Chapter 19 - Aldehydes and Ketones
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Nomenclature of Aldehydes
1) Suffix “-al” indicates aldehyde.
2) Parent chain must include aldehyde.
3) Aldehyde has priority (always position 1).
4) Aldehyde on ring is carbaldehyde.
Nomenclature of Ketones
1) Suffix “-one” indicates ketone.
2) Ketone has priority over alcohol, alkene, alkyne.
3) Ketone as substituent is “oxo.”
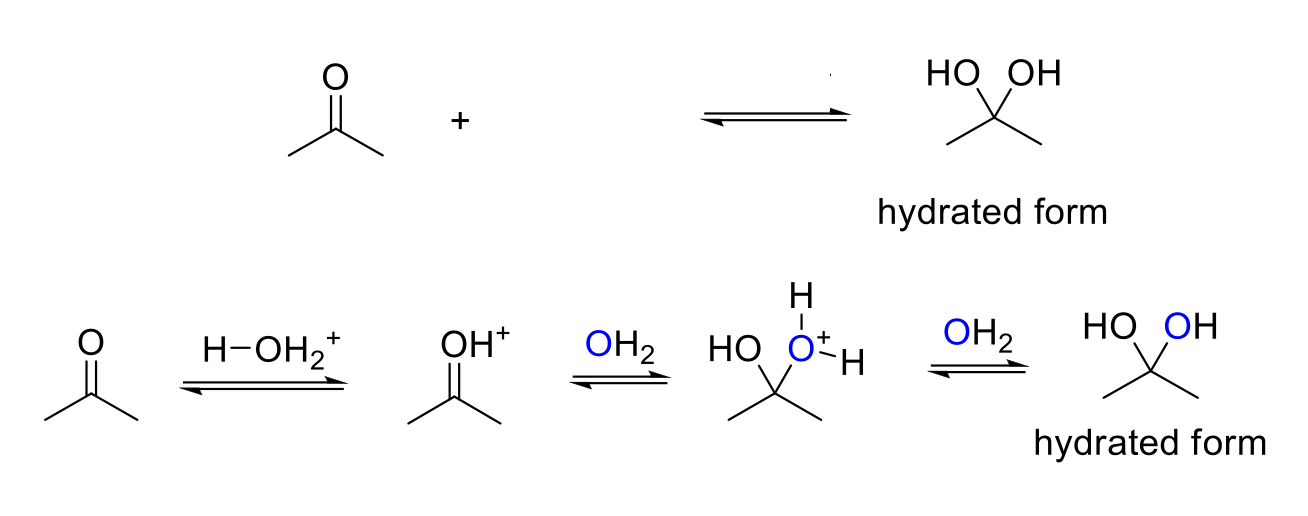
Acid-Catalyzed Hydration
Notes:
Acid conditions = H3O+
Adds two -OH to the carbonyl carbon.
Mechanism:
Acid protonates ketone/aldehyde. Water attacks the carbonyl carbon. -OH2 is deprotonated.

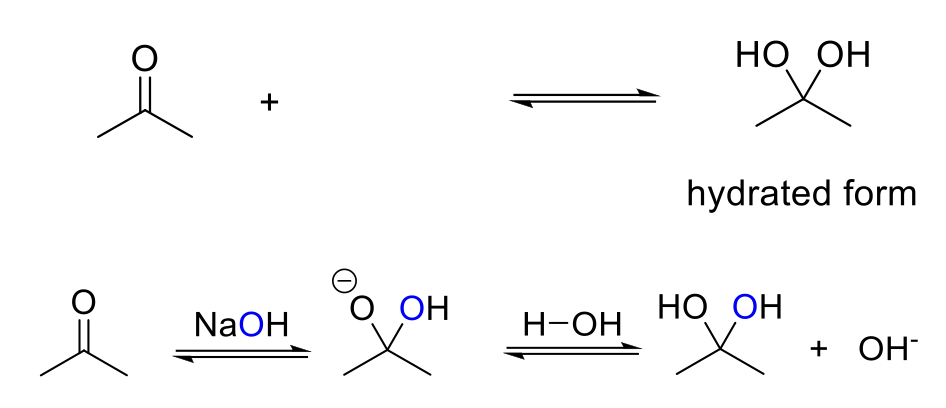
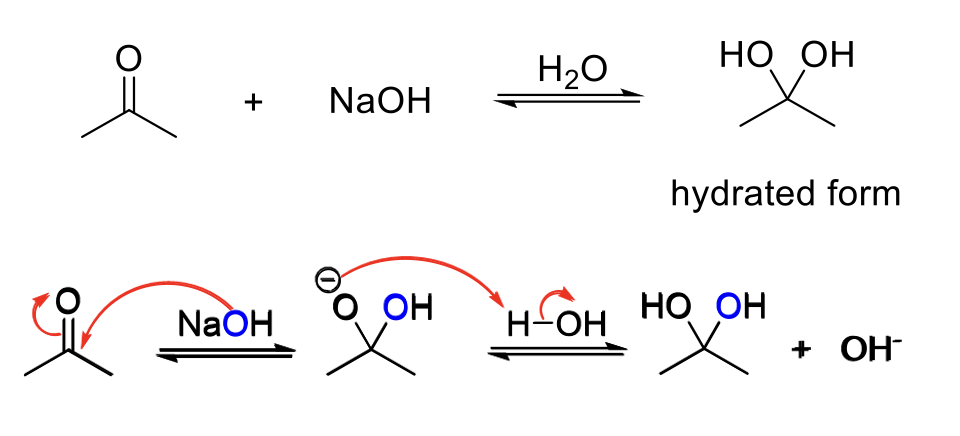
Base-Catalyzed Hydration
Notes:
Acid conditions = OH-
Adds two -OH to the carbonyl carbon.
Mechanism:
OH- attacks the carbonyl carbon. -O- is protonated.
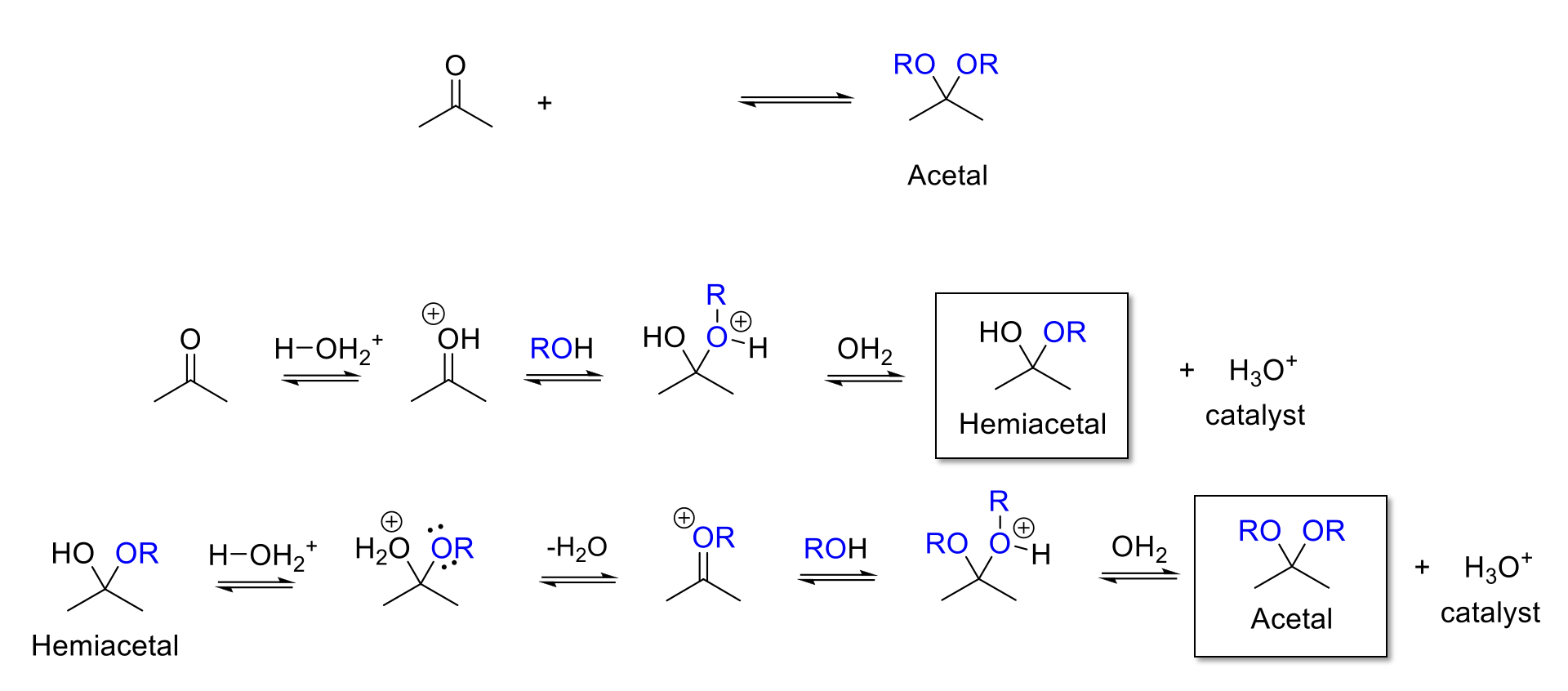
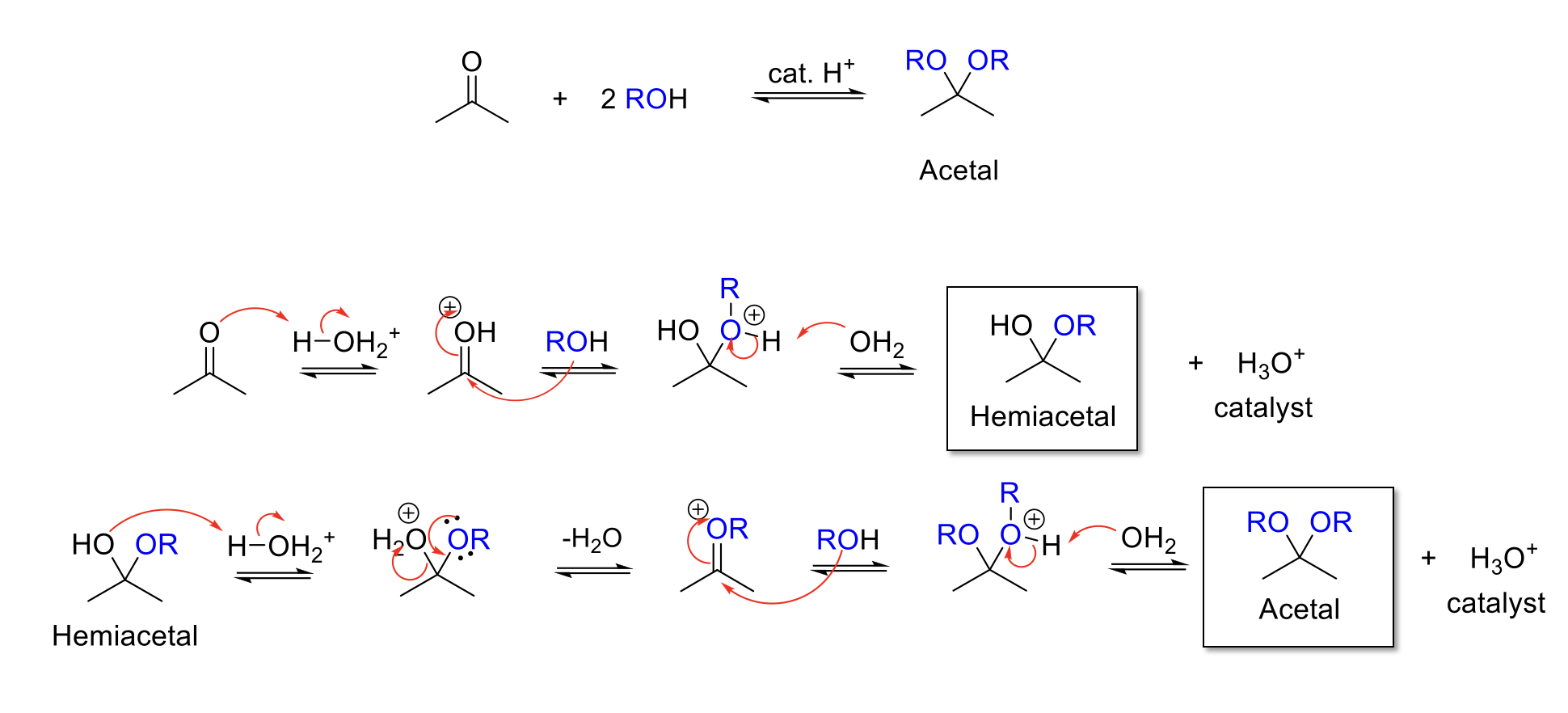
Acetal Formation
Notes:
Acid conditions = H3O+
Adds two -OR to the carbonyl carbon.
Forms a hemiacetal intermediate.
Mechanism:
Acid protonates ketone/aldehyde. Alcohol attacks carbonyl carbon. -ROH+ is deprotonated.
-OH is protonated. Reformation of C=O bond kicks out H2O. Alcohol attacks carbonyl carbon. -ROH+ is deprotonated.

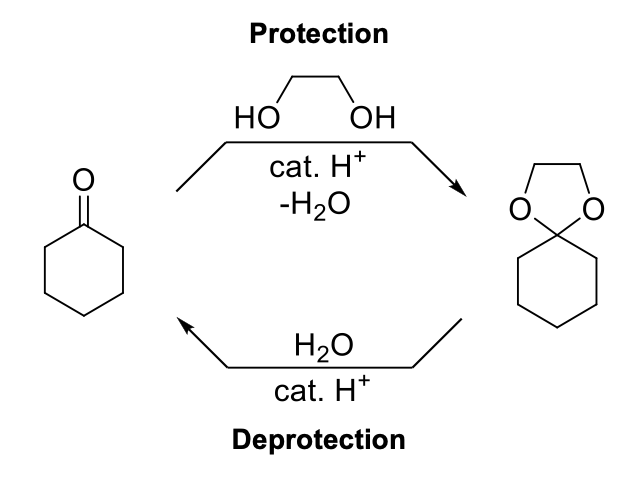
Acetal as a Protecting Group
Notes:
Acetal (R-O) bond is inert under basic conditions. Can be used to protect aldehydes and ketones.
Mechanism:
Same as acetal formatiom.
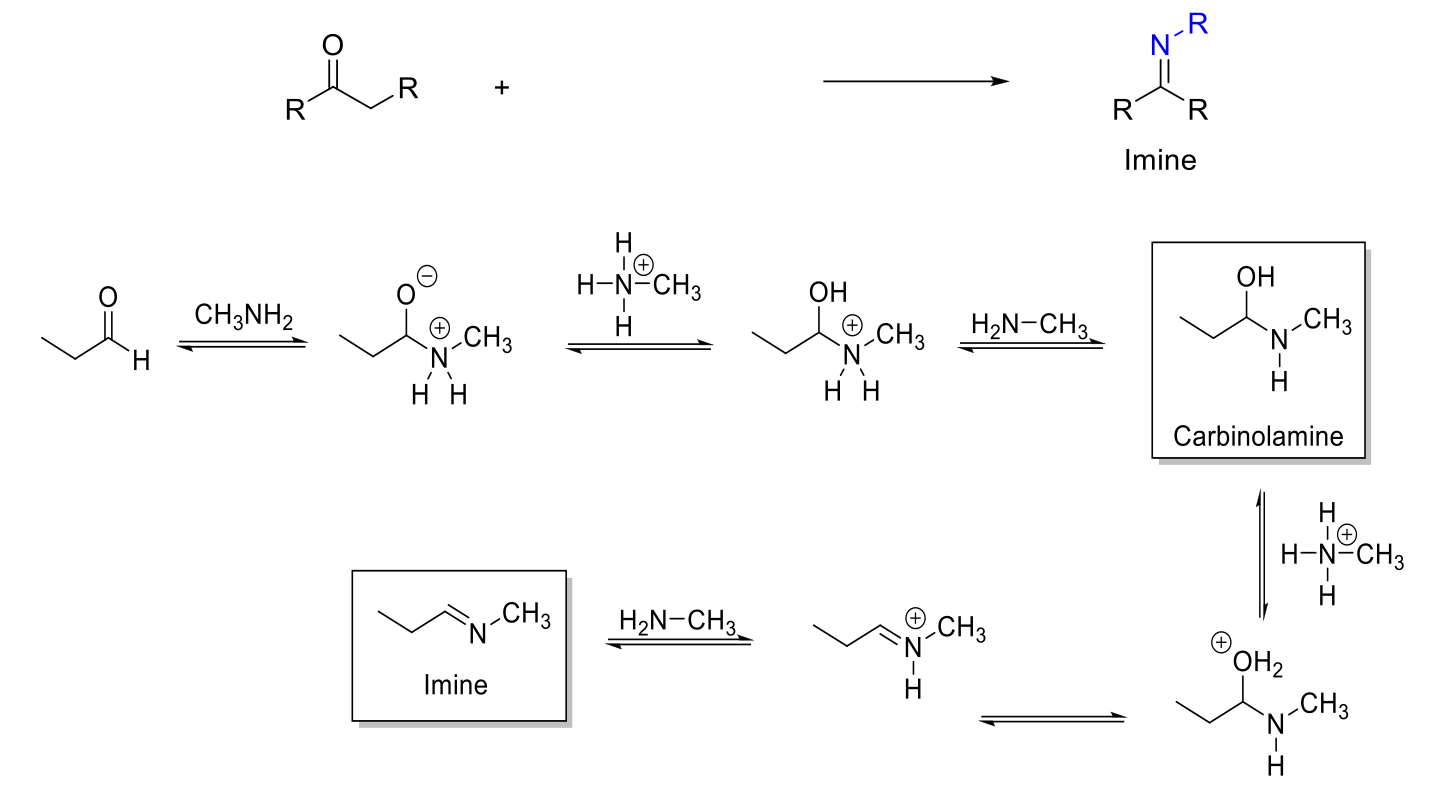
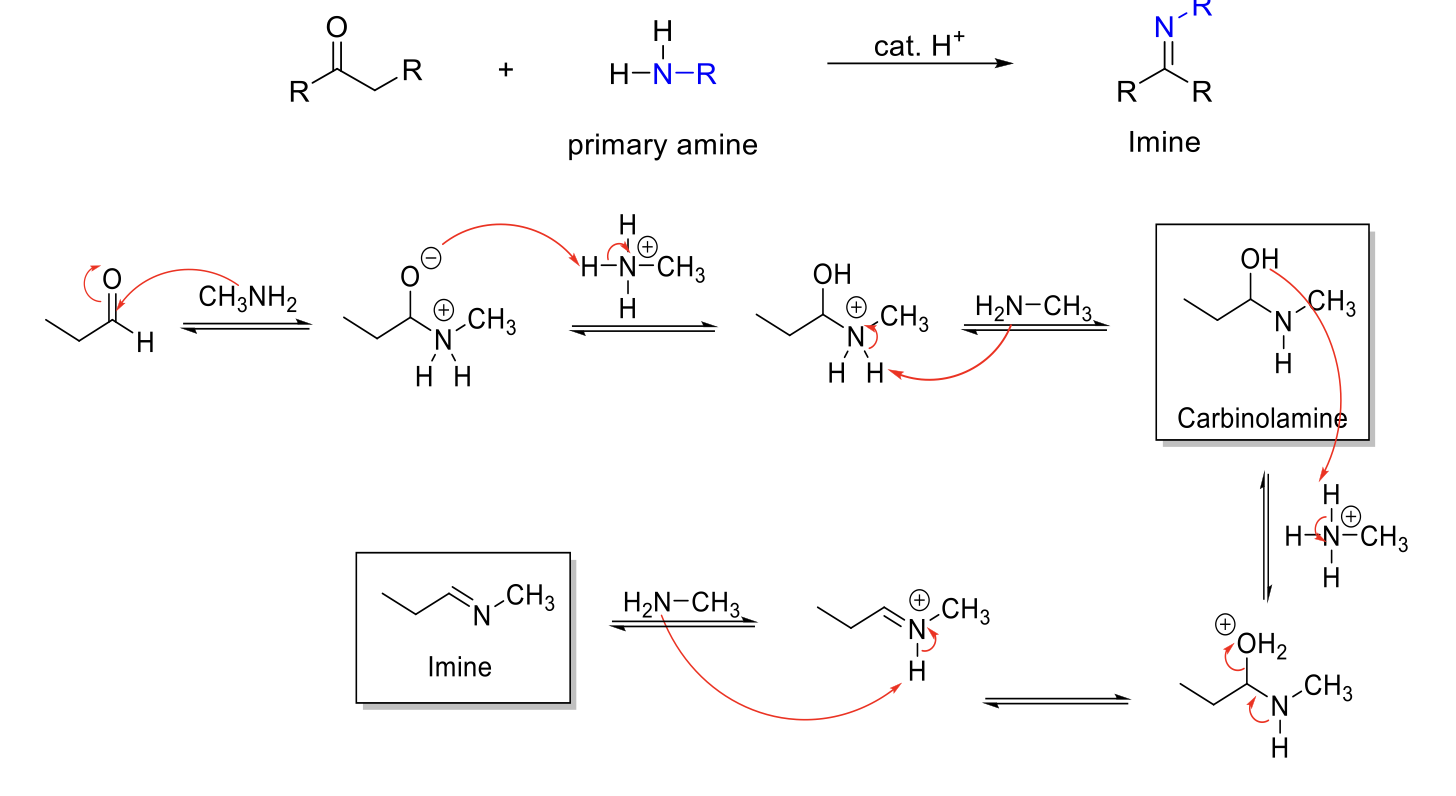
Imine Formation
Notes:
From primary amines (H2N-R, H2N-OH, H2N-NH2).
pH dependent - Max rate at ~4.5.
Forms imines (C=N double bond).
Carbinolamine intermediate.
Mechanism:
RNH2 attacks carbonyl carbon. -O- is protonated. R2NH2+ is deprotonated. Carbinolamine formed.
-OH is protonated. Formation of C=N bond kicks out H2O. R=NRH+ is deprotonated.
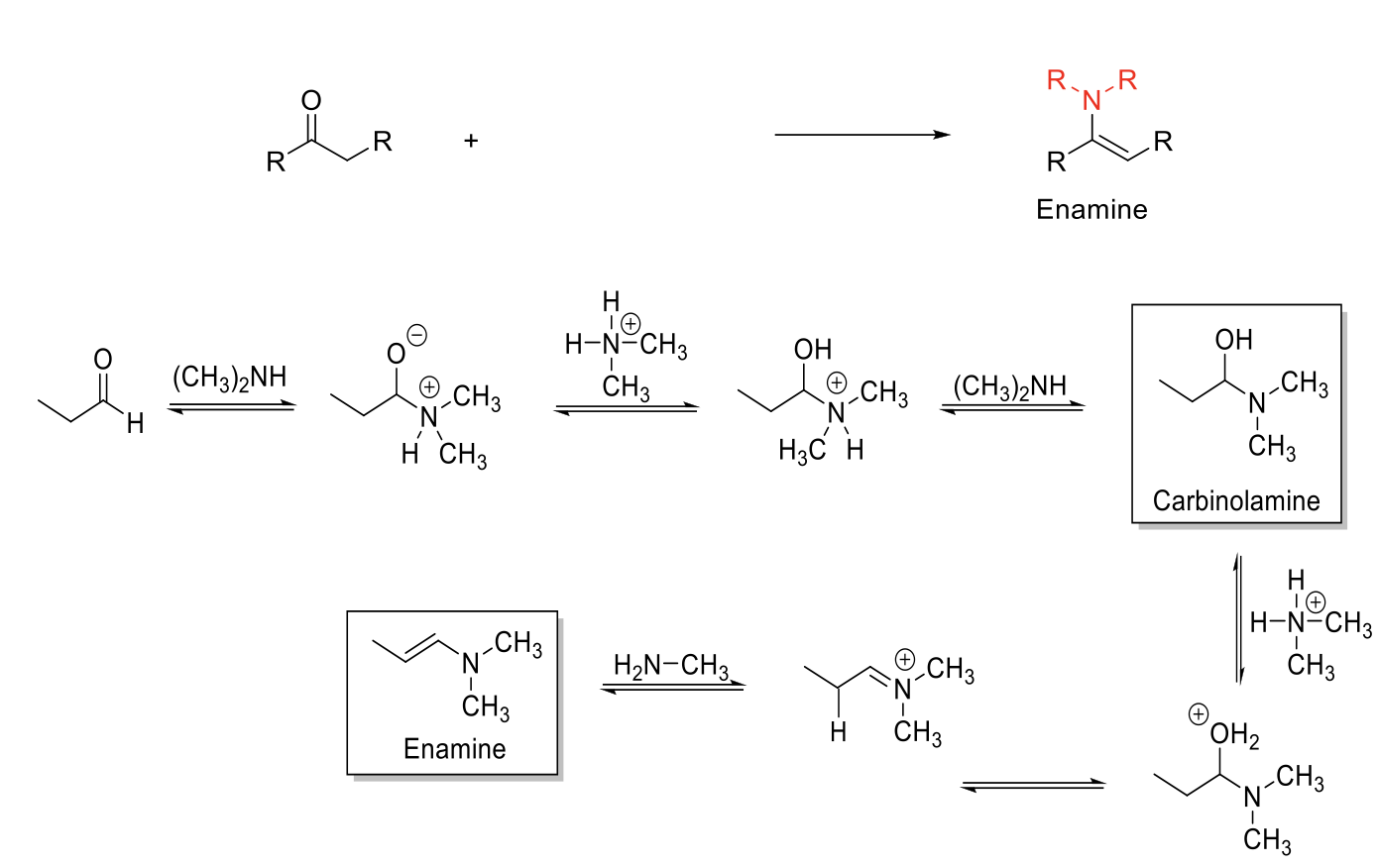
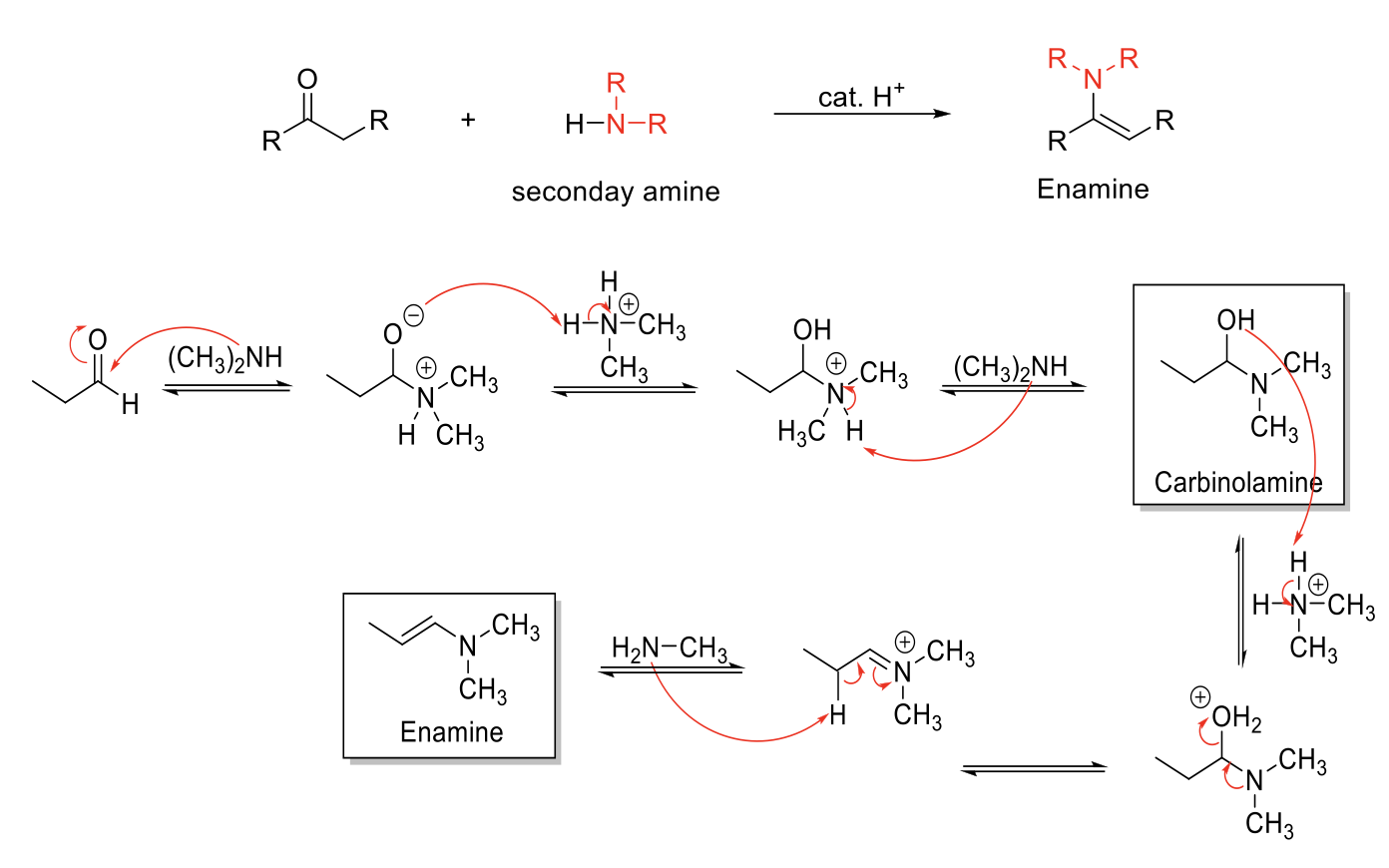
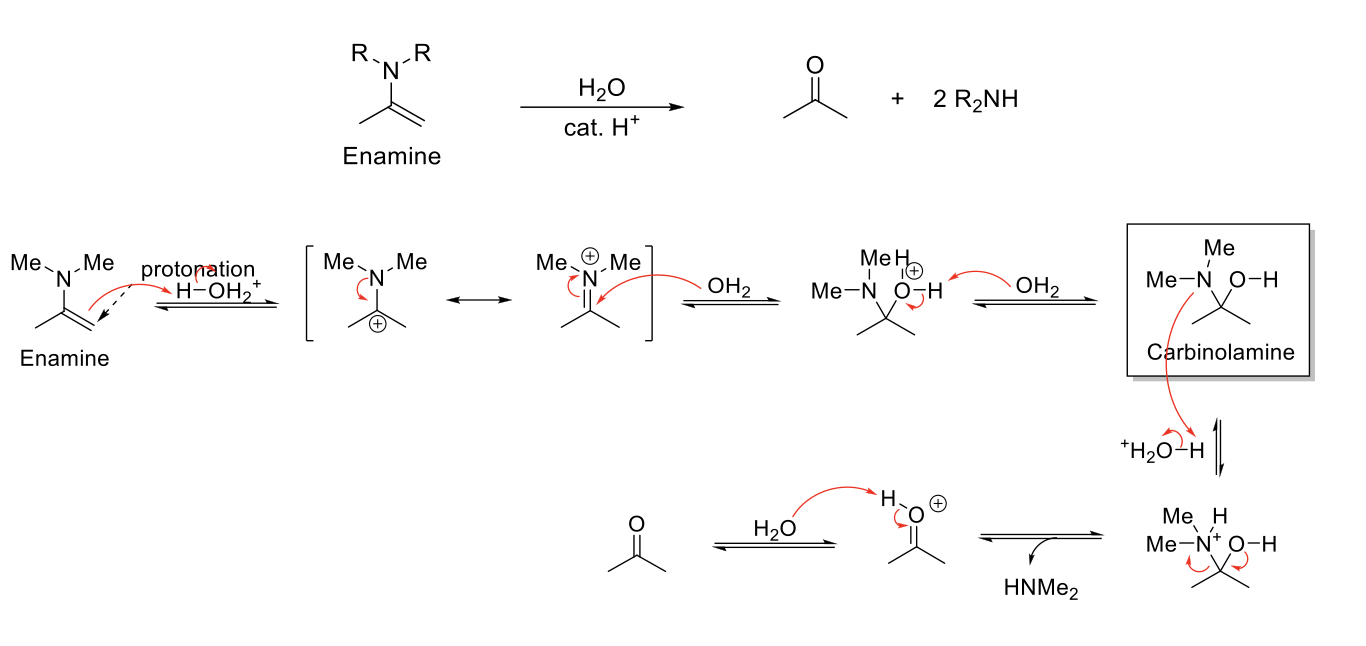
Enamine Formation
Notes:
From secondary amines (HN-R2)
Forms Enamines (Amine next to C=C).
Carbinolamine intermediate.
Mechanism:
R2NH attacks carbonyl carbon. -O- is protonated. R3NH1+ is deprotonated. Carbinolamine formed.
-OH is protonated. Formation of C=N bond kicks out H2O. Elimination reaction to form C=C and removes NR3+ charge.
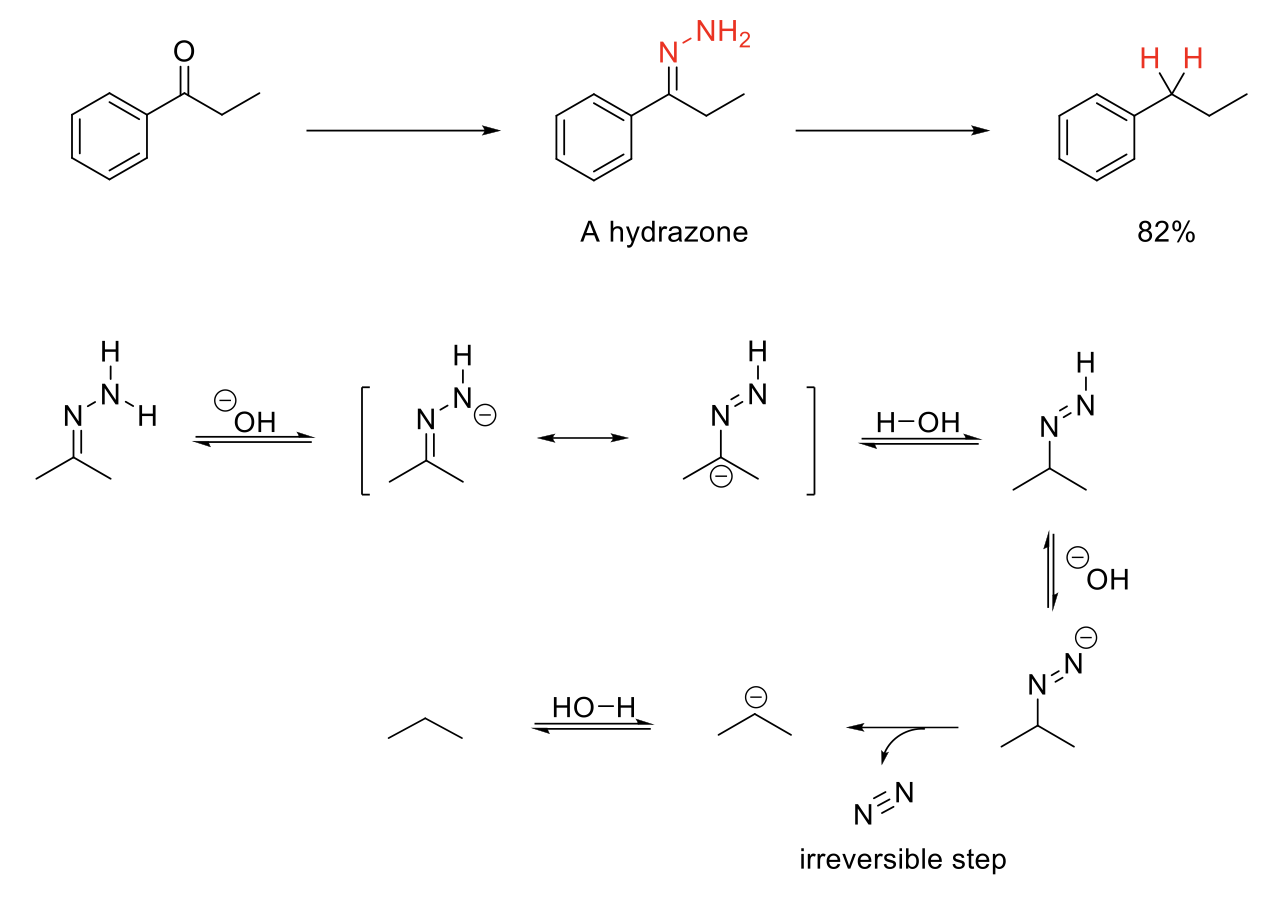
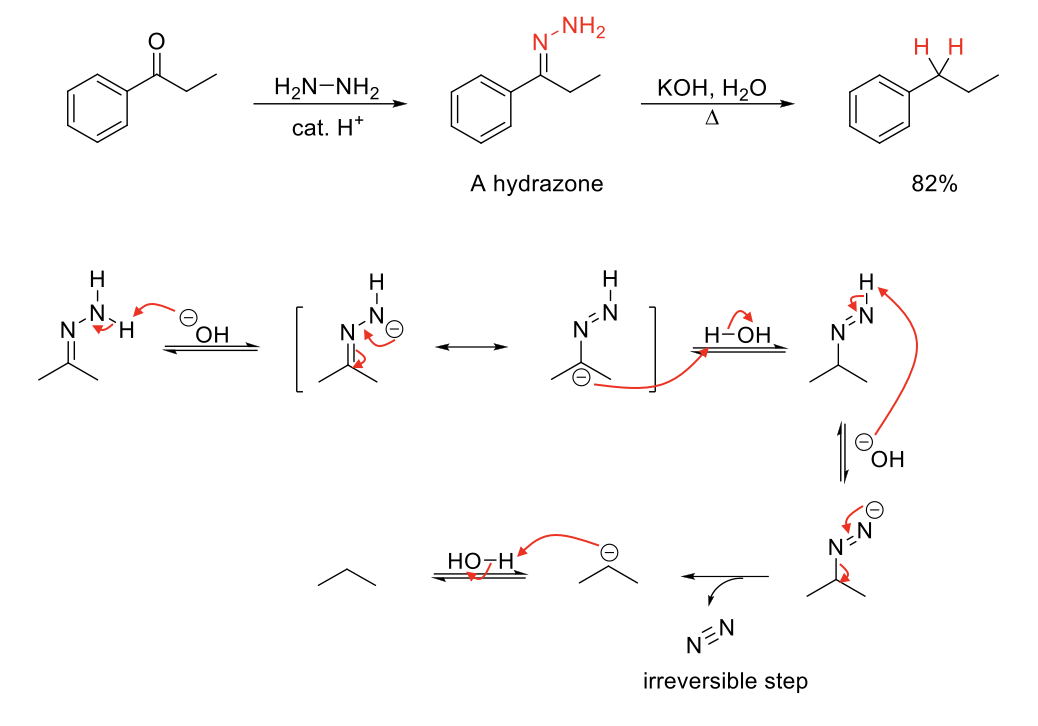
Wolfff-Kishner Reduction
Notes:
Imine fromation using H2N-NH2 forms a hydrozone which can undergo Wolff-Kishner Reduction.
Removes =N-NH2 and replaces it with 2 H.
Mechanism:
=N-NH2 is deprotonated. Resonance causes C to have - charge. - charge on C protonated. -N=N-H deprotonated. Formation of N=N bond kicks out N2. - charge on C protonated.
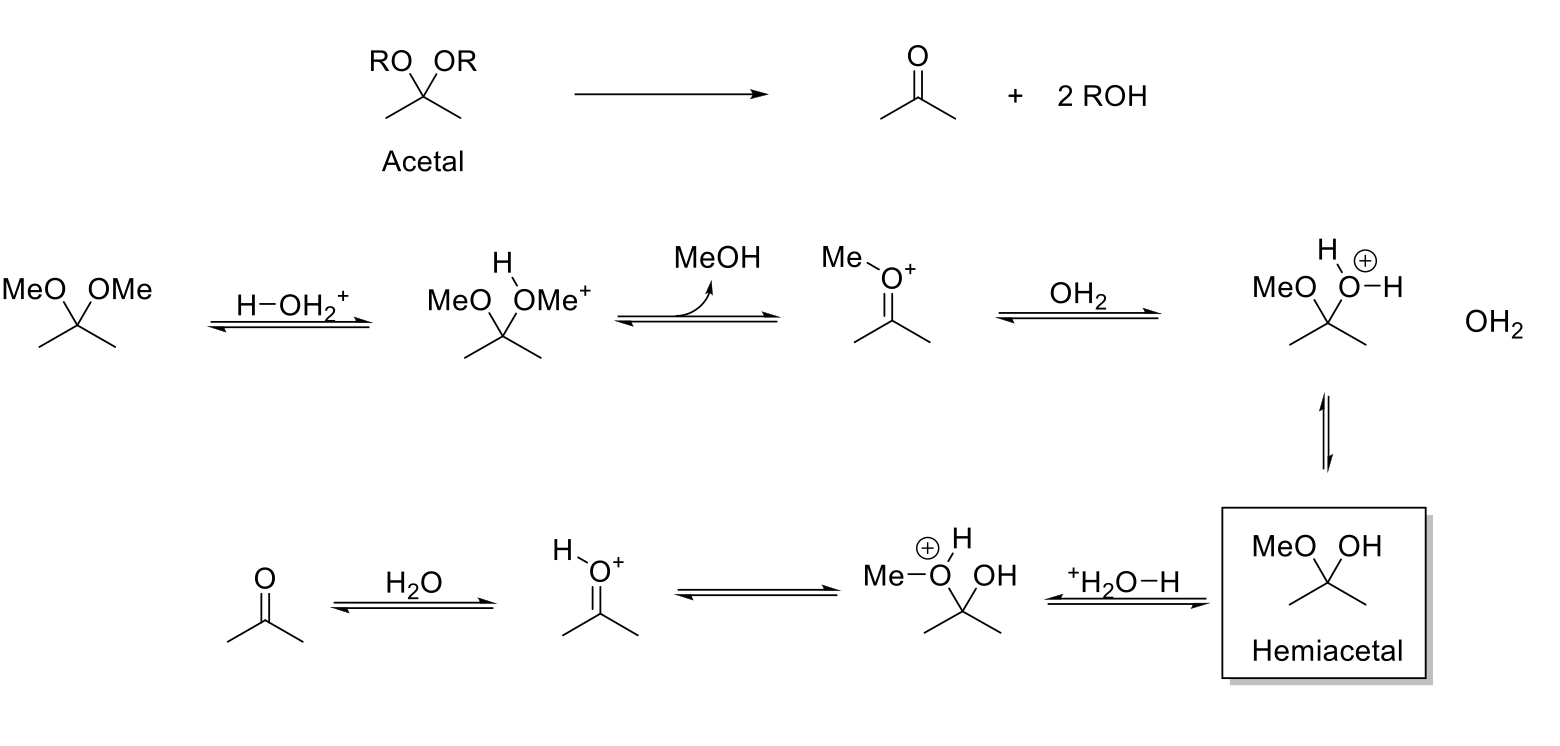
Acid-Catalyzed Hydrolysis of Acetals
Notes:
Acetal stable under basic conditions.
Reverse reaction of acetal formation.
Mechanism:
-OR is protonated. Formation of C=O bond kicks out HOR. H2O attacks carbonyl carbon. -H2O+ is deprotonated. -OR is protonated. Formation of C=O bond kicks out HOR. C=OH+ is deprotonated.
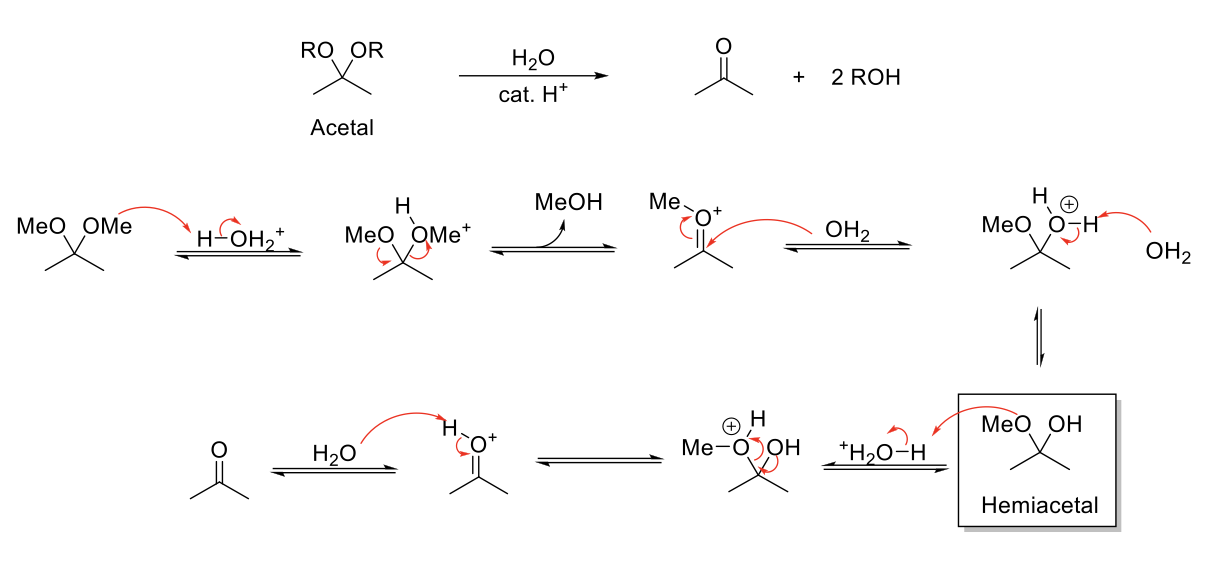

Acid-Catalyzed Hydrolysis of Imines
Notes:
Acetal stable under basic conditions.
Reverse reaction of imine formation.
Mechanism:
C=N-R is protonated. H2O attacks carbonyl carbon. -H2O+ is deprotonated. N is protonated. Formation of C=O bond kicks out H2NR. C=OH+ is deprotonated.
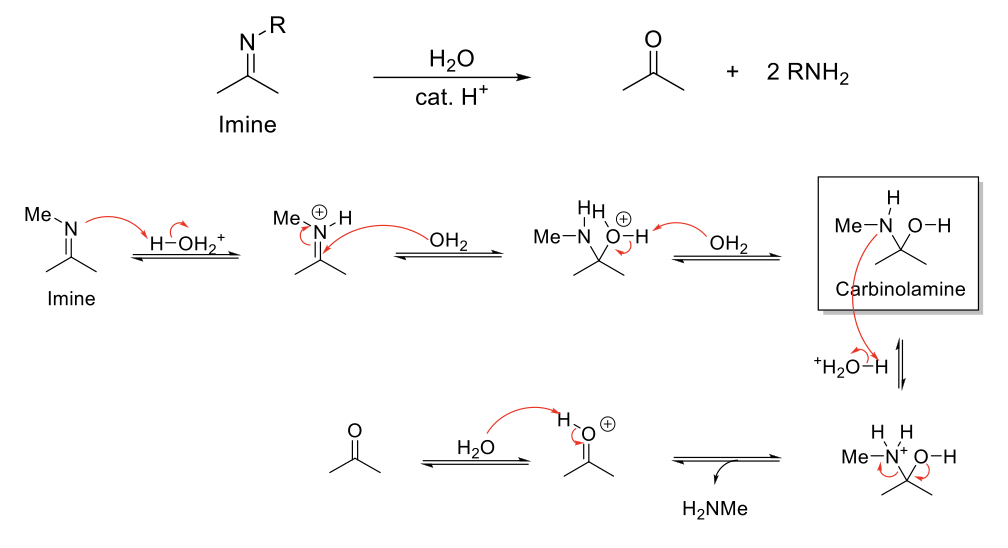
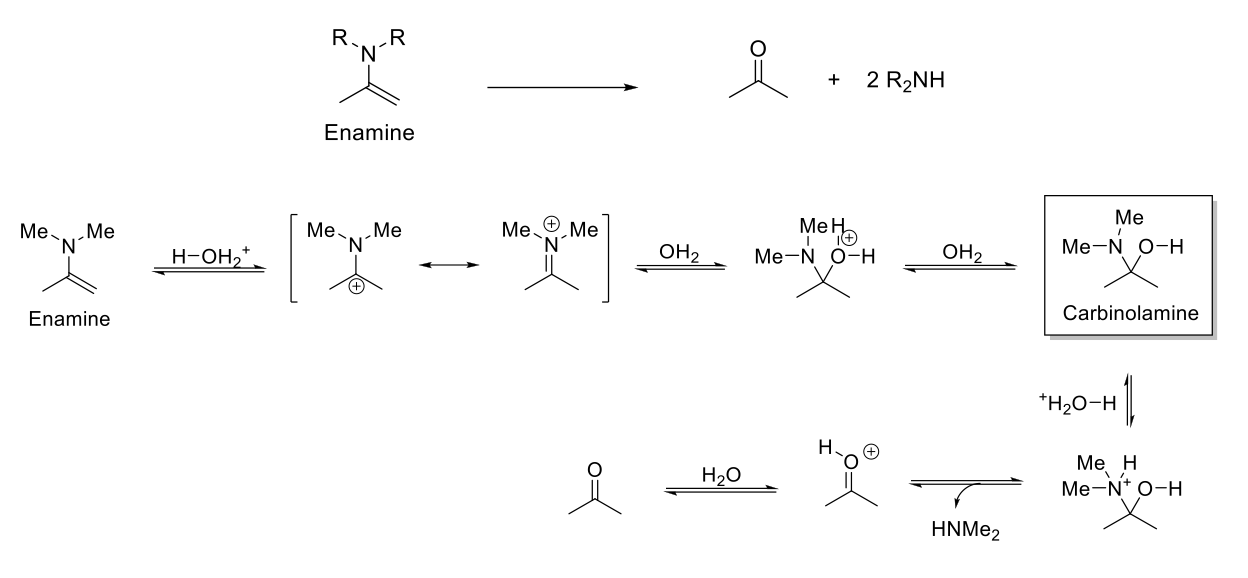
Acid-Catalyzed Hydrolysis of Enamines
Notes:
Acetal stable under basic conditions.
Reverse reaction of enamine formation.
Mechanism:
C=C double bond is protonated. Resonance when N=C reforms. H2O attacks C=N. -H2O+ is deprotonated. N is protonated. Formation of C=O bond kicks out HNR2. C=OH+ is deprotonated.
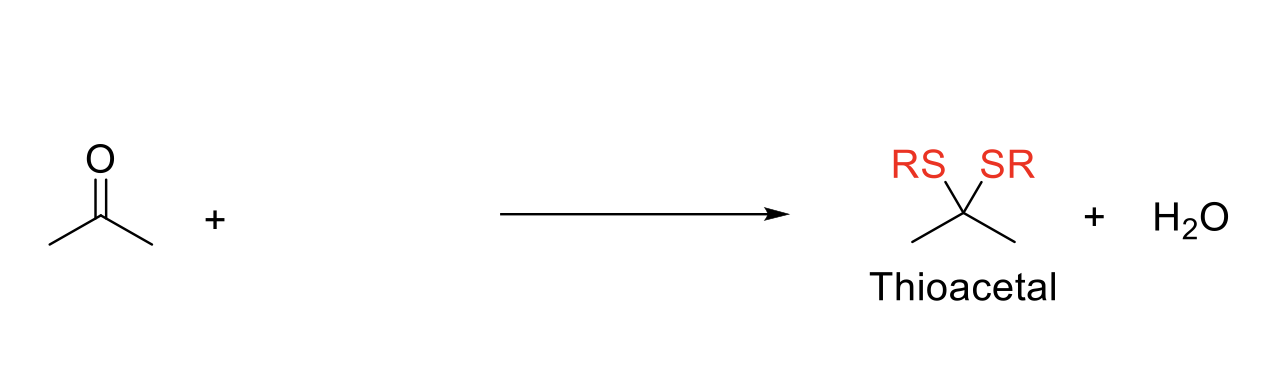
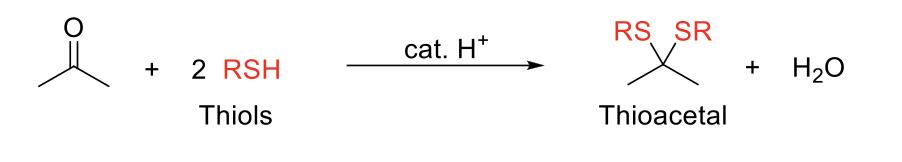
Thioacetal Formation
Notes:
Adds two -SR (thiols) to the carbonyl carbon.
Same mechanism as acetal formation.
Mechanism:
Same mechanism as acetal formation.
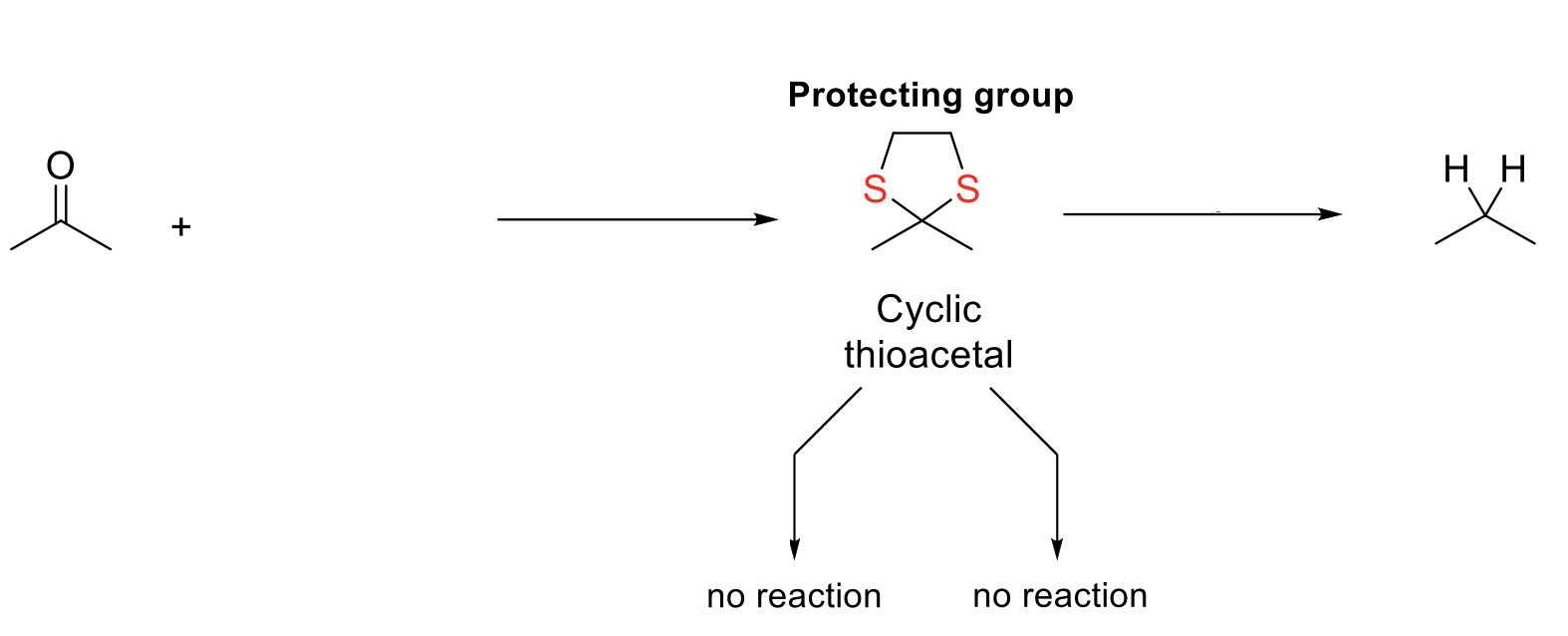
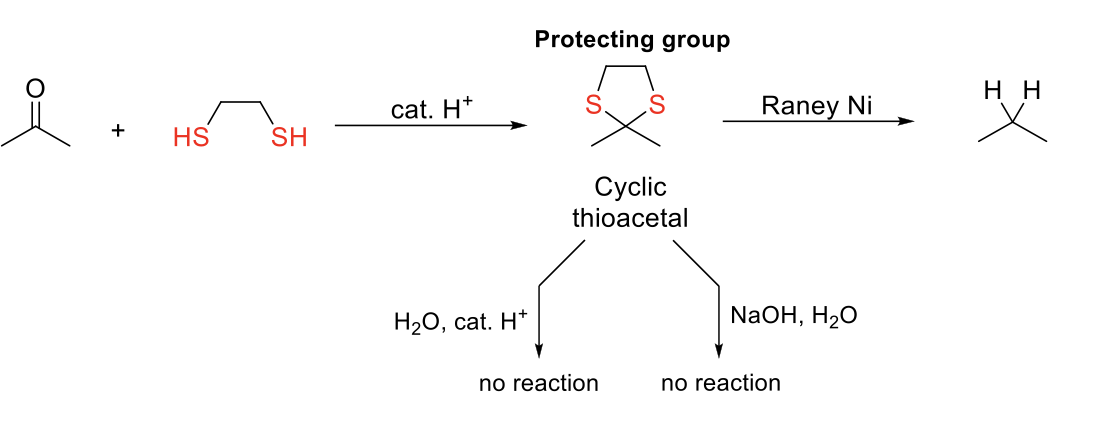
Desulfurization (Removal of Thioacetal)
Notes:
Cylic thioacetal is a good protecting group because it is stable in both acidic and basic conditions.
Mechanism:
Same mechanism as thioacetal formation.
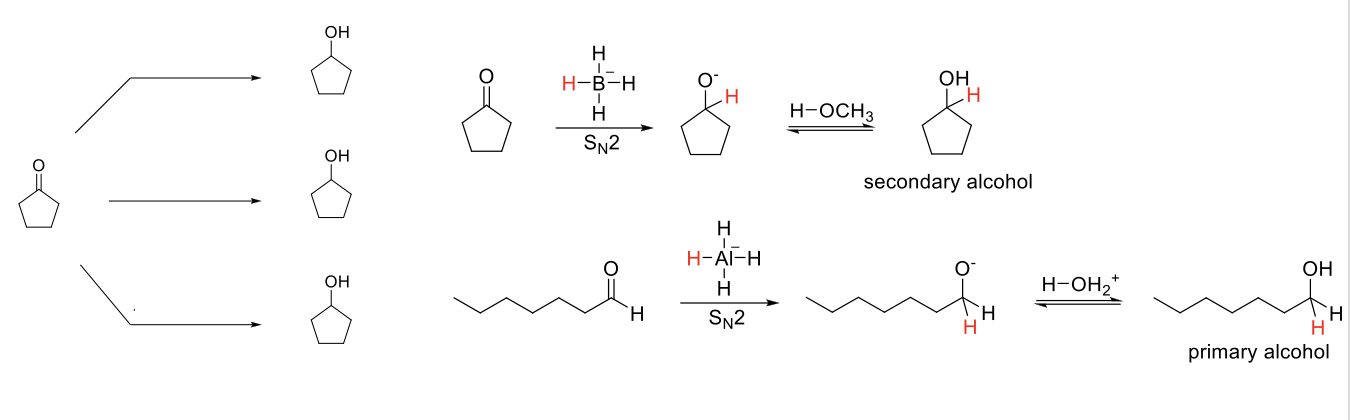
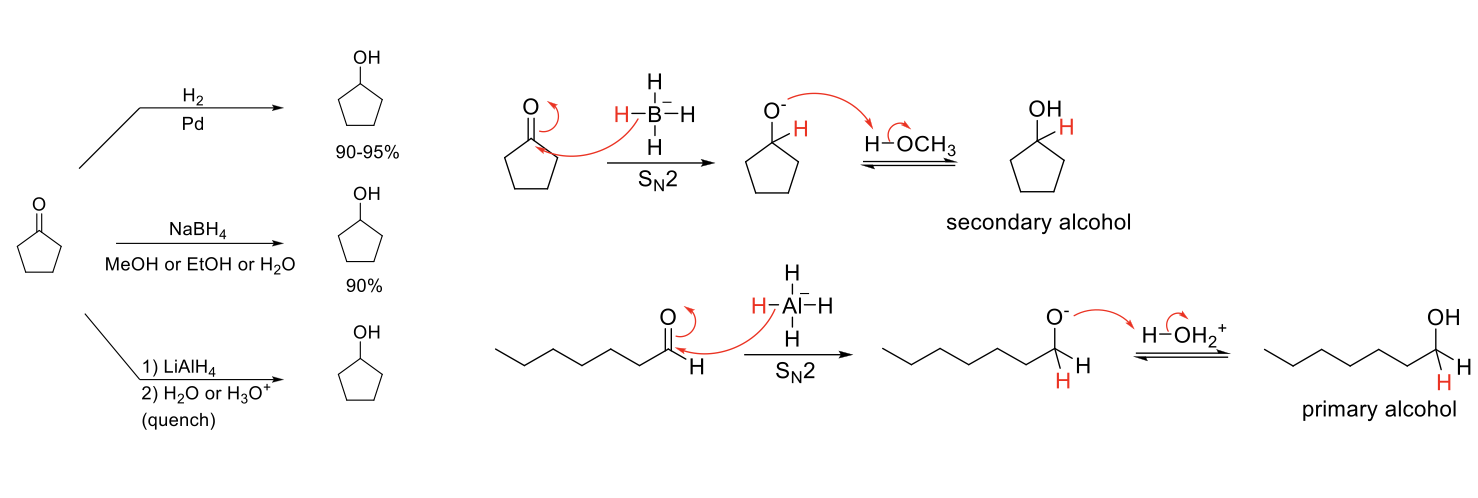
Reduction of Aldehydes/Ketones
Notes:
3 methods to reduce aldehydes/ketones.
Reduce aldehydes/ketones into alcohols by delivering H.
LiAlH4+ and protic solvents must be used in separate steps.
Mechanism NaBH4 / LiAlH4:
H added to carbonyl carbon. -O- protonated by protic solvent.
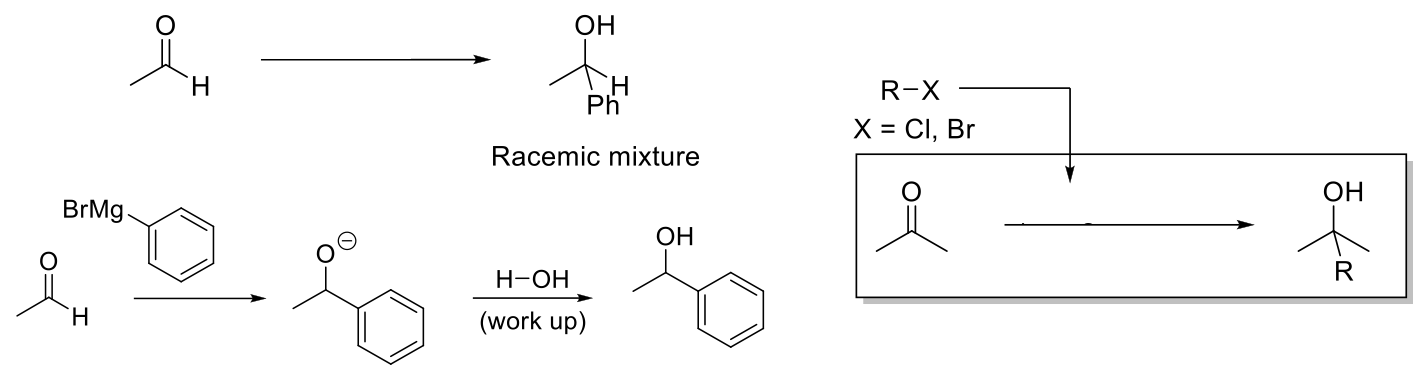
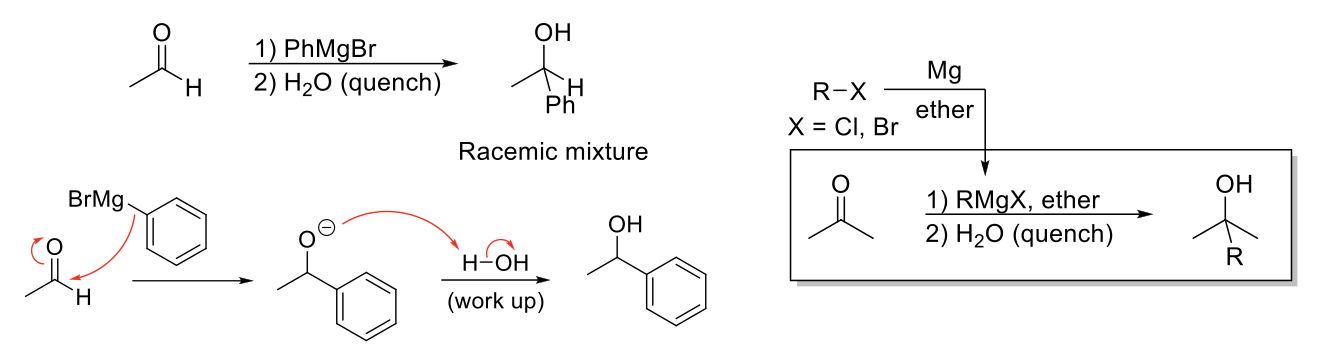
Grignard Reagents
Notes:
Reduces aldehydes/ketones into alcohol by delivering an alkyl group.
Grignard reagents synthesize by adding Mg + ether to an alkyl halide.
Mechanism:
High nucleophilic C attacks carbonyl carbon and delivers alkyl group. -O- protonated by protic solvent.
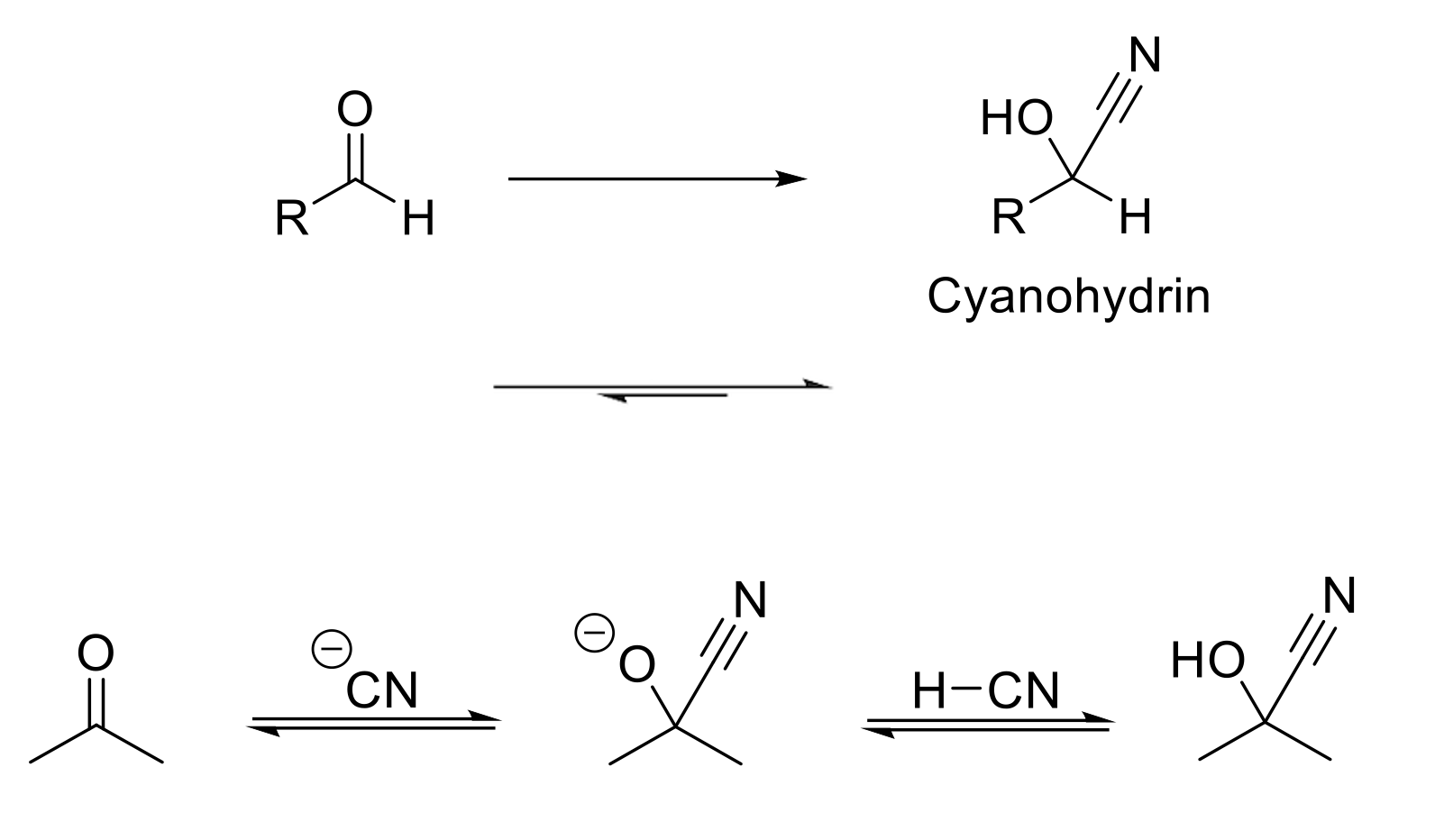
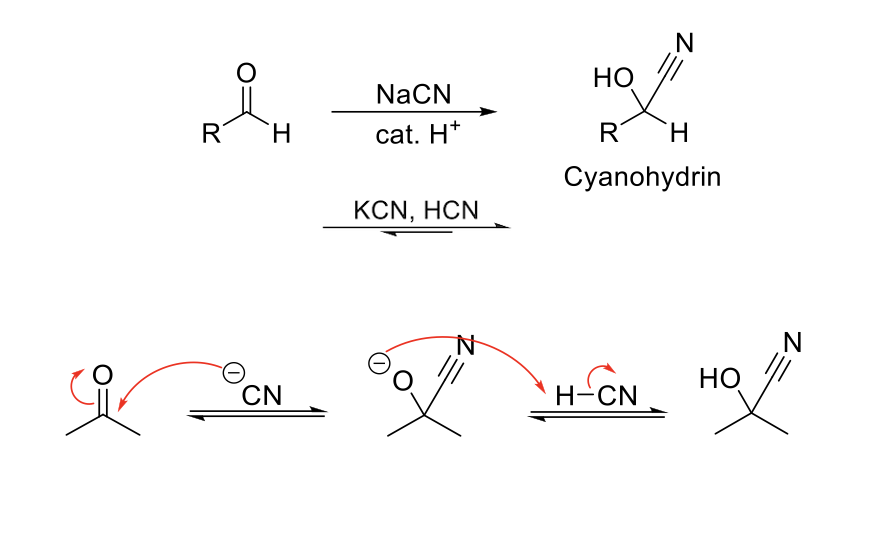
Cyanohydrin Formation
Notes:
Reduces aldehydes/ketones into alcohols by delivering a CN group.
Mechanism:
CN- attacks carbonyl carbon. -O- is protonated.
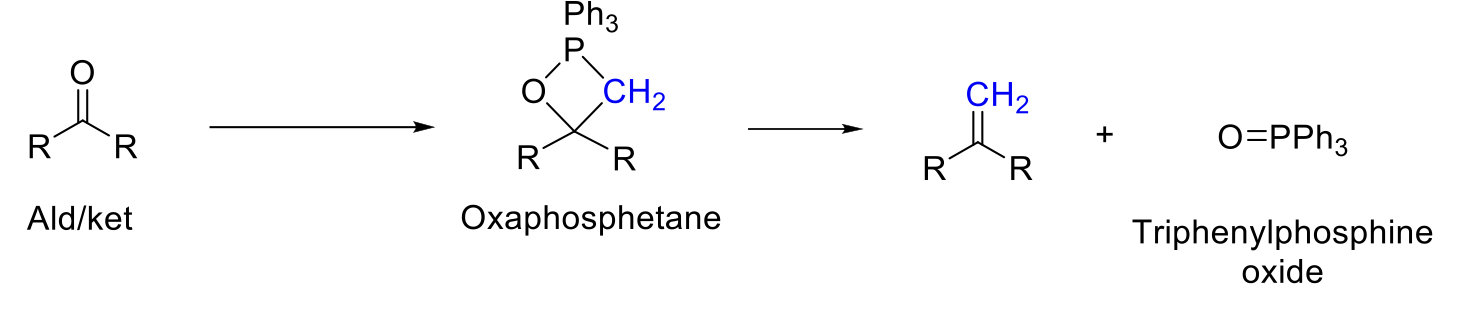
Wittig Reaction
Notes:
Unstabilized ylids (with alkyl group) = Z isomer (same)
Stabilized ylids (with EWG) = E isomer (opposite)
[2+2] Cycloaddition and retro [2+2] cycloaddition.
Driving force is O=P bond (triphenylphosphine oxide).
Mechanism:
CN- attacks carbonyl carbon. -O- is protonated.
![<p><strong>Notes:</strong></p><p>Unstabilized ylids (with alkyl group) = Z isomer (same)</p><p>Stabilized ylids (with EWG) = E isomer (opposite)</p><p>[2+2] Cycloaddition and retro [2+2] cycloaddition.</p><p>Driving force is O=P bond (triphenylphosphine oxide).</p><p></p><p><strong>Mechanism:</strong></p><p>CN<sup>-</sup> attacks carbonyl carbon. -O<sup>-</sup> is protonated.</p>](https://knowt-user-attachments.s3.amazonaws.com/d2373cee-1e1b-4d79-ae97-84090b7c5a35.png)
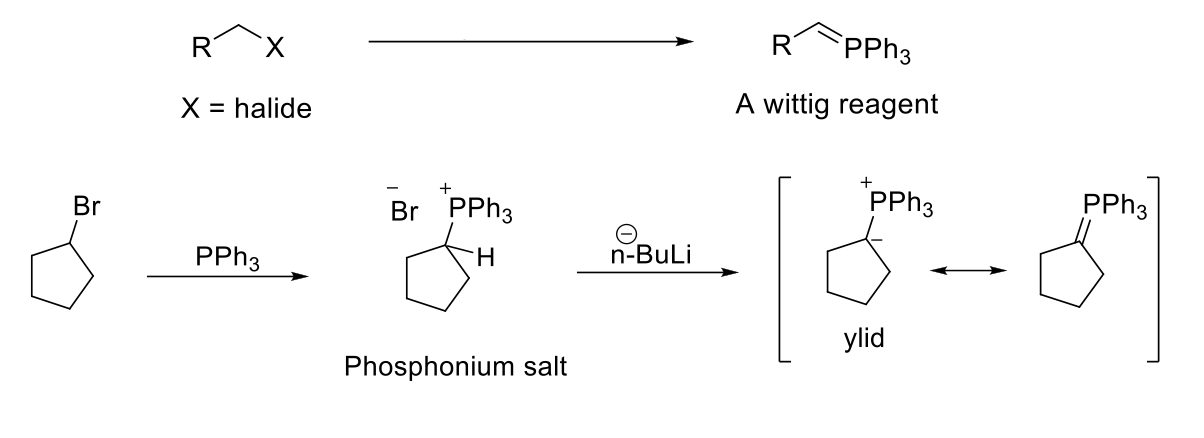
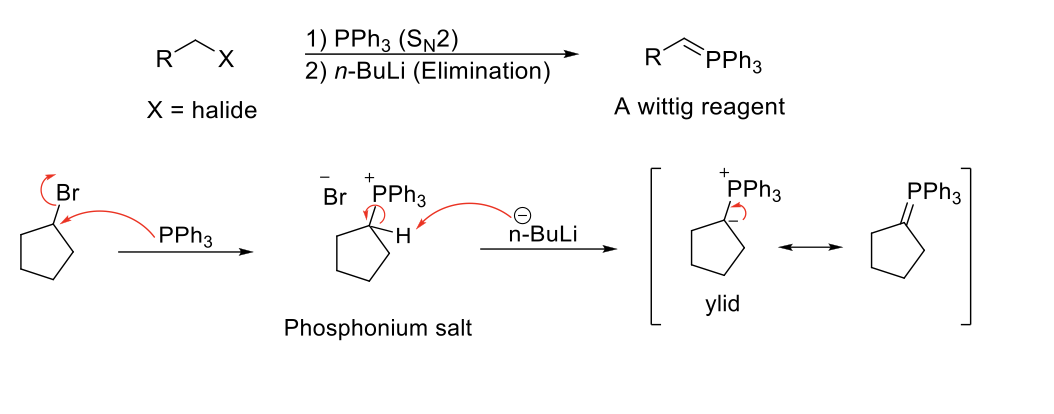
Preparation of Ylid Reagents for Wittig Reactions
Notes:
Unstabilized ylids (with alkyl group) = Z isomer (same)
Stabilized ylids (with EWG) = E isomer (opposite)
Must be done on methyl, primary, secondary R-X due to SN2.
Common bases used → NaH, n-BuLi, PhLi, NaNH2
Mechanism:
PPh3 attacks R-X and kicks X out (SN2). Base deprotonates C, leaving a negative charge on C and a positive charge on PPh3. Resonance occurs and double bond forms between C and PPh3
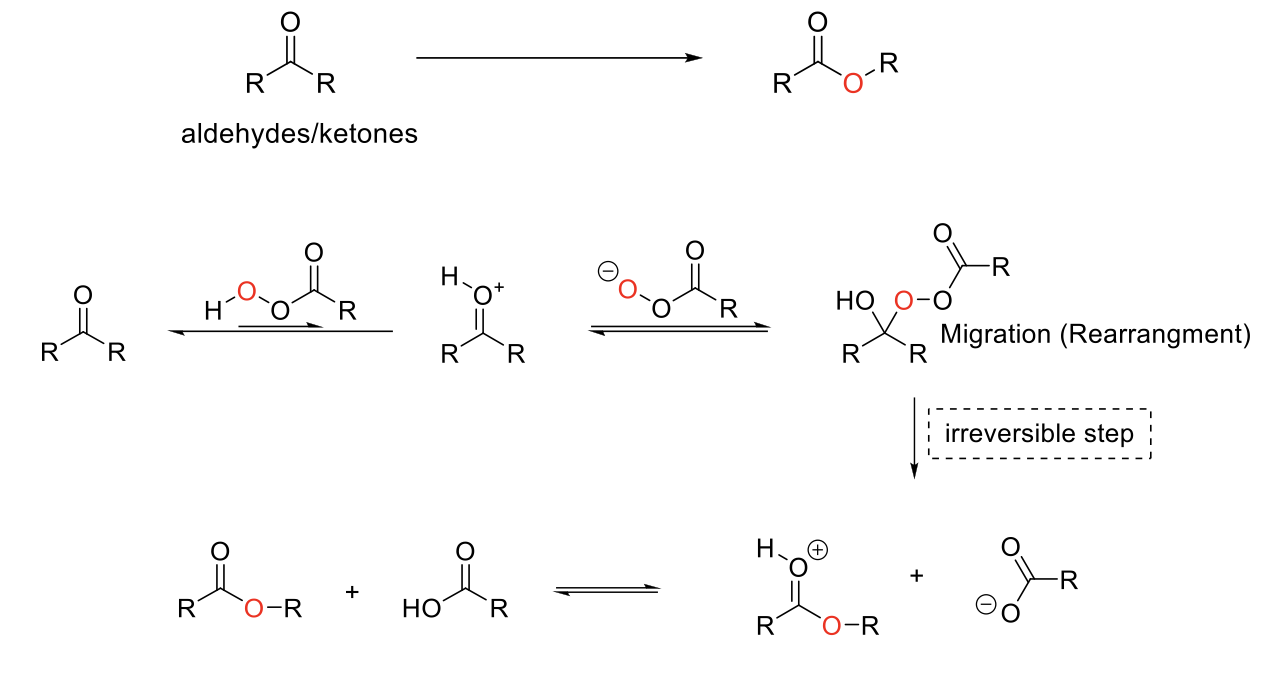
Baeyer-Oxidation Reaction
Notes:
Any peroxyacid can be used (ex: mCPBA).
Migration/irreversible step aptitude:
H > 3° > 2° > Ph > 1° > CH3
(Theory: Migrating group has ability to stabilzie + charge)
Forms an ester.
Mechanism:
Peroxyacid protonated aldehyde/ketone. Conjugate base of peroxy acid attacks carbonyl carbon. Undergoes migration/rearrangment. C=OH+ is deprotonated.
