1. Cellular Injury & Adaptation
1/152
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
153 Terms
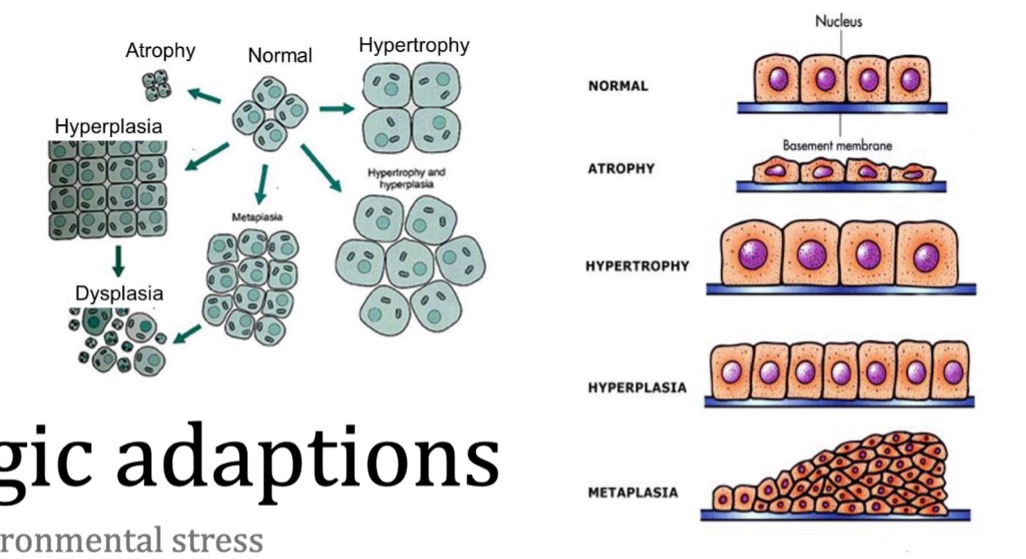
name the types of cell adaptation to environmental stress
hypertrophy
atrophy
hyperplasia
aplasia
hypoplasia
metaplasia
what is etiology?
causes of disease
what is pathogenesis?
mechanisms of disease
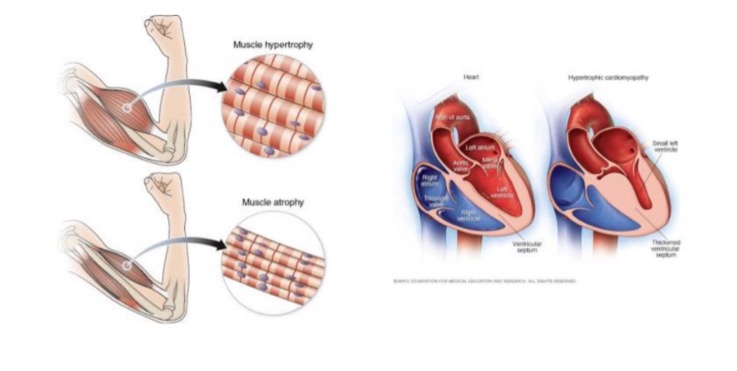
what is hypertrophy?
increase in organ/tissue size due to increased size of cells
on a cellular level, hypertrophy results in what?
increased protein synthesis
increased size or number of intracellular organelles
cellular adaptation due to increased workload
what are examples of hypertrophy?
skeletal muscle - increased mass associated with exercise
cardiac muscle - enlargement of L ventricle in hypertensive heart disease
nerve tissue
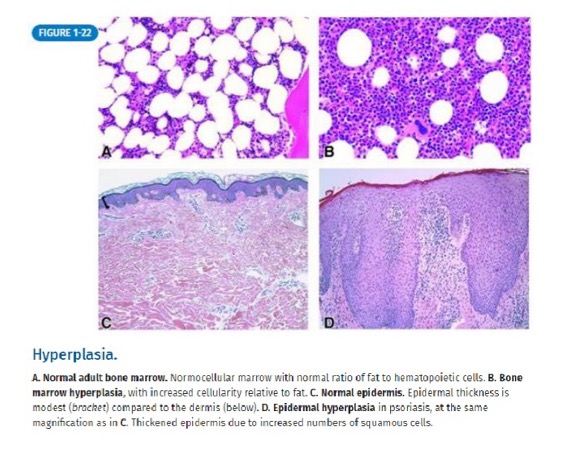
what is hyperplasia?
increased organ/tissue size due to increased number of cells
what are examples of hyperplasia?
breast during pregnancy - glandular proliferation
pathologic hyperplasia (can progress to dysplasia and cancer) - endometrial hyperplasia (exception is benign prostatic hyperplasia, which does not increase risk for prostate cancer)
what is dysplasia?
abnormal growth and development of cells within a tissue or organ
true or false: hyperplasia and hypertrophy often occur together
true
e.g. uterine enlargement in pregnancy is caused by both hypertrophy and hyperplasia of uterine smooth m. cells
true or false: an increase in stress leads to increase in organ size
true
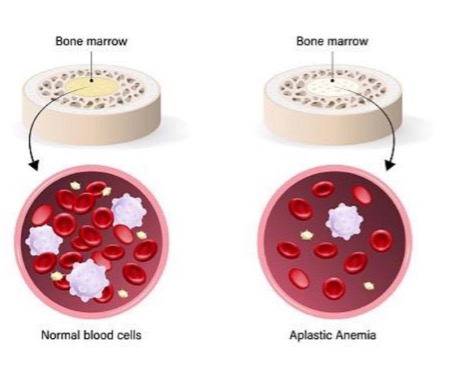
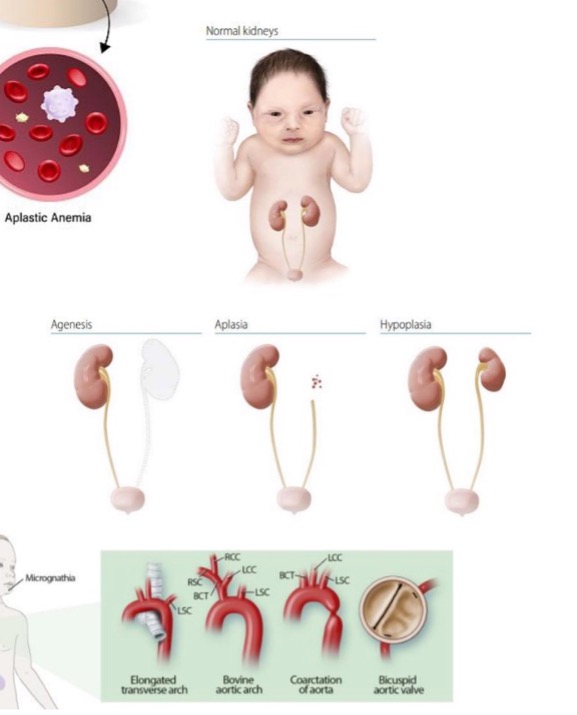
what is aplasia?
failure of cell production
“no development” (started but didn’t get far)
true or false: primordial tissue is present in aplasia
true
what is an example of aplasia?
aplastic anemia - bone marrow stem cells present but fail to produce sufficient blood cells
what is agenesis?
cell production never started
primordial tissue absent, organ not formed at all, earlier developmental failure
what is an example of agenesis?
unilateral renal agenesis (during embryogenesis)
what is hypoplasia?
decrease in cell production
(hypoplasia = “under development,” started and got further than aplasia but not all the way)
how does hypoplasia affect organ size?
small size
what is an example of hypoplasia?
streak ovary in Turner syndrome during hypoplasia
(fibrous tissue with little or no germ cells, degree of development may be defined as aplasia or hypoplasia)
(not a Q) kidney agenesis vs aplasia vs hypoplasia
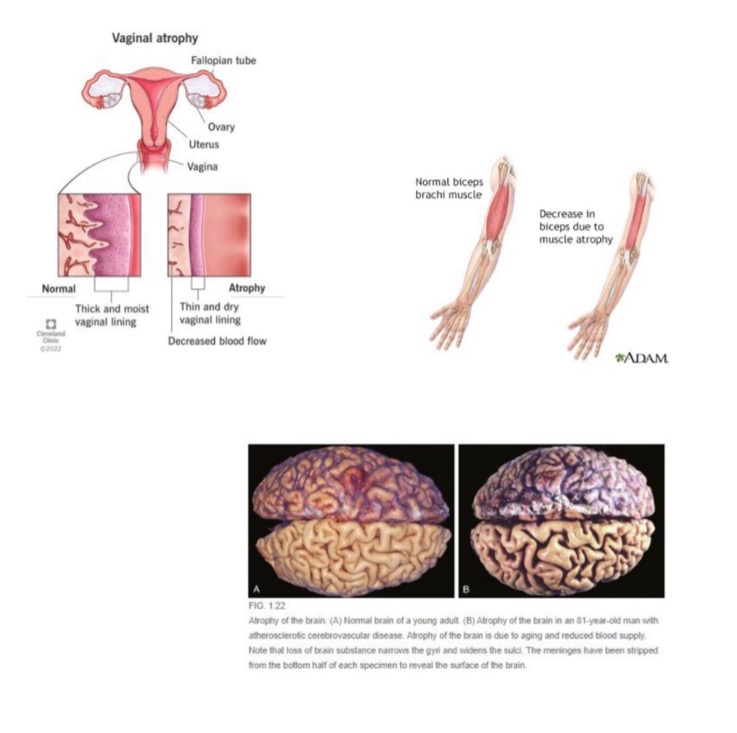
what is atrophy?
decreased organ or tissue size due to decrease in size and number of cells
true or false: atrophy refers to a reduction in size after normal, matured growth has been attained
true
what causes decrease in number of cells in atrophy?
apoptosis
what causes decrease in size of cells in atrophy?
ubiquitin-proteasome degradation of cytoskeleton
autophagy of cell components
what are some causes of atrophy?
decreased hormonal stimulation (e.g. vaginal atrophy in menopause)
denervation (nerve injury in trauma, destruction of NMJ)
decreased nutrients/oxygen/blood supply
disuse
aging
(not a Q) atrophy
what is metaplasia?
replacement of one differentiated tissue by another
metaplasia usually occurs between what cell types?
usually one surface epithelium to another (squamous, columnar, urothelial)
why does metaplasia occur?
new metaplastic tissue better able to handle new stress
can metaplasia be reversed with the removal of the driving stressor?
yes
what are examples of metaplasia?
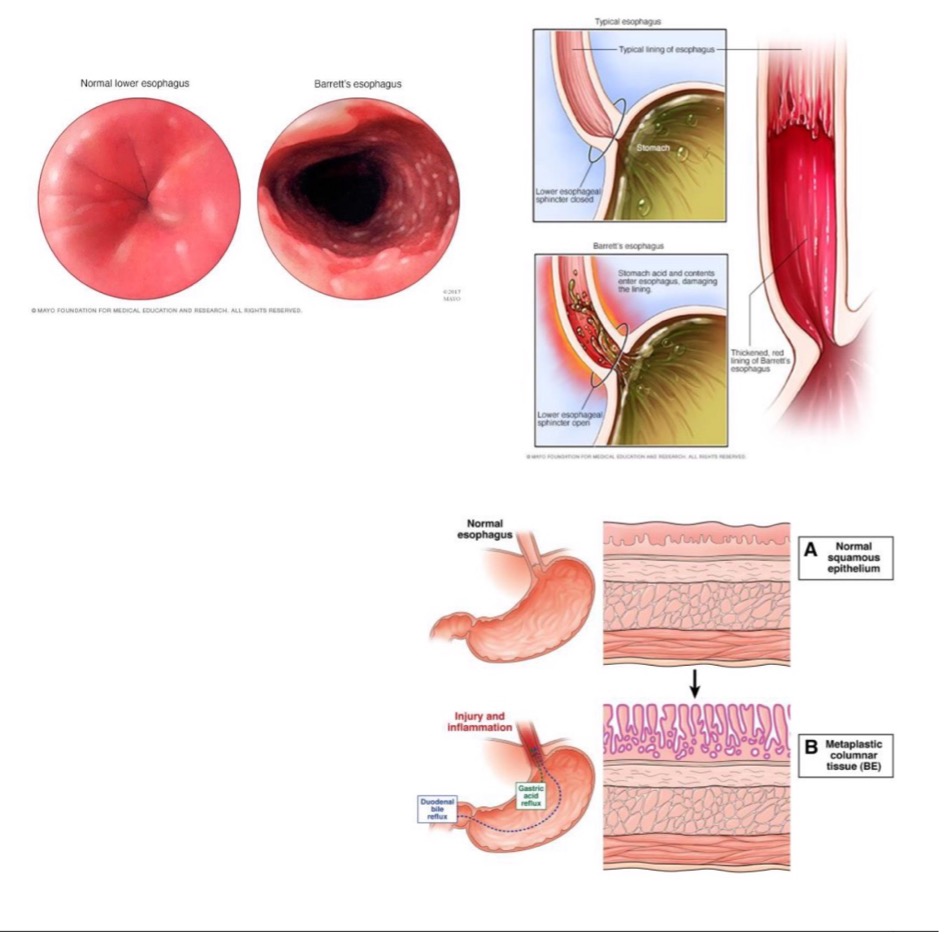
Barrett esophagus
squamous metaplasia
HPV infection of uterine cervix
how does Barrett esophagus occur?
esophagus normally lined by squamous epithelium (suited to handle friction of food bolus)
new stressor: acid reflux from stomach causes metaplasia
new cell type becomes mucin-producing columnar cells (better able to handle stress of acid)
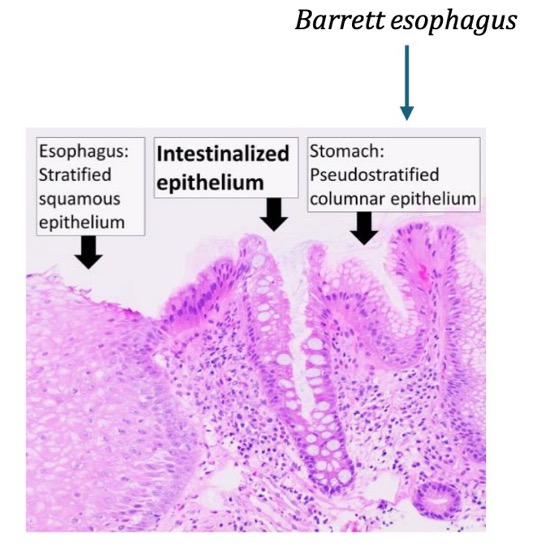
(not a Q) Barrett esophagus
how does squamous metaplasia occur?
bronchus is normally comprised of columnar epithelium
new stressor: chronic irritation from long-term tobacco use causes metaplasia
new tissue: squamous epithelium
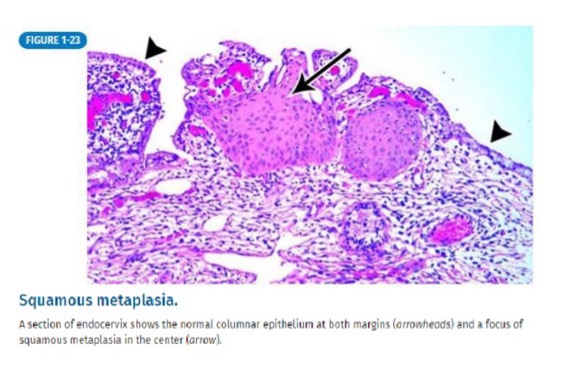
(not a Q) squamous metaplasia
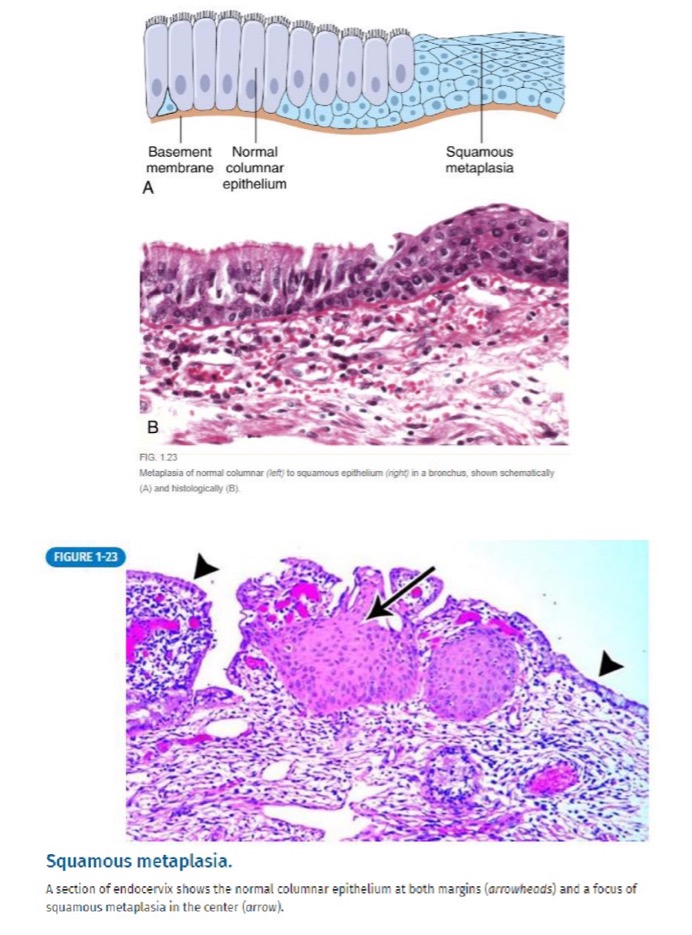
why does metaplasia occur with HPV infection of the uterine cervix?
glandular cells are normally lined by ciliated columnar epithelium
new stressor: HPV infection
new tissue: squamous epithelium
(not a Q) pathologic adaptations
name the 3 types of cellular injury
hypoxic cell injury
free radical cell injury
chemical cell injury
what may cause hypoxic cell injury?
cellular anoxia or hypoxia
name the mechanisms of hypoxic cell injury
ischemia
anemia
carbon monoxide poisoning
decreased perfusion (types of shock)
pulmonary disease
what organelle is affected first in hypoxic cell injury?
mitochondria
what occurs in the early stage of hypoxic cell injury
decreased oxidative phosphorylation and ATP synthesis
what are some consequences of decreased oxidative phosphorylation and ATP synthesis in early hypoxic cell injury?
failure of Na+/K+ pump
cellular and organelle swelling
increased buildup of Ca2+ in cell due to malfunction of Ca2+ pump
stimulation of phosphofructokinase activity (increased anaerobic glycolysis → lactic acid accumulation → decreased intracellular pH → denatures proteins and clumps nuclear chromatin)
what occurs in the late stage of hypoxic cell injury?
membrane damage (plasma membrane/organelles)
what are some consequences of membrane damage in late-stage hypoxic cell injury?
loss of membrane phospholipids
cell blebs (cell-surface deformity)
additional Ca2+ entering cell
intracellular enzymes/proteins released from necrotic cells into circulation
what is the “point of no return” in hypoxic cell injury?
cell death
what causes cell death in hypoxic cell injury?
irreversible damage to cell membrane
massive Ca2+ influx → calcification of mitochondria
true or false: vulnerability to irreversible hypoxic injury is dependent on tissue/cell type
true
how much time does it take for neurons to become vulnerable to irreversible hypoxic injury?
3-5 minutes
what types of neurons are most susceptible to irreversible cell death due to hypoxic injury?
Purkinje cells of cerebellum
neurons of hippocampus
how much time does it take for myocardial cells and hepatocytes to become vulnerable to irreversible hypoxic injury?
1-2 hours
(think 90-minute window for “door to balloon” for heart attack)
how much time does it take for skeletal muscle to become vulnerable to irreversible hypoxic injury?
4-6 hours
(limb tourniquet application recommended for max 4-6 hours before necrosis sets in)
what are free radicals?
molecules with a single unpaired electron in its outer orbital
what forms free radicals?
free radicals are the activated products of oxygen reduction reactions
name the mechanisms through which free radicals may cause cellular injury
peroxidation of lipids
oxidation of DNA and proteins
DNA damage implicated in aging and oncogenesis
name the mechanisms that generate free radicals
normal metabolism
oxygen toxicity
ionizing radiation
UV light
drugs/chemicals (e.g. barbiturate intoxication)
re-perfusion after ischemic injury
name the mechanisms that degrade free radicals
intracellular enzymes
exogenous/endogenous antioxidants
metal carrier proteins
spontaneous decay
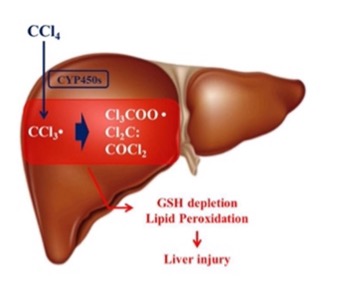
what is an example of chemical cell injury?
liver cell membrane damage caused by carbon tetrachloride (organic solvent used in dry cleaning in 1900s)
CCl₄ converted to CCl₃ → cell injury
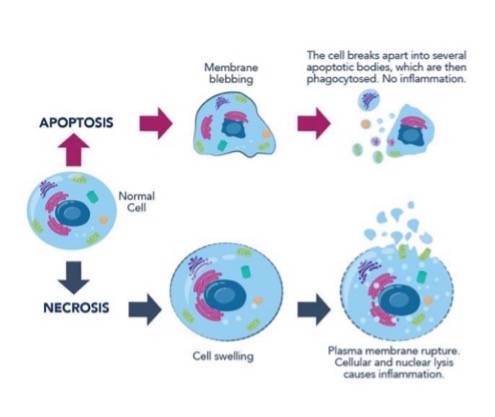
what are the types of cell death?
necrosis
apoptosis
what 3 things may happen to chromatin in cell death?
pyknosis
karyorrhexis
karyolysis
what is pyknosis?
condensation of chromatin in nucleus
in what type of cell death does pyknosis occur?
more characteristic in apoptosis but also happens in necrosis
what is karyorrhexis?
destructive fragmentation of nucleus
in what type of cell death does karyorrhexis occur?
apoptosis and necrosis
what is karyolysis?
complete dissolution of chromatin
in what type of cell death does karyolysis occur?
only necrosis
does NOT occur in apoptosis
name some characteristics of necrosis
enlarged cell size
pyknosis → karyorrhexis → karyolysis
disrupted plasma membrane
enzymatic digestion
frequent adjacent inflammation
name some characteristics of apoptosis
reduced cell size
fragmentation of nucleus into nucleosome-sized fragments
intact plasma membrane but altered structure
intact cellular contents, may be released in apoptotic bodies
no adjacent inflammation
is necrosis pathologic or physiologic?
invariable pathologic (culmination of irreversible cell injury)
is apoptosis pathologic or physiologic?
often physiologic (means of eliminating unnecessary cells)
may be pathologic after some forms of cell injury
what is necrosis?
degradative and inflammatory reactions occurring after tissue death
what is autolysis?
cellular degradation caused by intracellular enzymes
what is heterolysis?
cellular degradation by enzymes derived from extrinsic sources (bacteria or leukocytes)
name the types of necrosis
coagulative necrosis
liquefactive necrosis
caseous necrosis
gangrenous necrosis
fibrinoid necrosis
fat necrosis
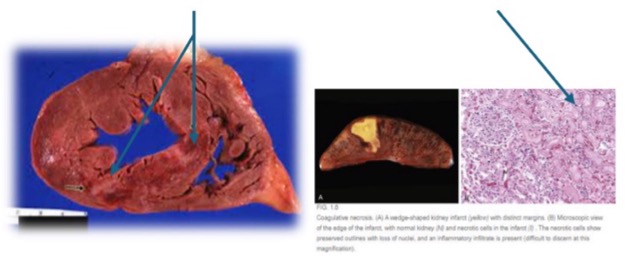
what occurs in coagulative necrosis?
tissue remains firm, cell shape/organ structure preserved
nucleus disappears (pyknosis, karyorrhexis, karyolysis)
what preserves cell shape/organ structure in coagulative necrosis?
coagulation of proteins
what causes coagulative necrosis?
ischemia
what are examples of coagulative necrosis?
heart or kidney necrosis (supplied by end arteries with limited collateral circulation)
anemic infarcts (white appearance)
(not a Q) coagulative necrosis
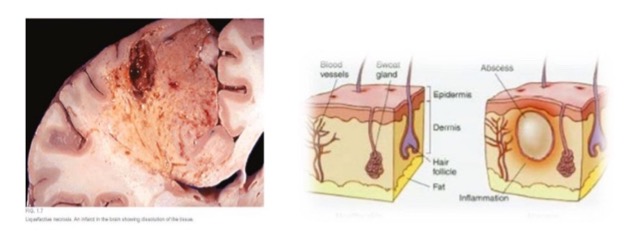
what is liquefactive necrosis?
necrotic tissue becomes liquefied by autolysis
enzymatic lysis of cells/protein
what causes liquefactive necrosis?
ischemia
what are examples of liquefactive necrosis?
CNS/brain
abscess (pus, localized collection)
(not a Q) liquefactive necrosis
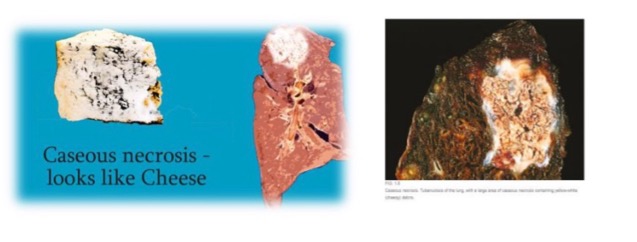
what is caseous necrosis?
a combination of coagulative and liquefactive necrosis
architecture not preserved but tissue not liquefied
what causes caseous necrosis?
granulomatous inflammation
infection of T lymphocytes, macrophages, and cytokines
what does caseous necrosis visually look like?
cottage cheese-like (caseous) appearance
what is an example of caseous necrosis?
tuberculosis
(not a Q) caseous necrosis
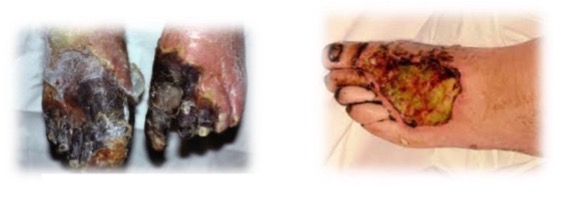
what is gangrenous necrosis?
not a distinctive pattern of cell death; refers to necrosis of a limb/bowel
what are the subtypes of gangrene?
wet gangrene
dry gangrene
what does wet gangrene involve?
includes infective heterolysis and liquefactive necrosis
what is usually involved in wet gangrene?
bacterial infection
what does dry gangrene involve?
includes coagulative necrosis without liquefaction
what causes dry gangrene?
usually ischemia without bacterial infection
true or false: vascular occlusion in lower extremity or bowel is a common cause of gangrene
true
(not a Q) gangrenous necrosis
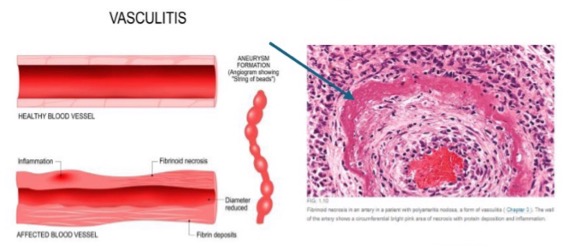
what is fibrinoid necrosis?
deposition of fibrin-like proteinaceous material in arterial walls
what is fibrinoid necrosis associated with?
immune-mediated vascular damage
what may cause fibrinoid necrosis?
malignant hypertension
vasculitis
(not a Q) fibrinoid necrosis