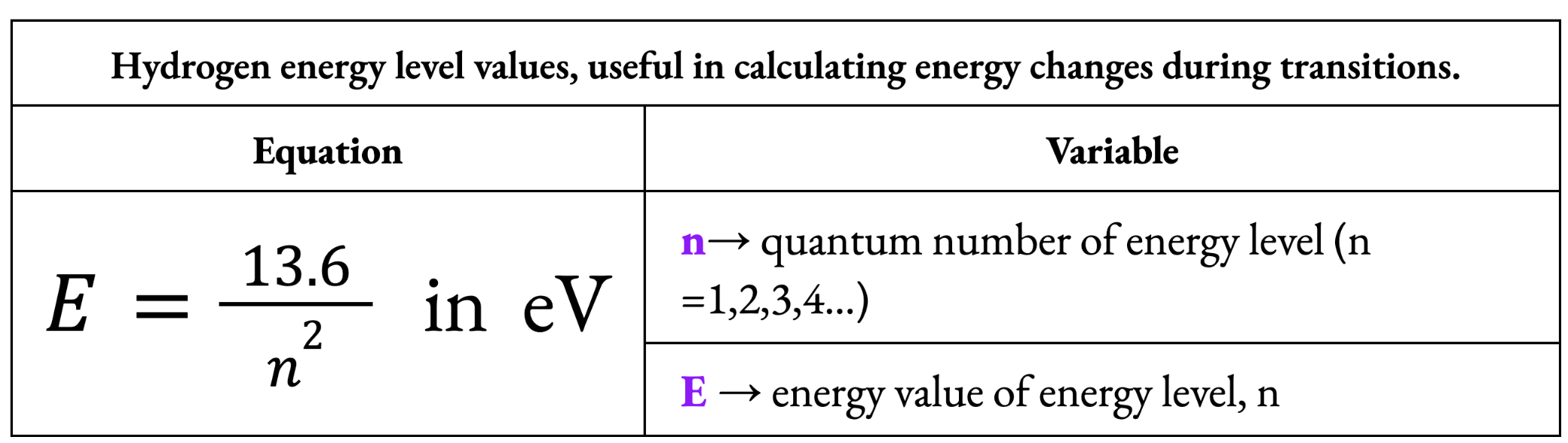
Photoelectric Effect
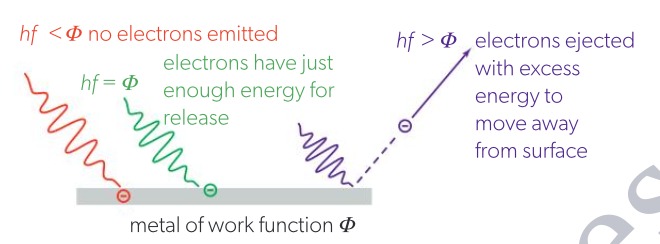
The ejection of electrons from a metal when LIGHT (any form of electromagnetic radiation) is incident on its surface, as long as the light is equal to or greater than the work function. Note:
Each photon reacts with only one electron (these electrons are on the metal lattice’s surface)
If the energy of light (a.k.a photon) is just equal to the work function, electrons are only brought to the metal surface as they do not have the kinetic energy to leave the surface.
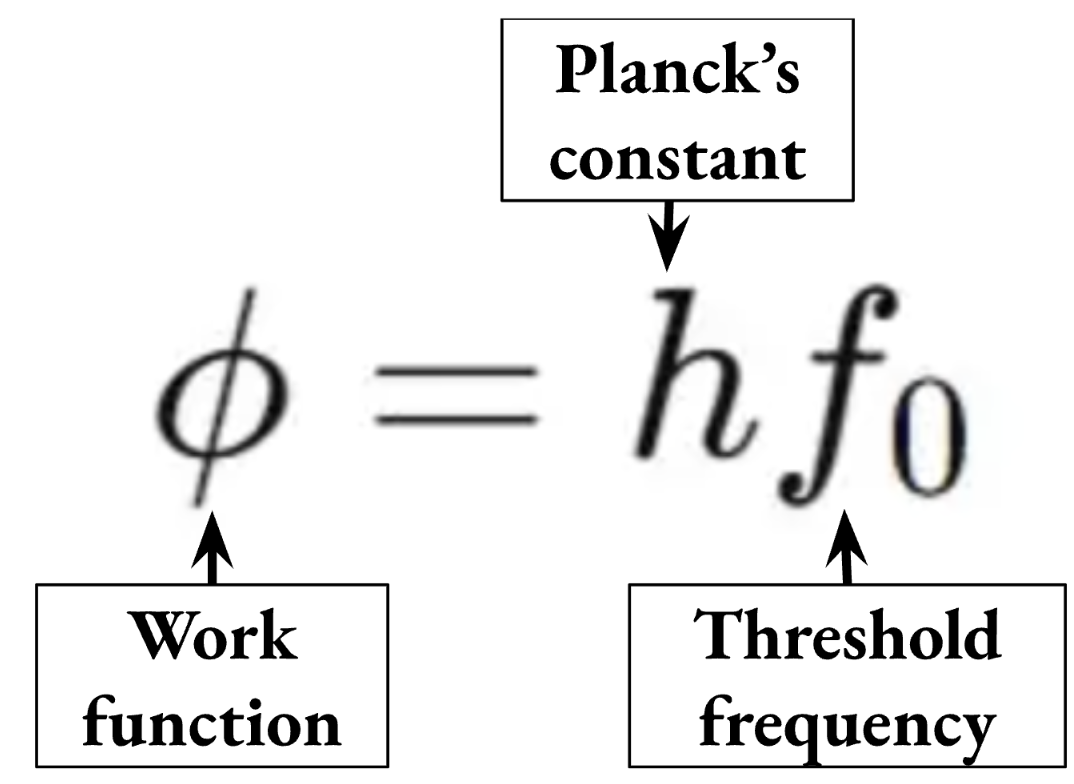
Threshold Frequency (photoelectric effect)
The minimum frequency of light required to emit electrons from metal.
No matter the light’s intensity, if the threshold frequency is not met, NO electrons will be emitted.
Intensity determines ONLY the amount of photoelectrons emitted.
The threshold frequency is specific to each type of metal.
Work Function
The minimum energy required to liberate electrons from a metal’s surface.
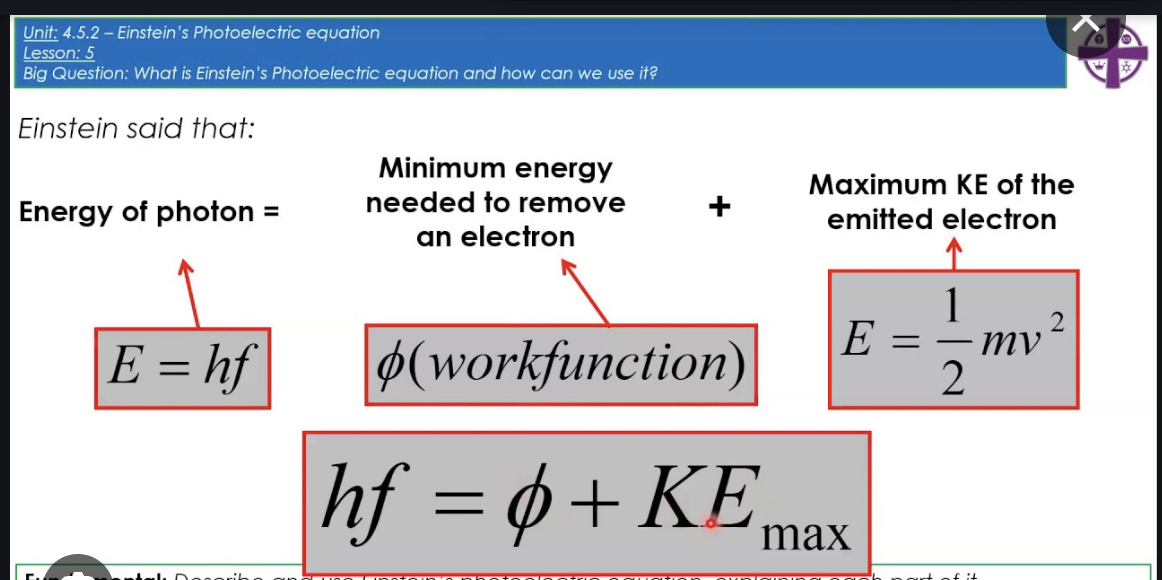
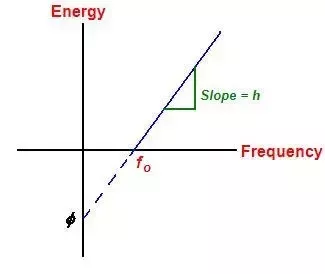
How do I know the maximum kinetic energy of an electron emitted due to the photoelectric effect?
From the image we can see that:
Emax = energy of photon - work function
= hf - (\phi)
Here, f is the frequency of light incident on the metal
Graph of electron’s kinetic energy vs. frequency!
Stopping Potential!
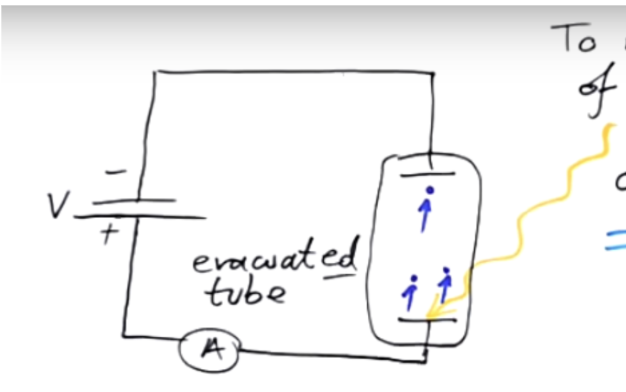
Stopping potential is the smallest voltage (applied in the opposite direction to photoelectron flow) that stops even the fastest electrons — the ones with the most kinetic energy — from reaching the anode in a photoelectric experiment.
It is the potential difference between the cathode and anode plates in the setup.
In the diagram, the battery terminals are reversed — the cathode of the battery connects to the anode plate, making it negative.
This reverse voltage repels the emitted electrons, pulling them back toward the cathode plate.
When the stopping potential is high enough, it exactly cancels out the electrons’ kinetic energy — so no current flows.
Since this voltage stops the fastest electrons, the work done by the field equals their maximum kinetic energy:
W = qVe = Ekmax
W = eVs = Ekmax = hf - work fucntion
Where ,Vs is stopping potential.
Stopping potential depends ONLY ON THE FREQUENCY OF LIGHT.
The de Broglie Wavelength
The definition of a de Broglie wavelength is:
The wavelength associated with a moving particle
De Broglie suggested:
electrons travel through space as waves, and therefore exhibit wavelike properties, such as wavelength and diffraction
All particles—not just electrons—can display wave-like behavior. Any moving particle has an associated matter wave.
Wave-particle duality
The phenomenon where light can behave as both a particle (i.e. photons) and a wave
Particle-like behavior: This is demonstrated by the photoelectric effect and Compton scattering as Light interacts with matter, such as electrons, in a particle-like manner.
Wave-like behavior: Light also propagates through space as a wave evident in its diffraction and interference patterns observed in Young’s Double-Slit Experiment.
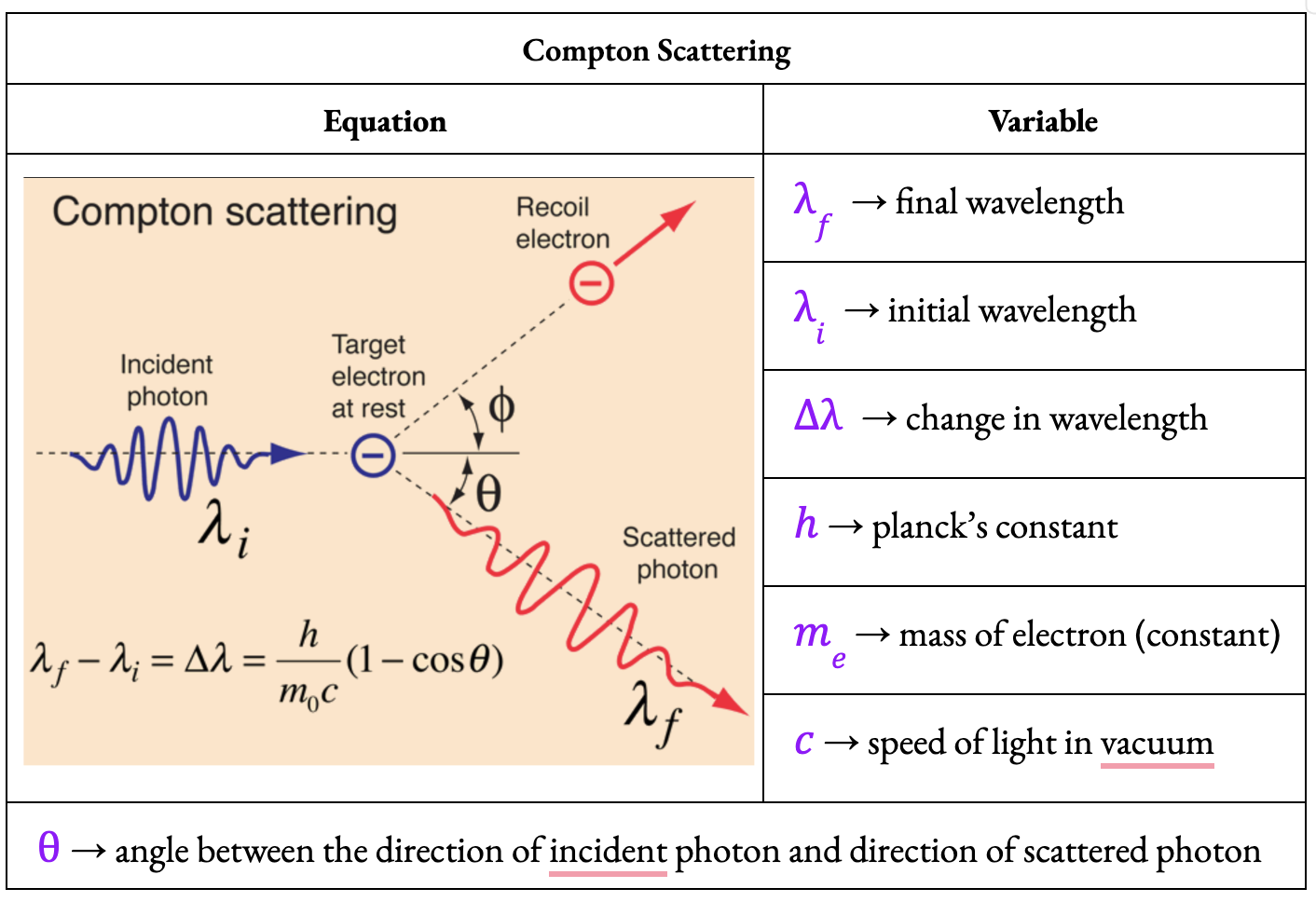
Compton Scattering
The Compton Effect is defined as:
The interaction of a high-energy photon with an orbital electron which causes an increase in the wavelength of the photon and the ejection of the electron
During the collision, the photon transfers some of its energy to the orbital electron, resulting in an inelastic collision. As a result:
The photon is deflected from its initial path
The photon's wavelength increases as its energy decreases
The electron involved is ejected from the atom
The electron and photon are deflected in different directions due to conservation of momentum.
Nuclide
A nuclide is a specific type of nucleus with a set number of protons and neutrons.
Isotopes: Two nuclides with same # of protons, different # of neutrons.
Isotones: Two nuclides with same # of neutrons, different # of protons.
Define radioactive decay.
Radioactive decay is the random and spontaneous disintegration of an unstable nucleus by emitting alpha, beta, or gamma radiation.
How is radioactive decay a random process?
Radioactive decay is a random process because the exact time a nucleus will decay cannot be predicted.
How is radioactive decay a spontaneous process?
Radioactive decay is a spontaneous process because the rate of decay is unaffected by external factors (such as temperature, pressure or chemical conditions).
Define background radiation
Background radiation is the ionising radiation that exists around us all the time.
Natural sources of background radiation include:
radon gas
cosmic rays from space
radioactive elements in rocks and soil
carbon-14 in living tissue
some food and drink
Artificial sources of background radiation include:
nuclear medicine
nuclear waste
nuclear fallout
nuclear accidents
Why do some nuclei emit radiation
Nuclei emit radiation when they are unstable due to having
an imbalance of protons or neutrons
an excess amount of energy
What effect does alpha decay have on a nucleus?
An alpha particle is a particle consisting of two protons and two neutrons; is identical to a helium nucleus:
When a nucleus emits an alpha particle, the effect on the nucleus is that:
Nucleon number decreases by 4
Proton number decreases by 2
Kinetic energy is transferred from the parent nucleus to the alpha particle and daughter nucleus
This occurs in massive nuclei that have a large proton-to-neutron ratio (too many protons).

What effect does beta-minus decay have on a nucleus?
A beta-minus particle is a high-energy electron emitted from the nucleus!
When a nucleus emits a beta-minus particle, the effect on the nucleus is that the:
nucleon number stays the same
proton number increases by 1
There is also the emission of an antineutrino.
This decay occurs when, an unstable nucleus has too many neutrons compared to protons.
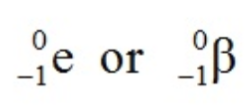
What effect does beta-plus decay have on a nucleus?
A beta-plus particle is a positron!
When a nucleus emits a beta-plus particle, the effect is:
nucleon number stays the same
proton number decreases by 1
There is also the emission of a neutrino.
This decay occurs when, an unstable nucleus has too many protons compared to neutrons
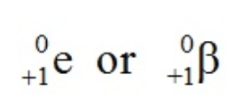
What effect does gamma decay have on a nucleus?
Gamma radiation is electromagnetic: (gamma with proton number 0, and nucleon number 0)
When a nucleus emits gamma radiation, the effect is:
nucleon number stays the same
proton number stays the same
The energy of the nucleus decreases.
This occurs when the nucleus with large amount of energy changes from an excited state to a lower energy level.

List the three types of nuclear radiation in order of increasing ionising power.
The types of nuclear radiation in order of increasing ionising power are:
gamma (least ionising, creates fewest ion pairs per cm)
beta (creates a moderate amount of ion pairs per cm)
alpha (most ionising, creates most ion pairs per cm)
List the three types of nuclear radiation in order of increasing penetrating power.
The types of nuclear radiation in order of increasing penetrating power are:
alpha (least penetrating, can be stopped by paper)
beta (can stopped by a few mm of aluminium)
gamma (most penetrating, can be reduced by a few mm of lead)
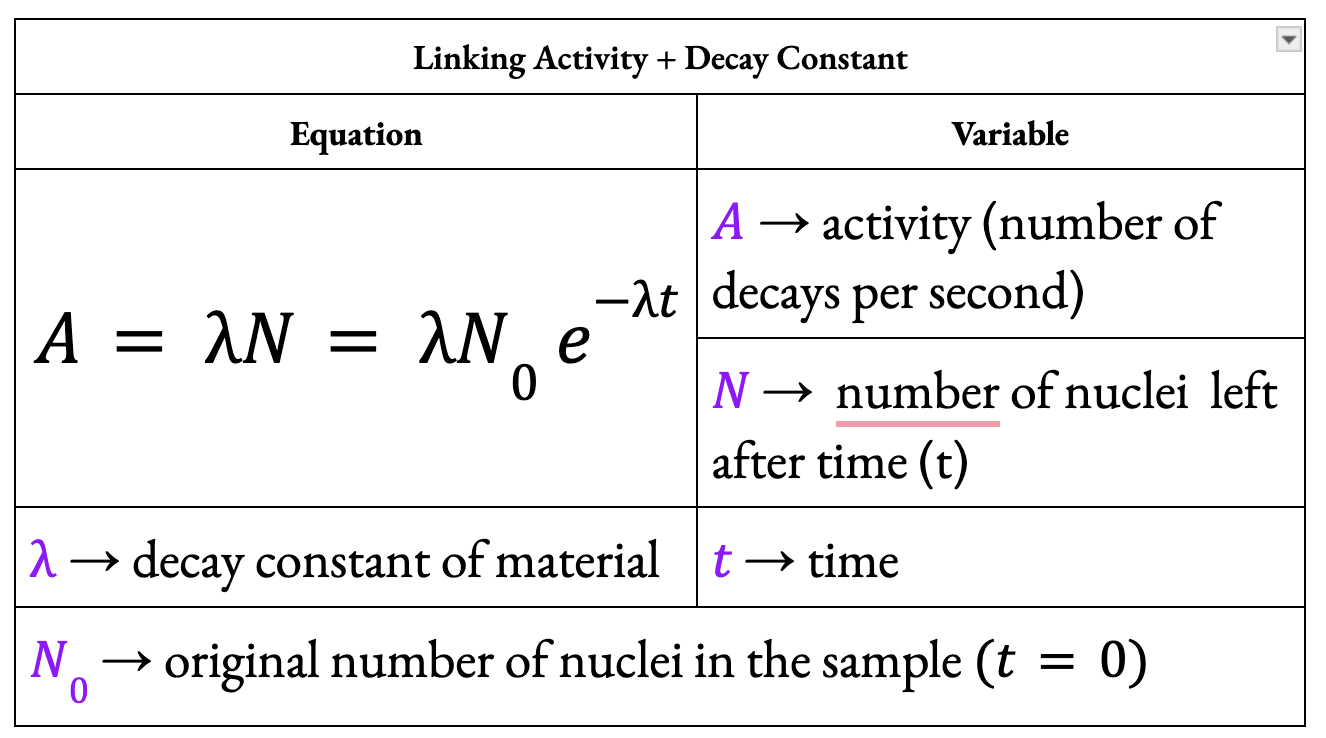
What is the activity of a radioactive source?
Activity is the number of nuclei which decay in a given time. It is measured in becquerels — One becquerel (Bq) is equivalent to a nucleus decaying every second.
What is the difference between activity and count rate?
Activity is the rate at which radiation is emitted, whereas count rate is the rate at which radiation is detected.
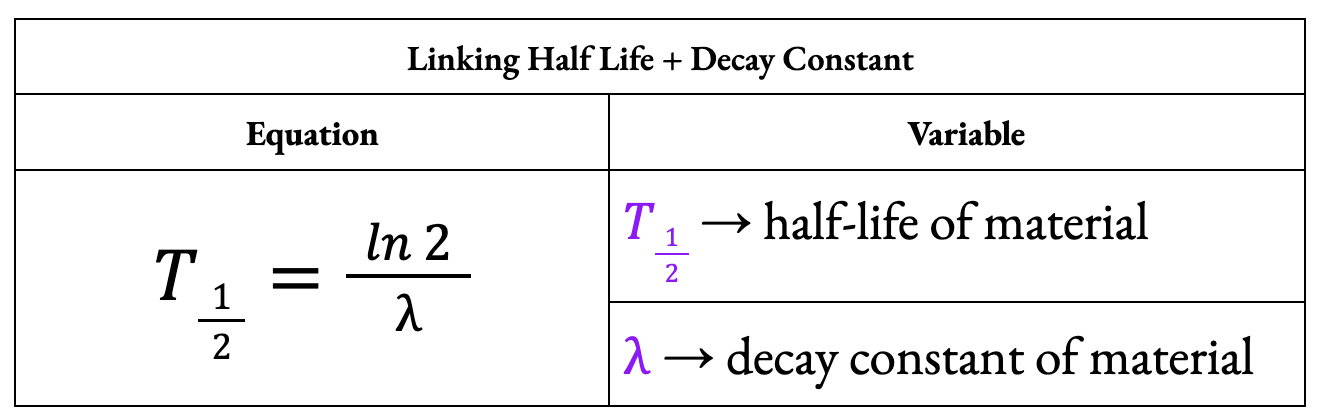
Define the term half-life.
Half-life is the time taken for half the undecayed nuclei of a particular isotope in any sample to decay.
It is also the time taken for the activity of a source to fall to half its original value.
Define the term decay constant.
The decay constant is the probability that an individual nucleus will decay per unit of time.
What is the equation linking activity and decay constant?
What is the equation linking half-life and decay constant?
Why does the activity of a sample decrease over time?
The activity of a sample decreases over time because:
unstable nuclei emit radiation to become more stable
after each decay, the number of unstable nuclei remaining decreases
activity is directly proportional to the number of nuclei, so activity decreases
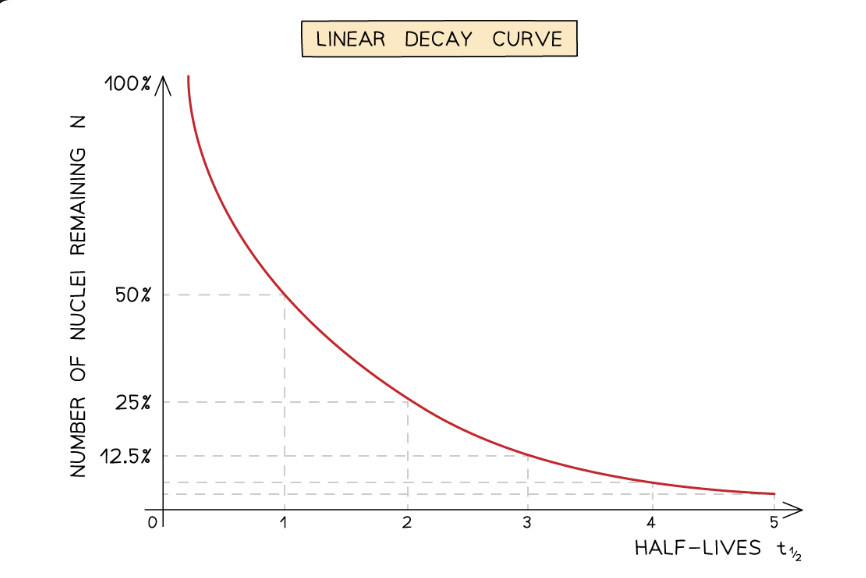
What relationship is represented by the linear decay curve?
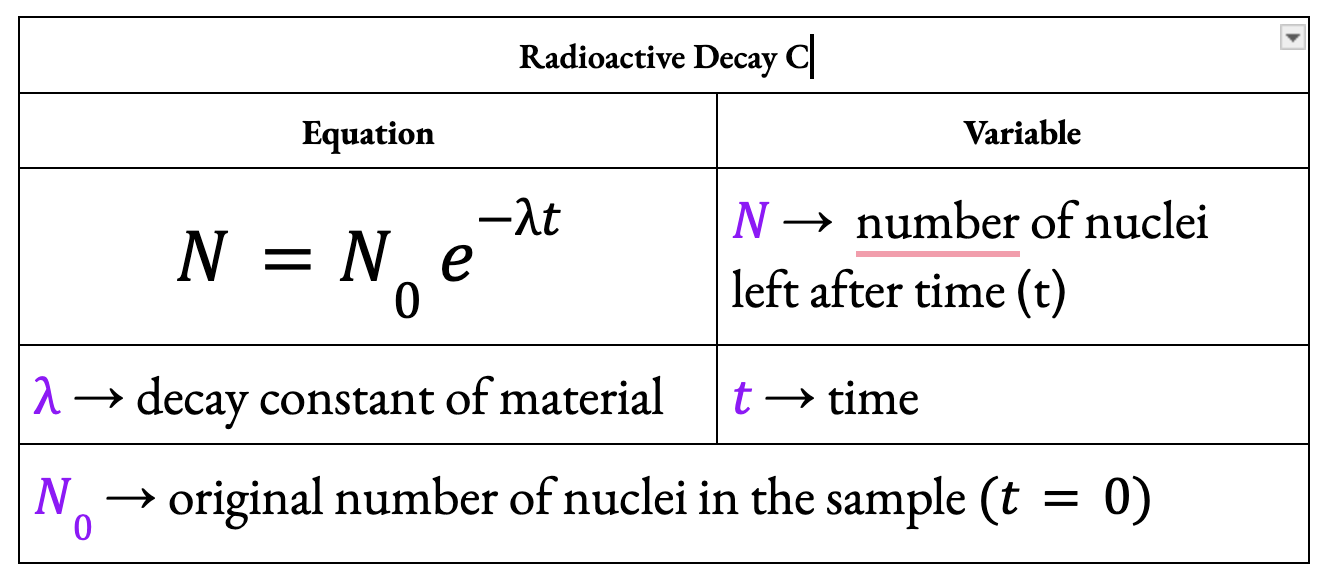
What is the law of radioactive decay?
The law of radioactive decay states that the rate of decay is proportional to the number of undecayed nuclei.
Define mass defect.
Mass defect is the difference between the mass of a nucleus and the total mass of all the constituent nucleons if they were separated.
Mass defect is caused by the energy released when a nucleus forms, or the energy needed to separate all the nucleons in a nucleus
True or False: Mass defect and binding energy are equivalent.
True
Define nuclear binding energy.
Nuclear binding energy is the energy required to separate a nucleus into its constituent nucleons.
What equation is used to convert mass to its equivalent energy?
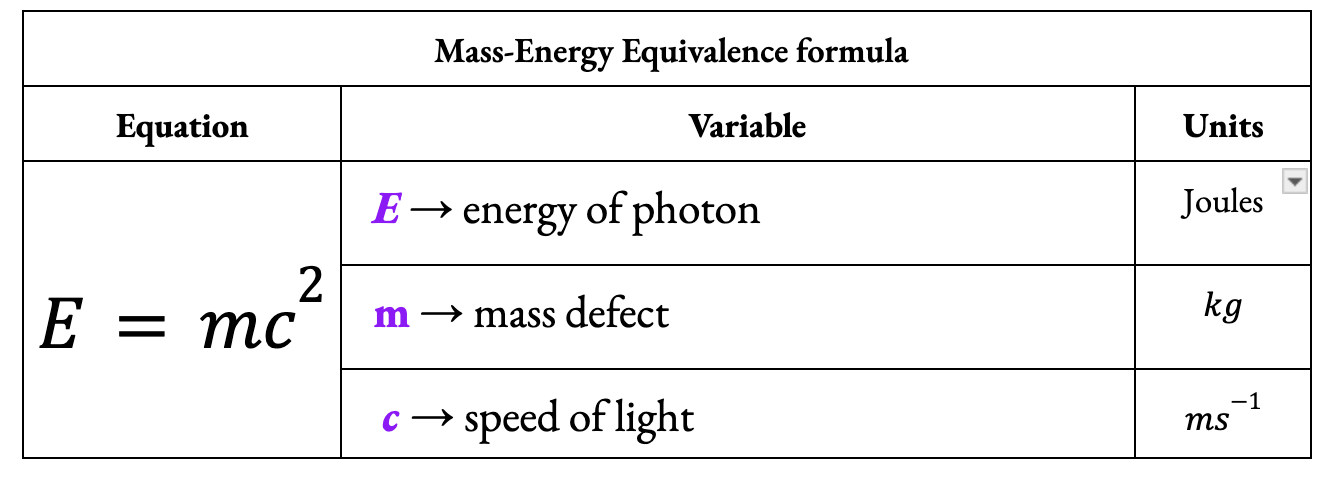
Define binding energy per nucleon.
Binding energy per nucleon is the binding energy of a nucleus divided by the number of nucleons.
Sketch the curve of binding energy per nucleon against nucleon number.
Include the regions of fusion, fission and the position of iron-56.
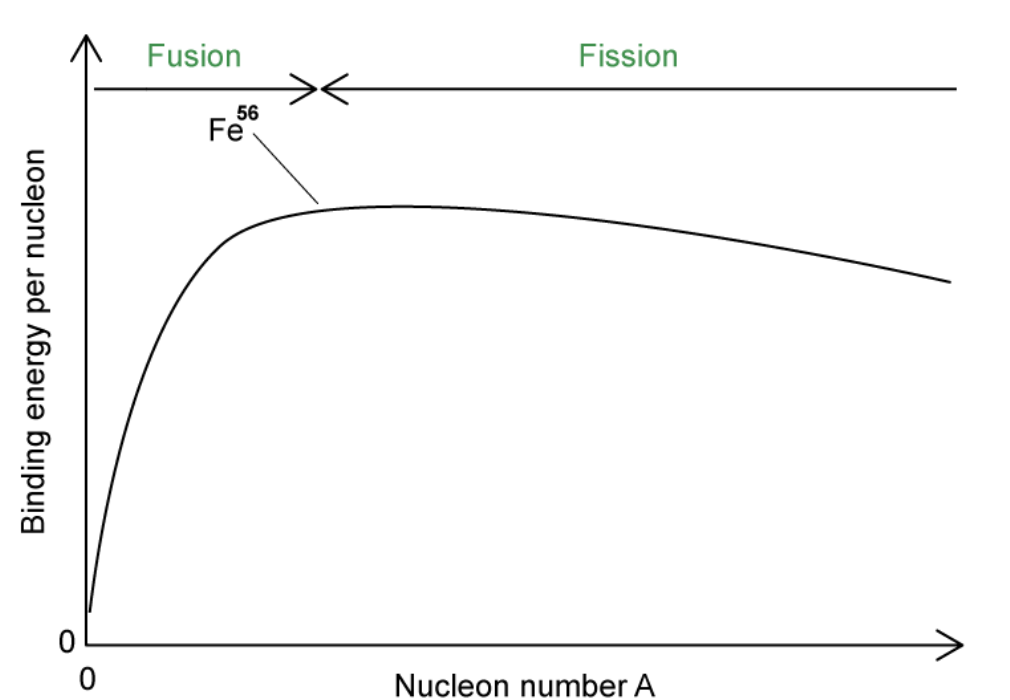
Why do heavy nuclei undergo fission?
Heavy nuclei undergo fission because they have high binding energies per nucleon, but this gradually decreases with A, making the heaviest elements the most unstable.
What makes a nucleus unstable?
too many neutrons
too many protons
too many nucleons (protons and neutrons)
too much energy
Why do heavy isotopes need more neutrons than protons to be stable?
Heavy isotopes need more neutrons than protons to be stable to add distance between protons to reduce the effects of electrostatic repulsion.
How are nuclear energy levels similar to electron energy levels?
Nuclear energy levels are similar to electron energy levels in that nuclei can exist in excited states and emit energy as photons when transitioning to lower states.
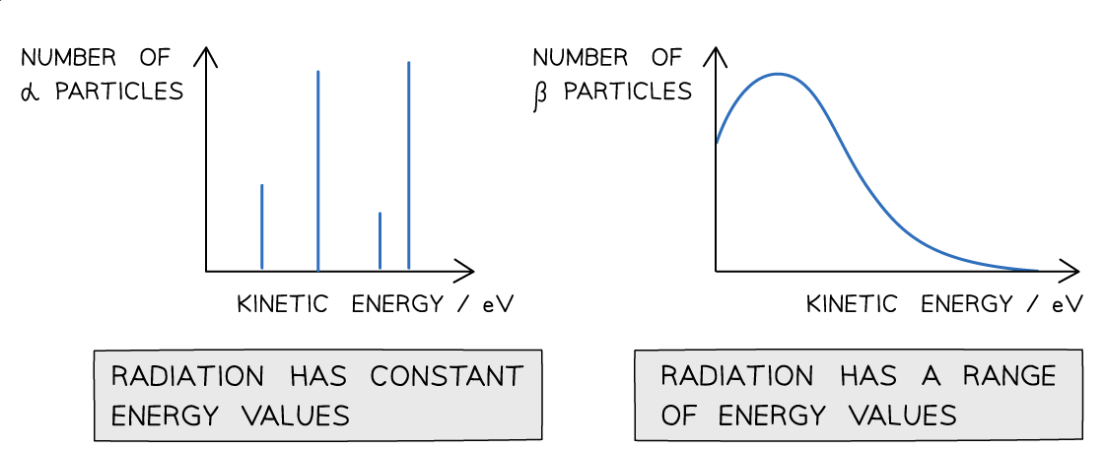
How do alpha and beta emissions differ in terms of energy distribution?
Alpha particles are emitted with discrete energies, while beta particles have a continuous range of energies.
Define nuclear fission.
Nuclear fission is the splitting of a large nucleus into two smaller nuclei. It can be:
Spontaneous i.e.it occurs randomly and without additional energy being put into the nucleus.
Neutron Induced i.e. it occurs, due to the absorption of a thermal (slow-moving) neutron. (not radioactive decay)
In what form is energy released from nuclear fission reactions?
In fission reactions, energy is released in the form of kinetic energy of the fragments as well as neutrons and gamma rays.
What is a nuclear chain reaction?
A nuclear chain reaction occurs when:
2 or 3 neutrons are emitted from a fission reaction
these neutrons cause further fission reactions
more neutrons are emitted
the rate of fission reactions increases
What is a thermal neutron?
A thermal neutron is a neutron which is in thermal equilibrium with the moderator.
What happens if the amount of nuclear fuel in a fission reactor is too low?
If the amount of nuclear fuel in a fission reactor is below the critical mass, chain reactions will stop or slow down.
This is because neutrons can escape the material without hitting any more nuclei.
What happens if the amount of nuclear fuel in a fission reactor is too high?
If the amount of nuclear fuel in a fission reactor is above the critical mass, chain reactions increase rapidly.If uncontrolled, this can lead to a huge release of energy and even a nuclear explosion.
What is the purpose of control rods in a nuclear reactor?
The purpose of control rods is to absorb neutrons.
What is the purpose of the moderator in a nuclear reactor?
The purpose of a moderator is to slow down neutrons.
Why must neutrons be slowed down in a nuclear reactor?
In a nuclear reactor, neutrons must be slowed down so they can react with the uranium fuel efficiently.
This is because slow neutrons are more likely to be absorbed by a uranium nucleus than fast neutrons
What is the purpose of shielding in a nuclear reactor?
The purpose of shielding is to absorb hazardous radiation.
What are the advantages of nuclear power?
Some of the advantages of nuclear power are:
it produces no greenhouse gases or chemical pollution
it is a highly reliable method of electricity generation
nuclear fuel is widely available and has an extremely high energy density
What is nuclear waste?
Nuclear waste refers to the leftover radioactive materials associated with the production of nuclear power. Different waste materials can be classed as
low-level waste (eg. Contaminated clothing)
intermediate-level waste
high-level waste (eg. spent fuel rods)
Treatment of Nuclear Waste!
Low-level Waste
encasing it in drums filled with concrete
storing it underground for a few years
eventually disposing of it in landfill sites
Intermediate-level waste
encasing it in steel drums filled with concrete
storing it underground in a secured facility
High-level waste
recovering useable isotopes of uranium and plutonium
encasing it in reinforced steel containers filled with concrete or vitrified with molten glass
storing in underwater cooling tanks initially
storing it deep underground in a secured facility
What is nuclear fusion?
Nuclear fusion is the joining of two smaller nuclei to form one larger nucleus, releasing a large amount of energy in the process. IT takes place in core of stars
Why do nuclei require very high kinetic energies in order to fuse?
overcome the repulsive coulomb forces between protons
get close enough for the strong nuclear force to act
In what form is energy released from nuclear fusion reactions?
In fusion reactions, energy is released in the form of kinetic energy of the nuclei produced as well as gamma rays and neutrinos.
What two conditions are required for nuclear fusion to occur?
High temperature
High pressure or density
What is radiation pressure?
Radiation pressure is a pressure that arises due to the transfer of momentum from photons to the surrounding matter.
How do main sequence stars remain in equilibrium?
Main sequence stars remain in equilibrium because fusion reactions generate radiation pressure which acts outwards and balances the inwards-acting gravitational force.
When does a protostar become a main sequence star?
A protostar becomes a main sequence star when nuclear fusion reactions are initiated in its core and the inward force of gravity is balanced by the outward pressure from the fusion reactions.
Initial Stages of Star’s life cycle
Nebula (cloud of dust and gas)
Protostar
Main sequence star
The stages that follow depend on the mass of the main sequence star that forms.
When does a main sequence star turn into a red giant?
A main sequence star turns into a red giant when hydrogen in the star's core begins to run out and the star begins to fuse helium into heavier elements.
What is the endpoint of a high masses star lifecycle?
A high mass star will eventually explode as a supernova and become a neutron star or a black hole.
What is a supernova?
A supernova is a large exploding star, that occurs when a star much more massive than the Sun reaches the end of its red supergiant stage. It collapses, becomes very unstable and explodes.
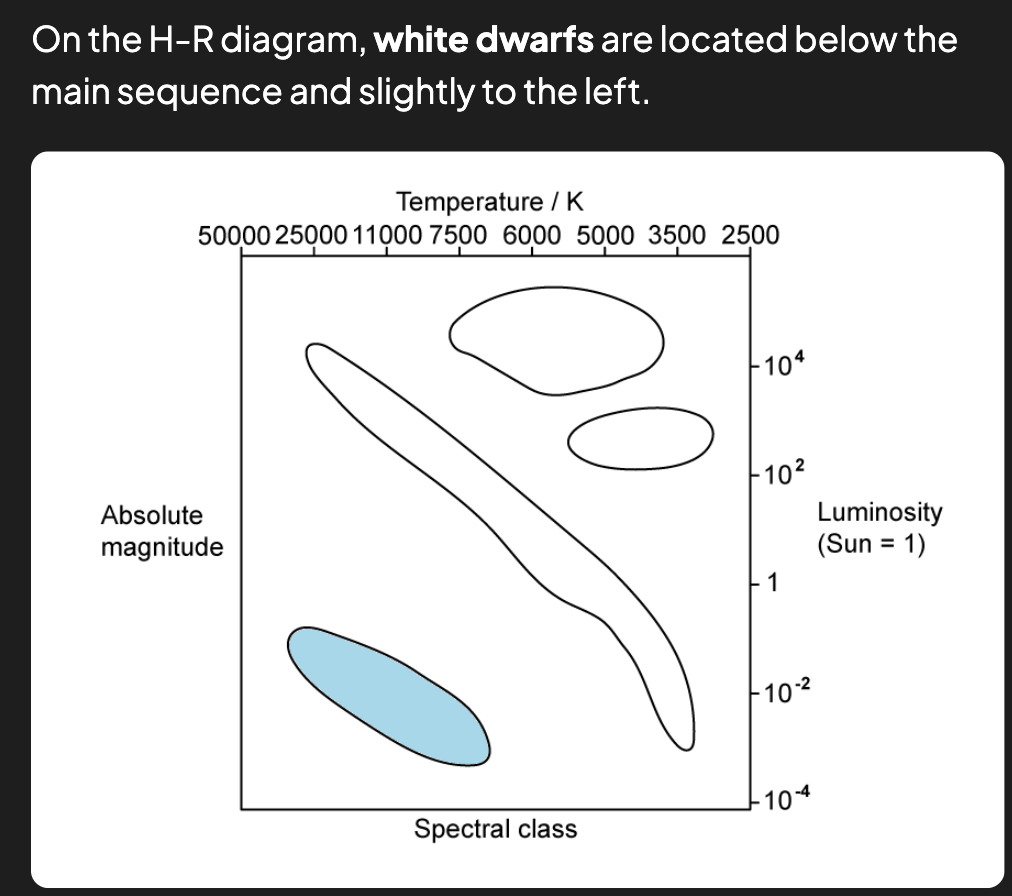
What is a Hertzsprung-Russell (HR) diagram?
The Hertzsprung-Russell (HR) diagram is a plot of a star's luminosity on the y-axis and its surface temperature on the x-axis.
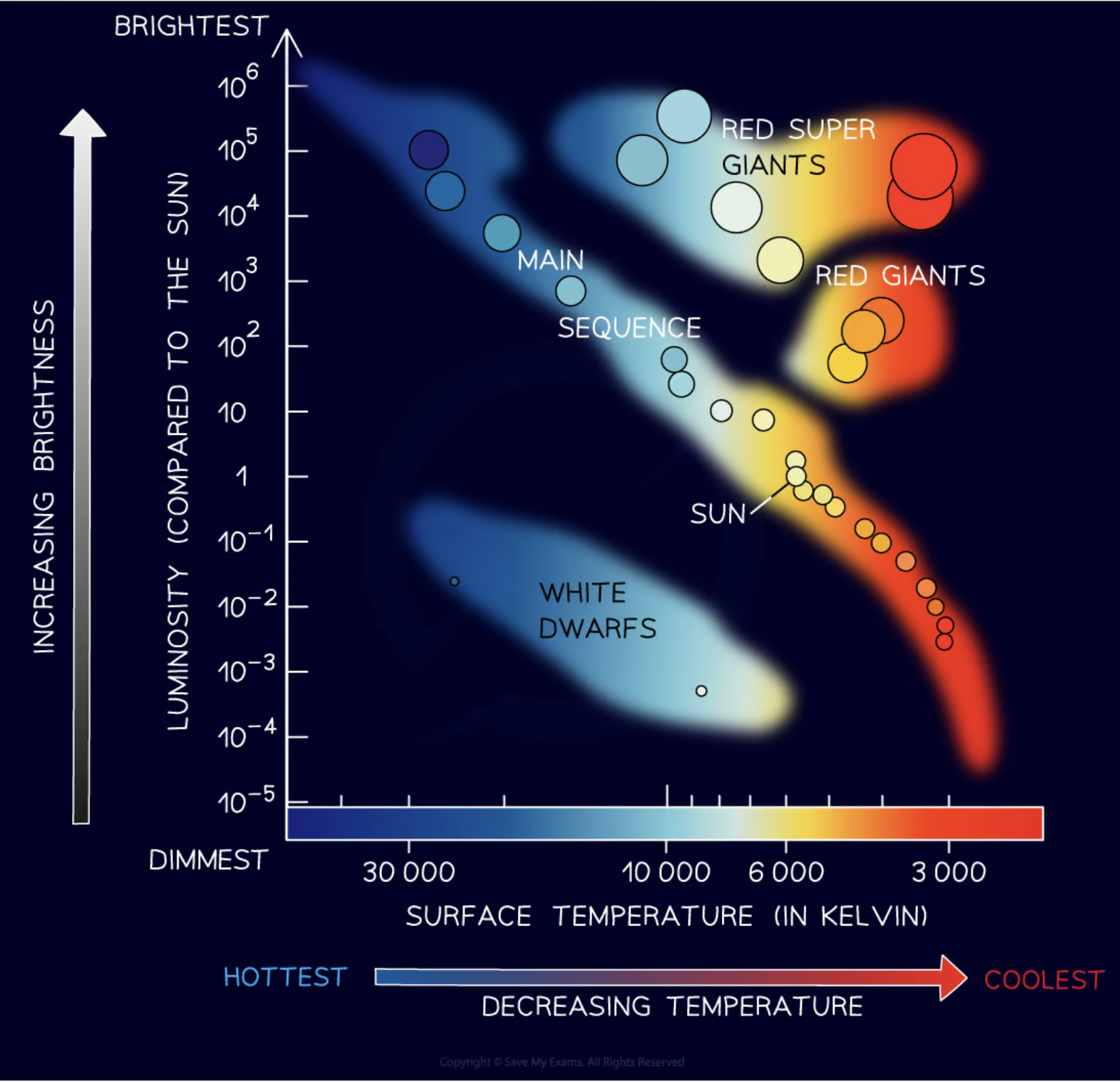
What type of stars are the hottest and dimmest on the H-R diagram?
The hottest and dimmest stars on the H-R diagram are white dwarfs.
What type of stars are the coolest and brightest on the H-R diagram?
The coolest and brightest stars on the H-R diagram are red supergiants.
Why do stars emit absorption spectra?
Stars emit absorption spectra because:
the hot, dense core produces a continuous spectrum
the cool, low-density gas in the outer layers absorbs photons
the resulting spectrum is a set of dark lines on a coloured background
How can the chemical composition of a star be determined?
The chemical composition of a star can be determined by analysing its absorption spectrum and identifying elements by their unique pattern of spectral lines.
Define 1 astronomical unit (AU).
One astronomical unit (AU) is the mean distance between the centre of the Earth and the centre of the Sun.
1 AU is equal to approximately 1.5 × 1011 m. (Data Booklet)
Define 1 light-year (ly).
One light-year is the distance travelled by light in one year.
1 ly is equal to approximately 9.5 × 1015 m.
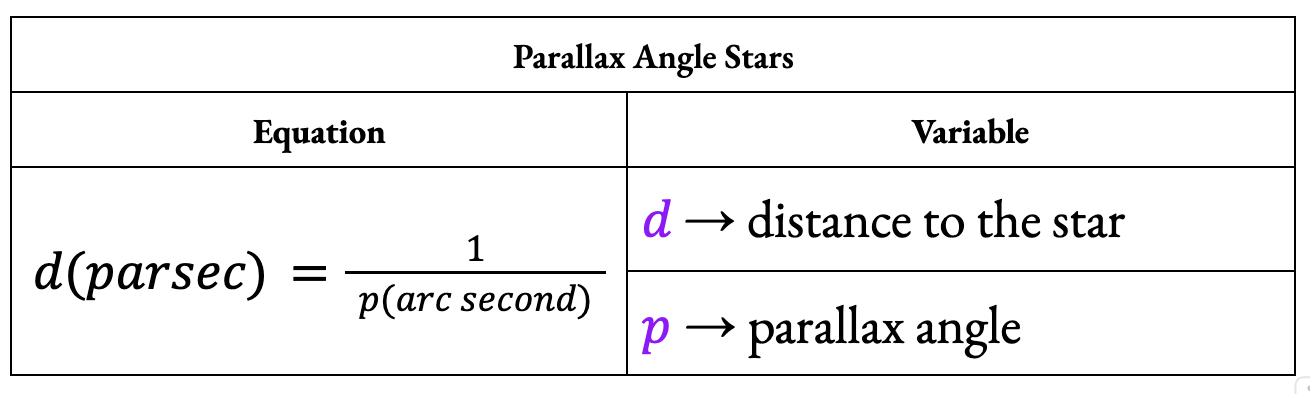
Define 1 parsec (pc).
One parsec is a unit of distance that gives a parallax angle of 1 second of an arc.
1 pc is equal to approximately 3.1 × 1016 m.
What is stellar parallax?
Stellar parallax is the displacement in the apparent position of a nearby star against a background of distant stars when viewed from different positions of the Earth during its orbit around the Sun.
Stellar parallax is accurate for measuring distances up to about 100 parsecs.
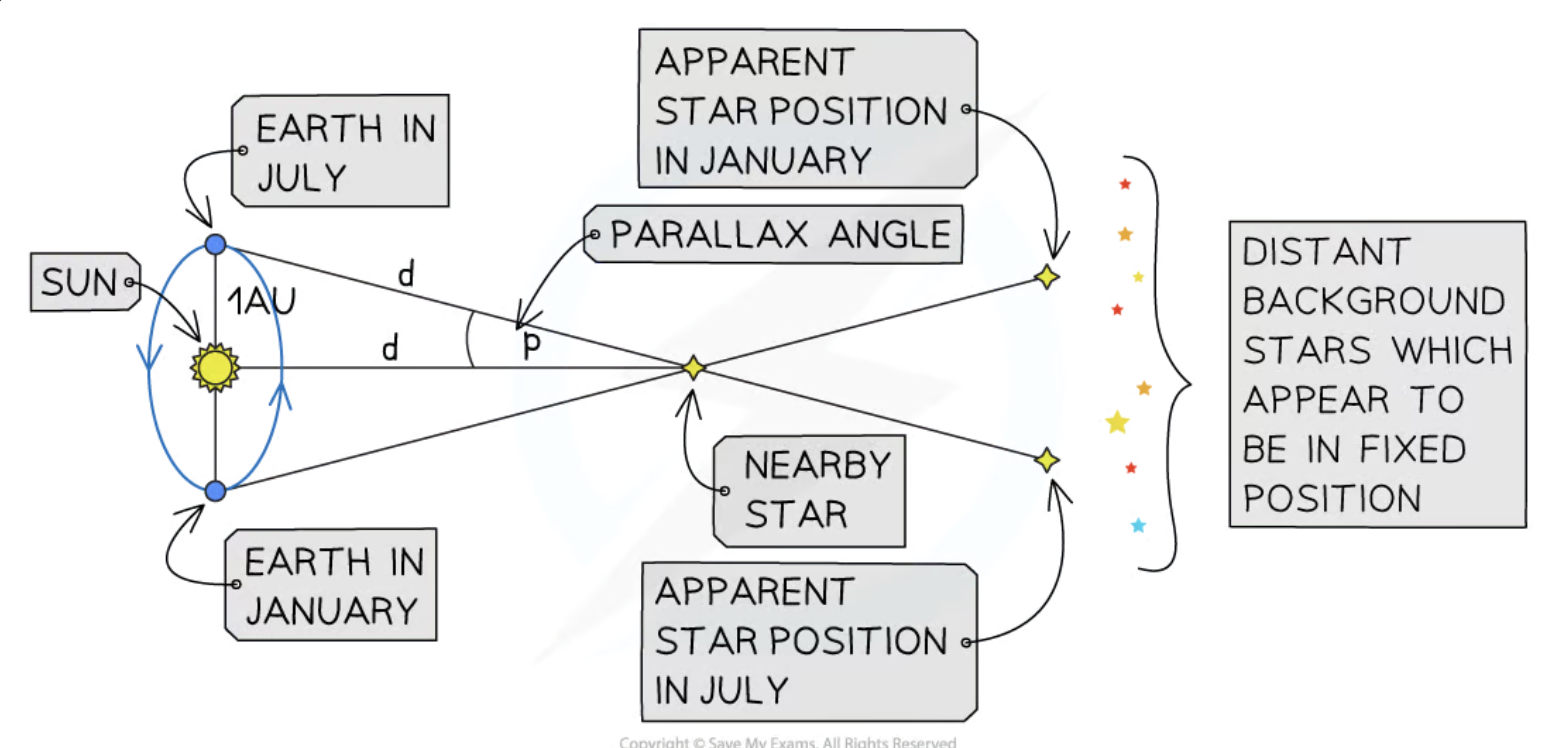
What is the equation for calculating the distance to a star using parallax angle?