Biochemistry Basics POGIL
1/41
Earn XP
Description and Tags
AP Bio flashcards from ms sys assignment
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
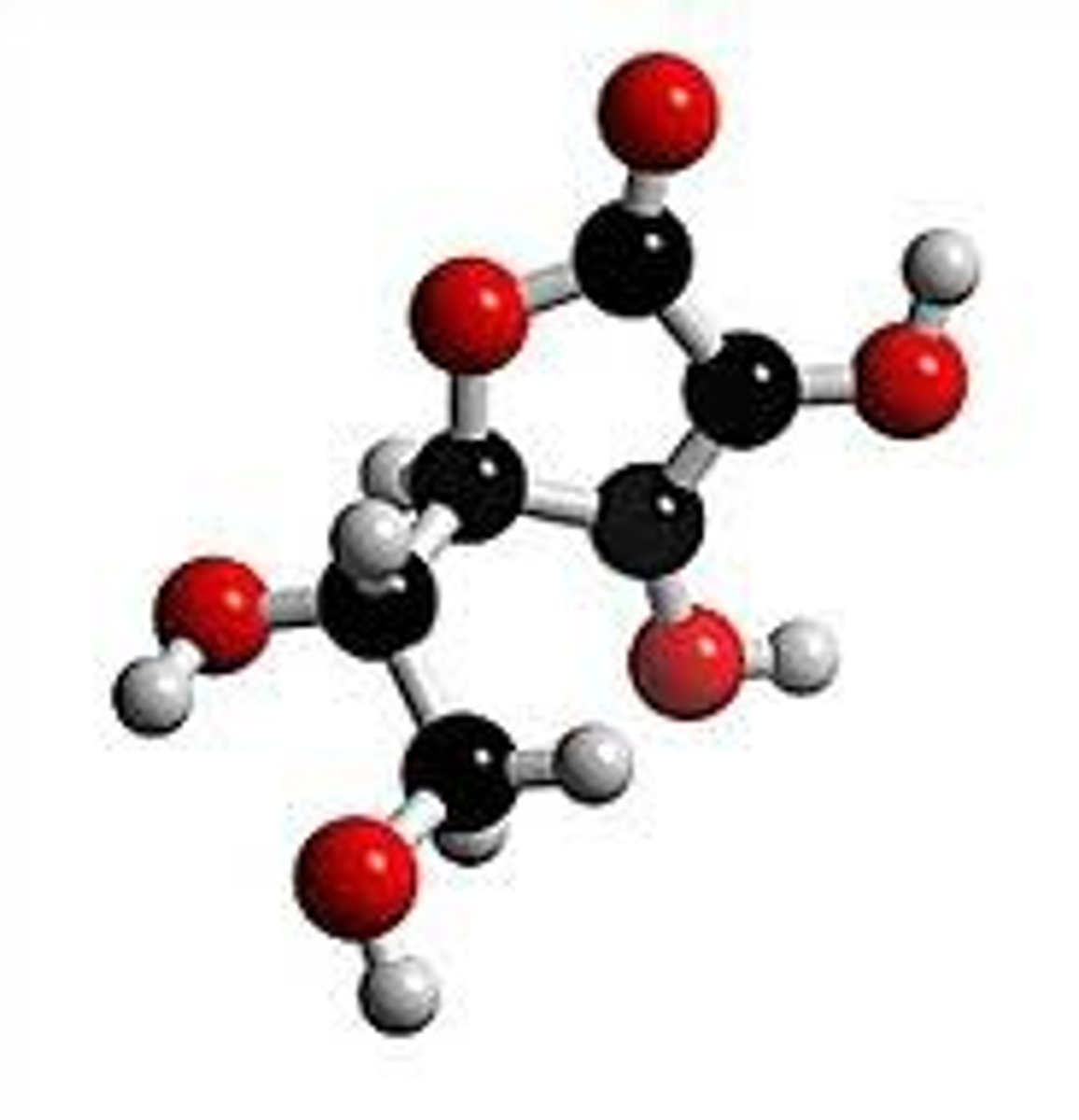
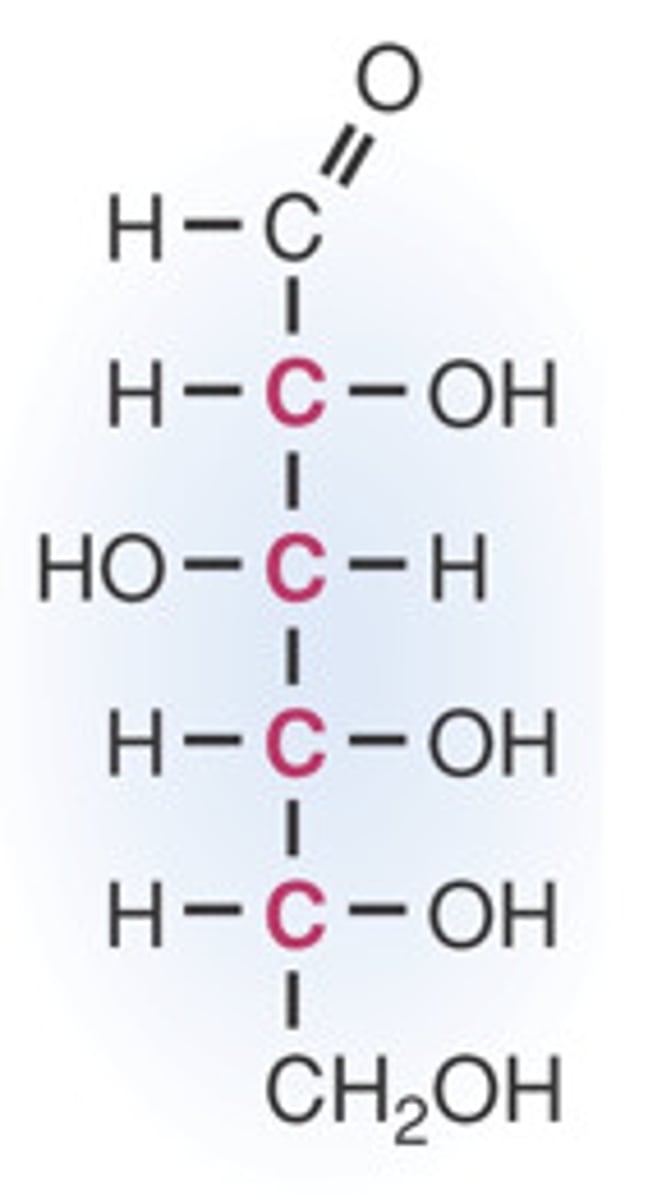
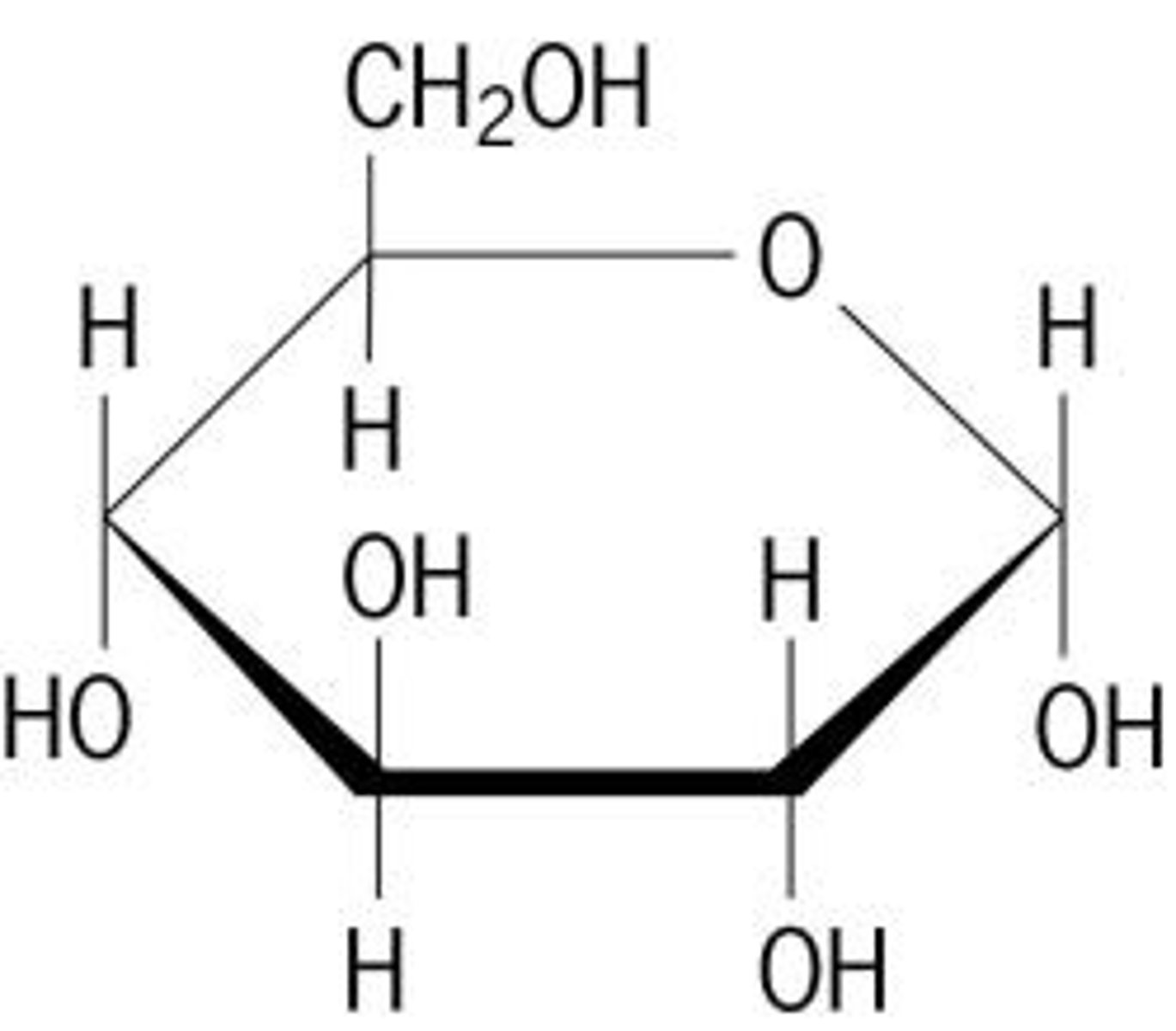
ball and stick, lewis structure, line drawing
molecular drawings
ball and stick
lewis structure
line drawing
4
# of bonds carbon can form
ball and stick
molecular drawing that shows the shape
carbon and hydrogen
elements that are missing from line drawings
points
where are carbons in the line drawing?
line
simplest molecular drawing
nitrogen and oxygen
elements that make a molecule polar
very electronegative
why do oxygen and nitrogen make molecules polar
yes just fewer
can nonpolar molecules have oxygen and nitrogen atoms?
polarities are similar
things will dissolve best when their
polar
is water nonpolar or polar
nonpolar
is oil polar or nonpolar
polar molecules
dissolves well in water
nonpolar molecules
dissolves well in oil
hydrophilic
polar molecule
hydrophobic
nonpolar molecule
water loving
hydrophilic
water fearing
hydrophobic
acid
A substance that increases the hydrogen ion concentration of a solution, lactic acid donate a hydrogen proton to water
base
A substance that decreases the hydrogen ion concentration in a solution, takes a hydrogen proton
<7
the pH of a lactic acid solution
7
pH of an amino acid
>7
pH of dopamine
7
pH of lactose
solid lines
covalent bonds are represented by
dotted lines
hydrogen bonds are represented by
polar
type of molecule that will form hydrogen bonds with itself or water
hydroxyl
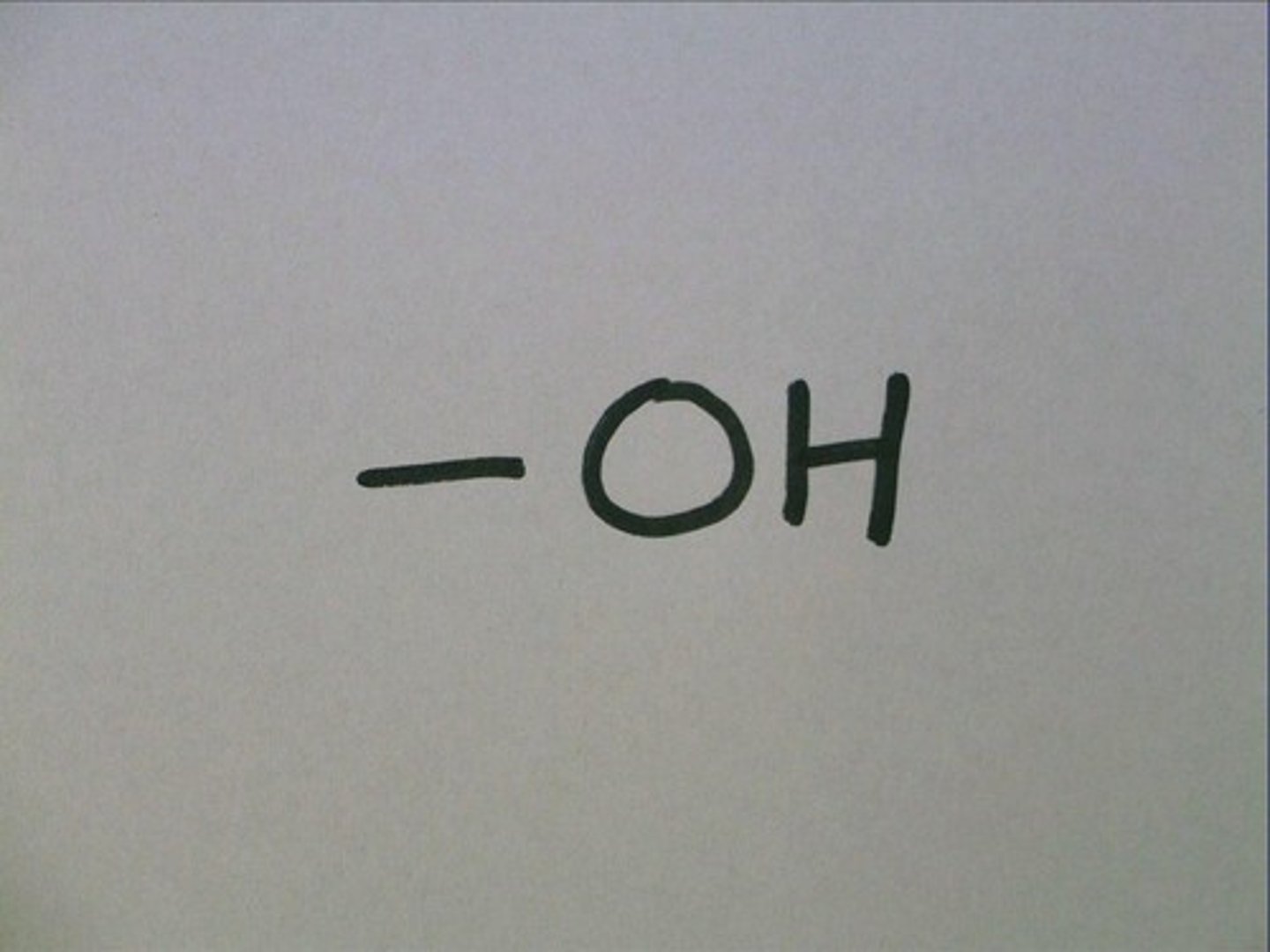
carboxyl
-COOH
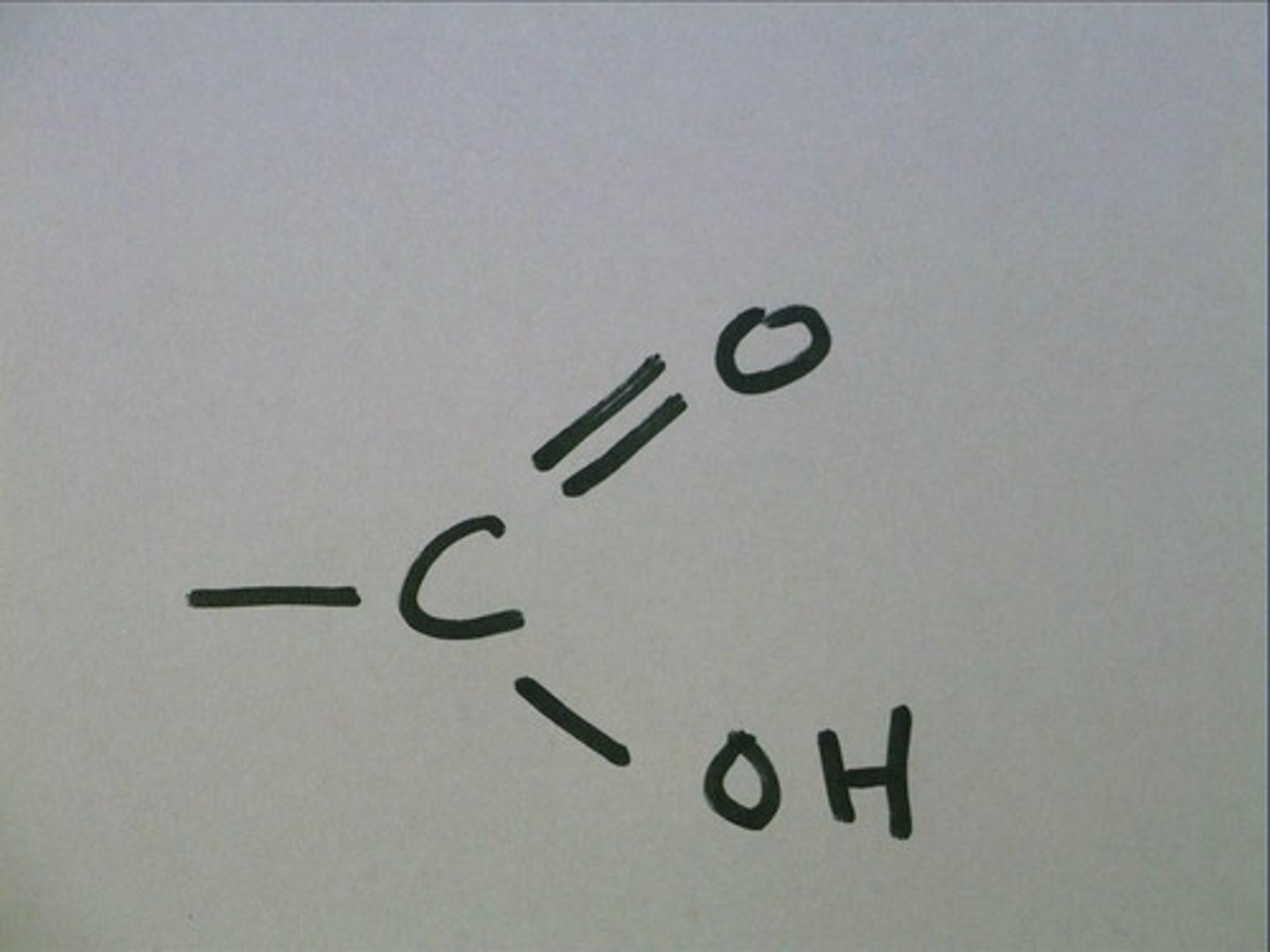
carbonyl
C=O
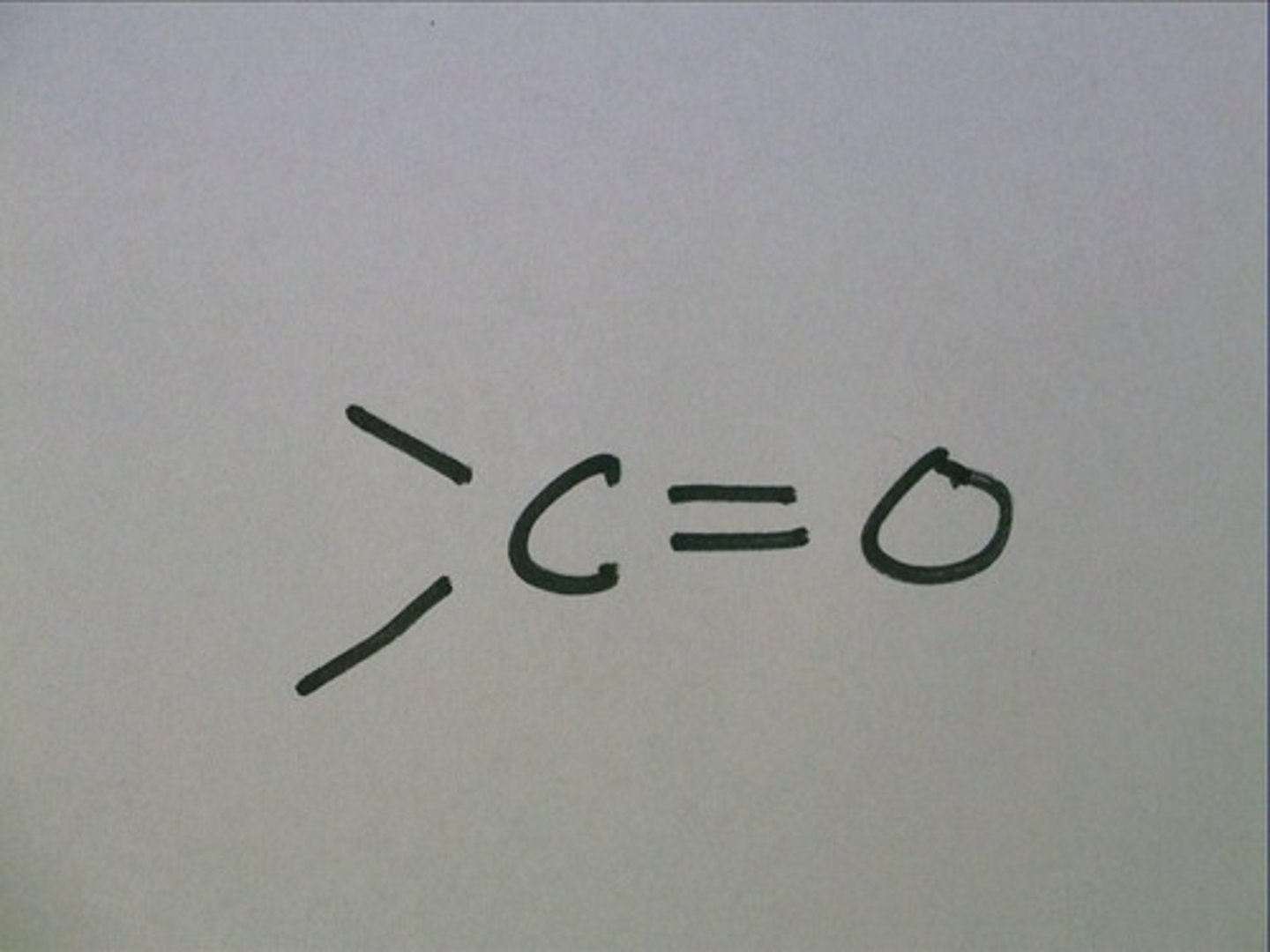
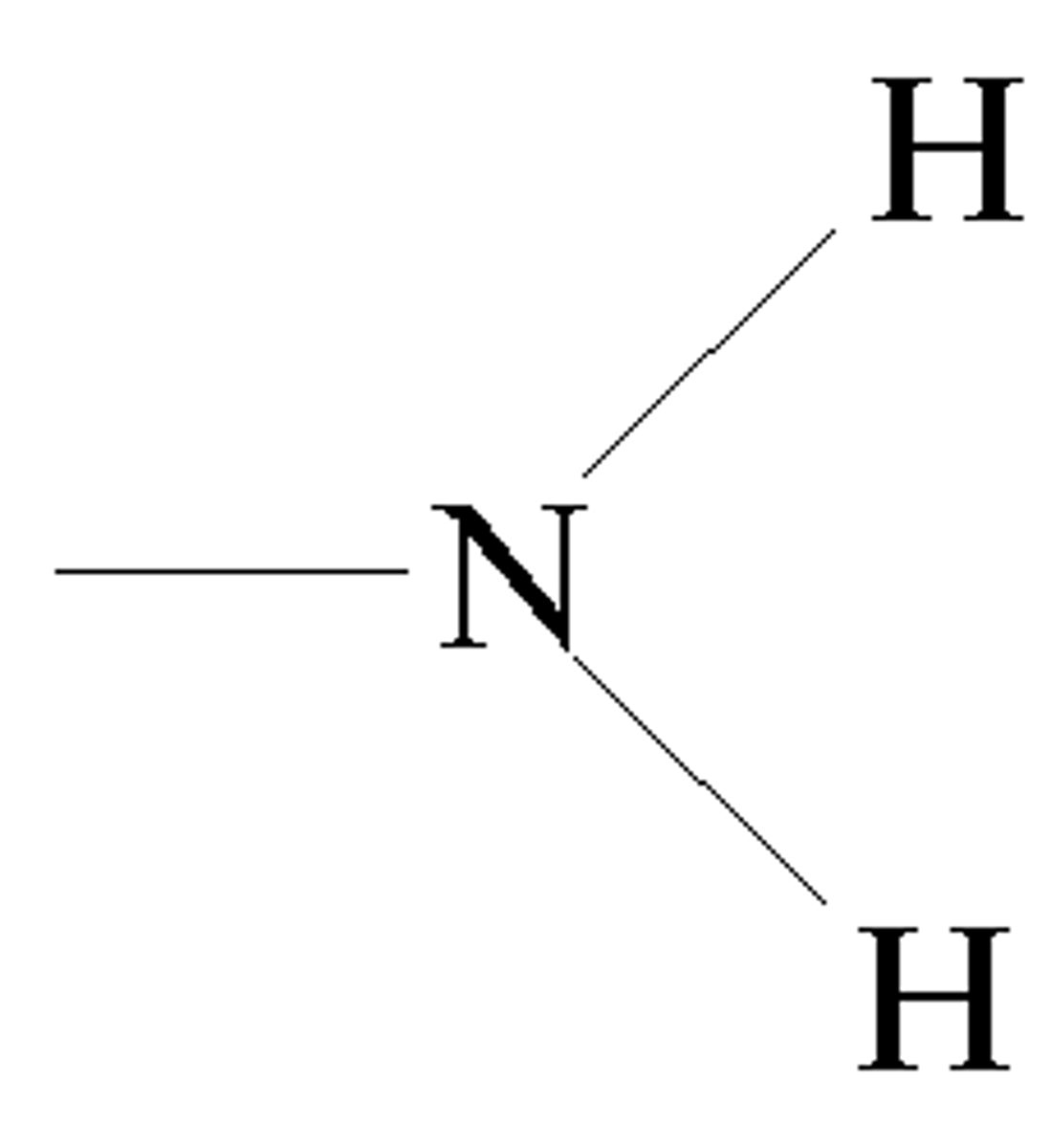
amino
NH₂
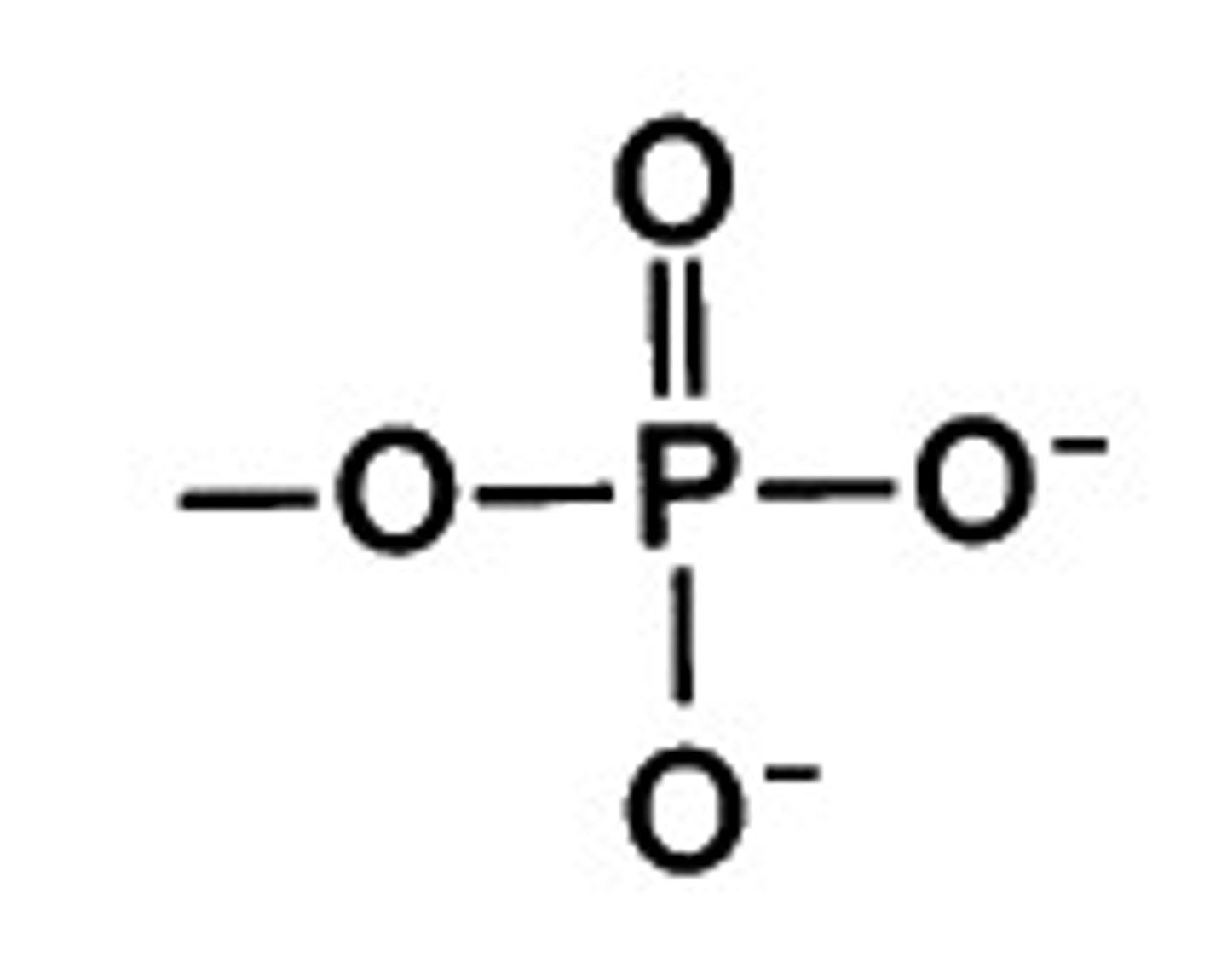
phosphate
-PO₃
sulfhydryl
-SH
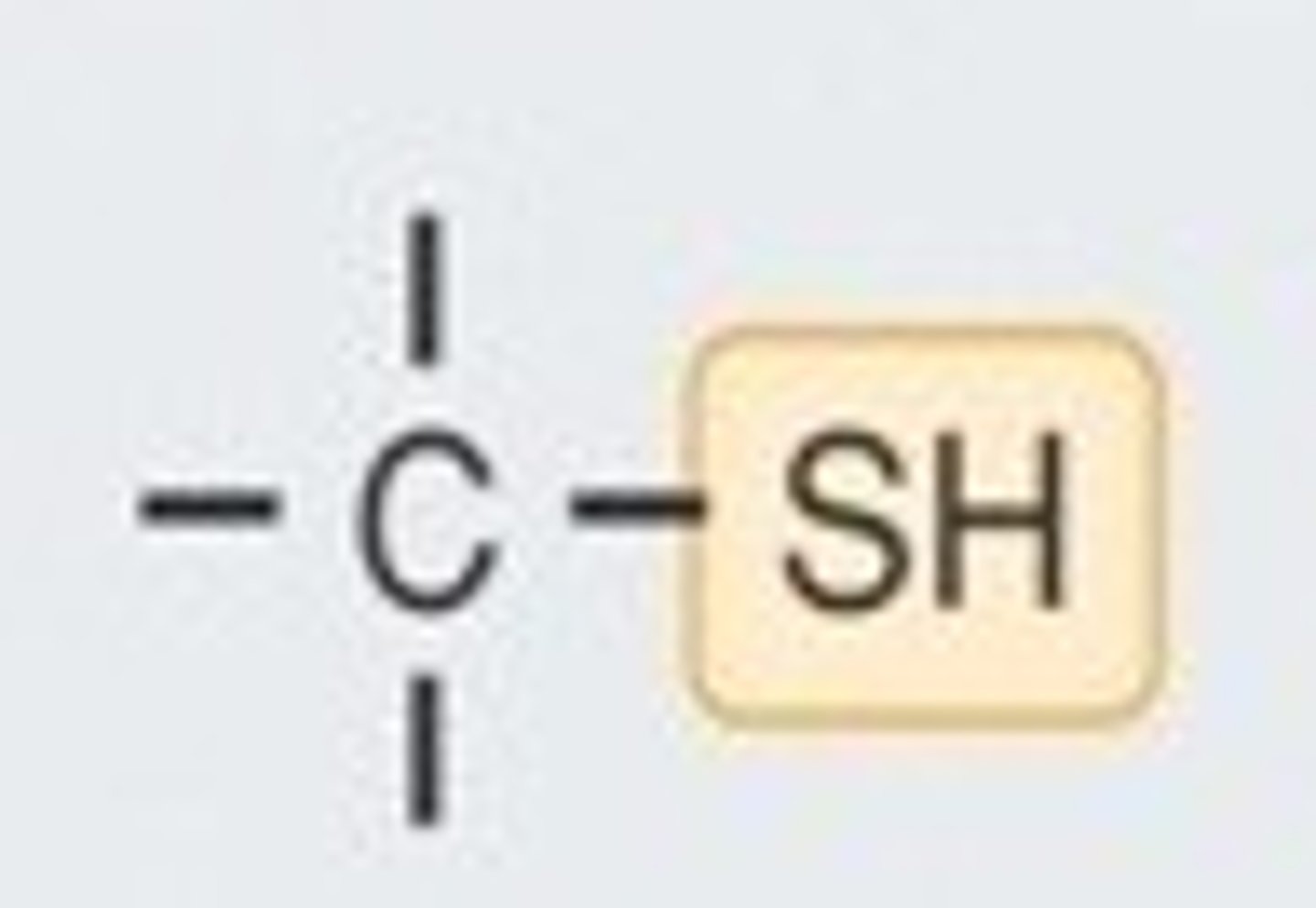
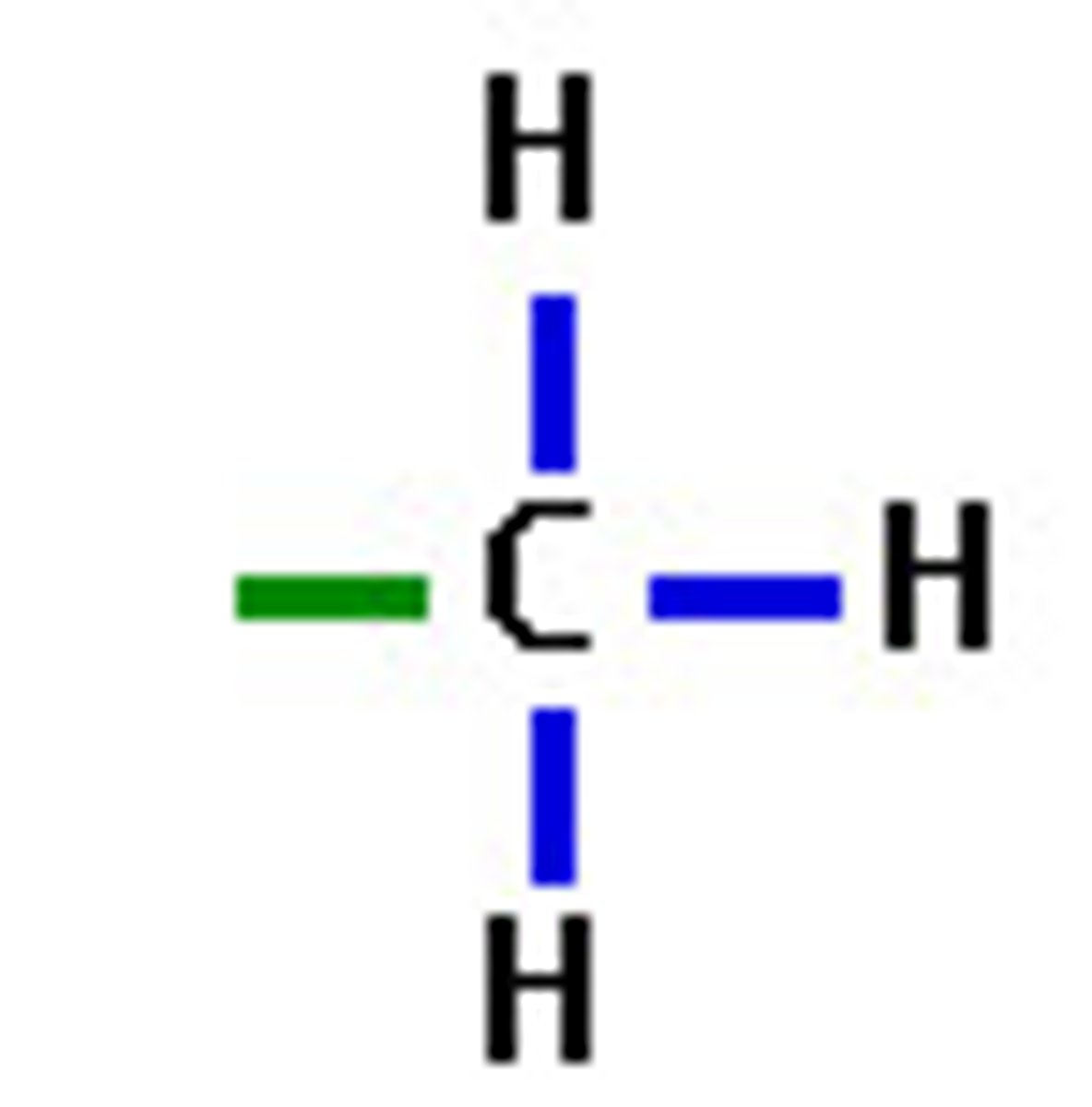
methyl
-CH₃
hydroxyl
alcohol, and other things that end in -ol contain; helps dissolve molecules such a sugars, polar, increases solubility
carboxyl
fatty acids and sugars; acidic properties because it is a source of H+, polar
carbonyl
ketones and aldehydes, polar
ketone
double bonds in middle
aldehydes
double bonds on end