Types of Strain
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
When does max steric strain happen?
When two methyl groups are completely eclipsing
Gauche
Methyl groups near each other
Flagpole-Flagpole
H atoms close to each other
1,3-Diaxial
Methyl group in axial position
Anti-Conformer Strain
No steric or torsional strain
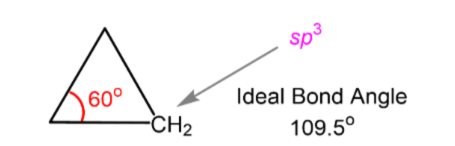
Bond Angle Strain
Deviation from expected bond angle of sp3 carbon
Torsional Strain
Always occurs in eclipsed conformations, never occurs in staggered conformations
Strains Present in Cyclopropane
BAS + TS
Strains Present in Cyclobutane
BAS + TS
Strains Present in Cyclopentane
TS
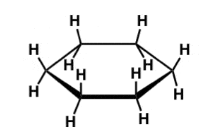
Planar Cyclohexane Strains
BAS + TS
Chair Cyclohexane Strains
No BAS, TS or SS
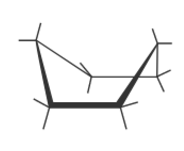
Boat Cyclohexane Strains
SS (flagpole-flagpole) + TS
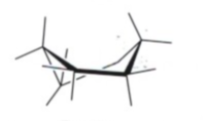
Twist Boat Cyclohexane Strains
MINIMAL BAS + TS
Half Chair Cyclohexane Strains
TS + BAS