3.8 aldehydes and ketones
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
describe oxidation reactions of aldehydes
relatively easy to oxidise, even by weak oxidising agents like Cr2O7/H+ and Tollen’s reagent
readily oxidised to carboxylic acids
aldehyde + [O] → carboxylic acid
during oxidation, a H atom is removed from the C atom double bonded to O
describe the conditions required when oxidising aldehydes
heat under reflux with excess oxidant to ensure as much aldehyde as possible is oxidised to carboxylic acid
heat and distil to purify carboxylic acid
describe oxidation reactions of ketones
cannot be oxidised easily, only oxidised by powerful oxidising agents
the C atom double bonded to O is not bonded to any H atoms, so no H atoms are available to remove during oxidation
how can you distinguish between an aldehyde and a ketone?
warm with Tollen’s reagent
with aldehyde: colourless to silver mirror
with ketone: no visible change
warm with Fehling’s solution
with aldehyde: blue to brick red precipitate
with ketone: no visible change
describe the redox reactions that occur when an aldehyde is reacted with Tollen’s reagent
aldehyde is oxidised by Ag+ to carboxylic acid
aldehyde + [O] → carboxylic acid
Ag+ is reduced by aldehyde to metallic silver
Ag+ + e- → Ag
describe the redox reactions that occur when an aldehyde is reacted with Fehling’s solution
aldehyde is oxidised by Cu2+ to carboxylate ion under alkaline solutions (the solution is alkaline)
aldehyde + [O] → carboxylate ion-
blue Cu2+ is reduced by aldehyde to brick-red Cu+
Cu2+ + e- → Cu+
![<p>aldehyde is oxidised by Cu<sup>2+</sup> to carboxylate ion under alkaline solutions (the solution is alkaline) </p><ul><li><p><span>aldehyde + [O] → carboxylate ion</span><sup><span>-</span></sup></p></li></ul><p></p><p></p><p>blue Cu<sup>2+</sup> is reduced by aldehyde to brick-red Cu<sup>+</sup> </p><ul><li><p>Cu<sup>2+</sup> + e<sup>-</sup> → Cu<sup>+</sup></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/323d218c-9698-4756-87fa-bca1abe8ee6b.jpg)
why is Tollen’s reagent used over the less expensive acidified potassium dichromate (VI) as the oxidising agent to test for aldehydes?
dichromate(VI) will also oxidise primary and secondary alcohols
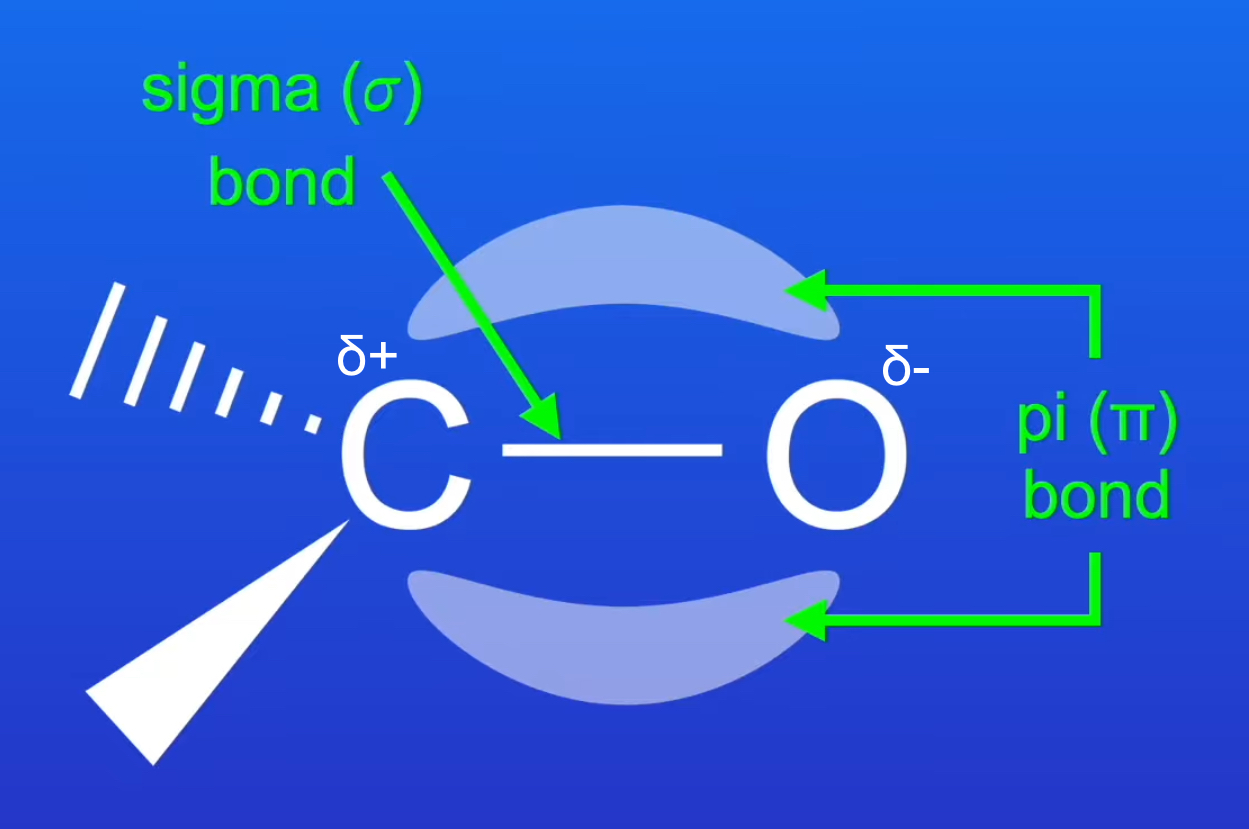
describe the structure and bonding in a carbonyl group
C=O double bond consists of a sigma bond and a pi bond.
the pi bond is formed from the sideways overlap of a 2p orbital from both the C atom and the O atom.
C=O bond is polar: O is δ- as O is more electronegative so attracts the electron density in the double bond towards itself, C is δ+
what is a nucleophile?
an electron pair donor
why are aldehydes and ketones able to undergo nucleophilic addition?
the C=O bond is polar, as O is more electronegative than C
the lone pair on the nucleophile is attracted and donated to the δ+ C
what products are formed when aldehydes and ketones are reduced by nucleophilic addition using NaBH4?
aldehydes reduced to primary alcohols
ketones reduced to secondary alcohols
what conditions are needed to reduce aldehydes and ketones by NaBH4?
warm with aqueous NaBH4
NaBH4 provides hydride ion H-
H2O provides hydrogen ion, H+
what are the equations for the reduction of aldehydes and ketones by nucleophilic addition using NaBH4?
aldehyde + 2[H] → primary alcohol
ketone + 2[H] → secondary alcohol
above the arrow: NaBH4/H2O
[H] represents the reducing agent
what is the chemical name for NaBH4?
sodium borohydride
also sodium tetrahydridoborate (III)
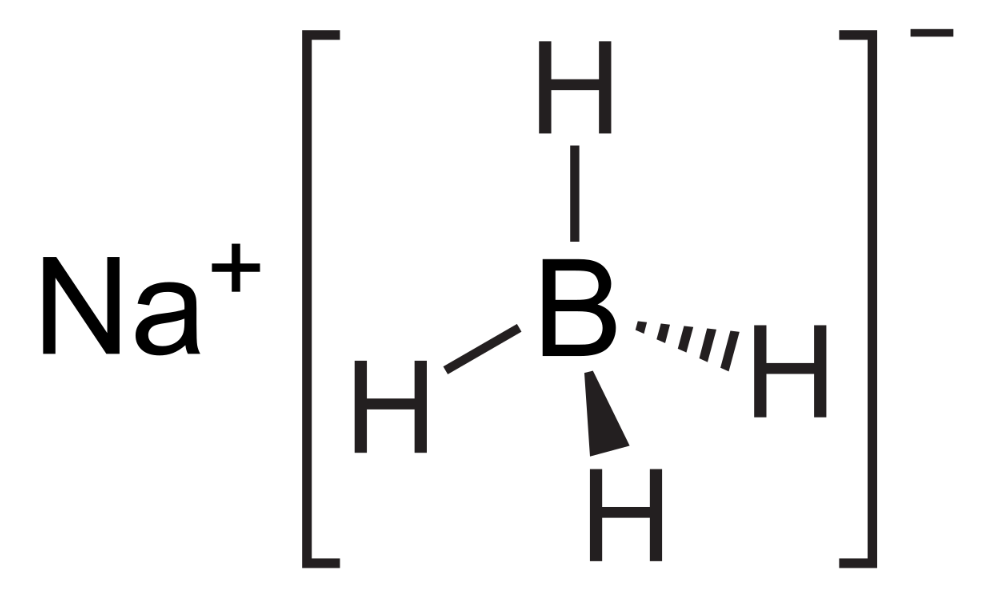
why don’t alkenes react with aqueous NaBH4?
H- ion acts as a nucleophile
alkenes have electron rich C=C
H- nucleophile is repelled by C=C / C=C only attacked by electrophiles
what products are formed when aldehydes and ketones are reduced by nucleophilic addition using HCN?
hydroxynitriles, hydroxyalkylnitriles
what conditions are needed to reduce aldehydes and ketones by HCN?
KCN and dilute acid, aqueous conditions
KCN provides cyanide ion CN-
dilute acid provides hydrogen ion H+
what are the equations for the reduction of aldehydes and ketones by nucleophilic addition using HCN?
aldehyde or ketone + HCN —> hydroxynitrile or hydroxyalkylnitriles
above the arrow: KCN/H2SO4
what are the hazards of using HCN?
HCN cannot be used directly as it:
is very toxic / poisonous
is hard to store as a gas
reacts to produce dangerous byproducts
KCN/H2SO4 used to generate HCN in the reaction mixture
what the pros and cons of using KCN instead of HCN?
KCN dissociates better than HCN to provide CN- nucleophile (HCN weak / [CN-] too low)
reaction with HCN is very slow
KCN is very toxic so the reaction is not carried out in the lab
why is this reaction extremely useful in organic synthesis?
it increases the carbon chain length
the product contains 2 reactive functional groups: hydroxyl (OH) and nitrile (CN)
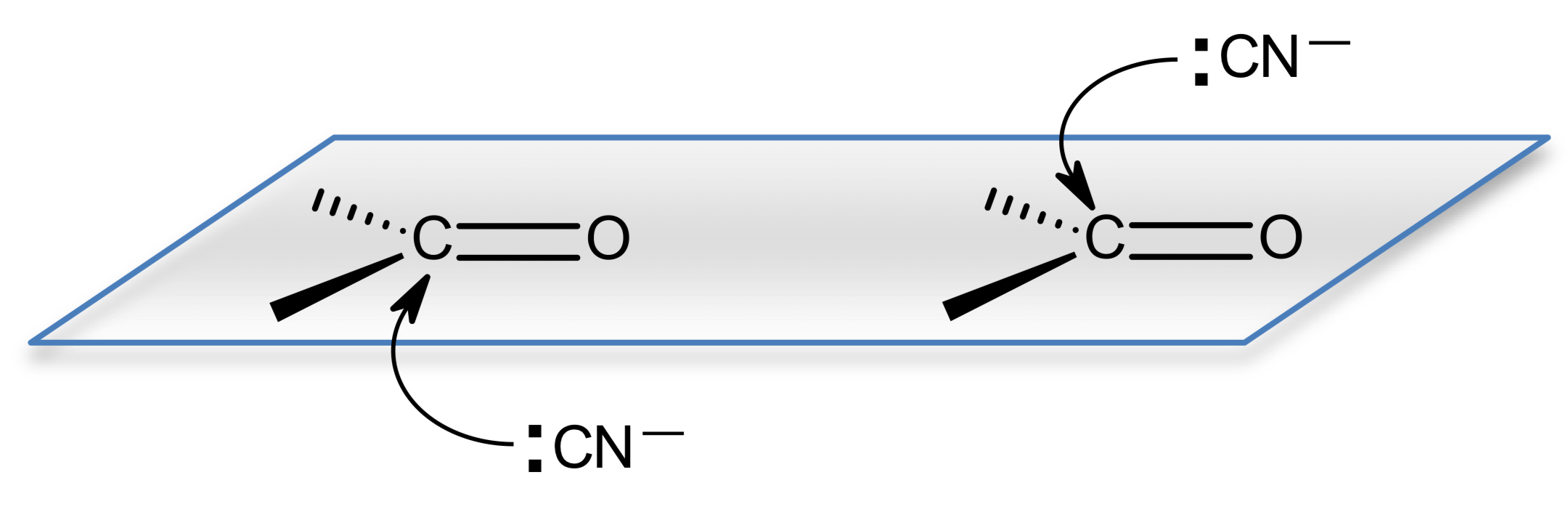
what happens when aldehydes and unsymmetrical ketones react with KCN/H+?
why does this happen?
they form mixtures of enantiomers because the product has a chiral centre
carbonyl group is planar
equal chance of CN- nucleophile attacking from above or below
equal amounts of both enantiomer is formed so a racemate is formed
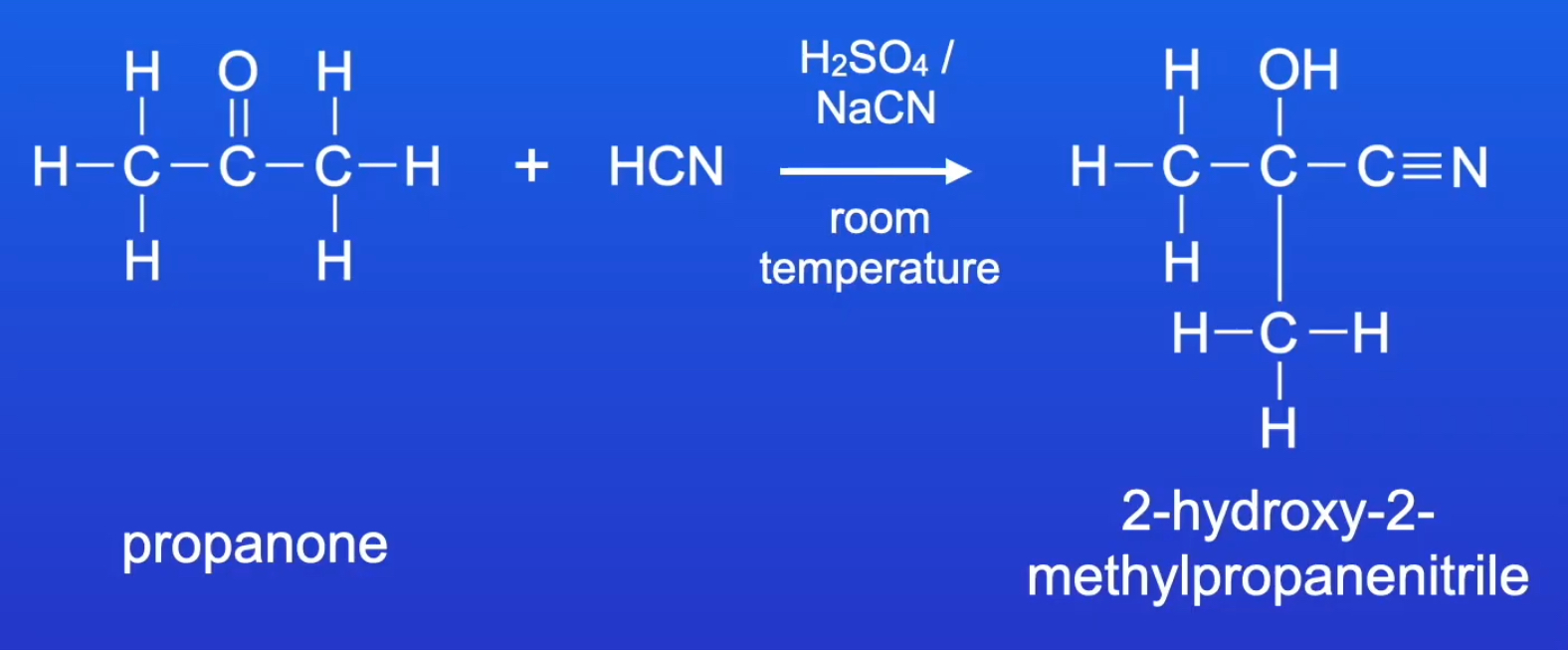
predict the product formed when propanone reacts with KCN/H2SO4
give the IUPAC name and the structural formula
2-hydroxy-2-methyl-propanenitrile
C(CH3)2(OH)CN
what is the structural formula of 2-hydroxybutanenitrile?
CH3CH2CH(OH)CN
how would the rate of reaction of HCN with propanone compare with the rate of reaction with propanal?
slower with propanone:
C of C=O is less δ+ because alkyl groups are electron-releasing / have positive inductive effect so hinder attack by :CN- nucleophile
faster with propanal:
easier to attack end of the chain