BIOL10002: Topic 2 Cells and Energy
1/142
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
143 Terms
a chemical reaction occurs when…
atoms have sufficient energy to combine or change their bonding partners
energy
the capacity to do work
occurs when a force operates on an object over a distance
bioenergetics
the study of how organisms manage their energy resources
forms of energy (5)
chemical-bond: stored in bonds
electrical: separation of charges
heat: transfer dur to temperature difference
light: electromagnetic radiation stored as photons
mechanical: energy of motion
types of energy in biology can be categorised as…
potential or kinetic energy
potential energy
the energy of state or position
can be stored in covalent bonds, as concentration gradient, as electrical charge imbalance
kinetic energy
energy of movement that does work or makes things change
heat causes molecular motions and can break chemical bonds
thermodynamics
the study of energy transformations
thermodynamics - closed system
In a closed system is isolated from its surroundings
thermodynamics - open system
energy and matter can be transferred between the system and its surroundings
most of the time biological systems are open systems as organisms exposed to external environments
first law of thermodynamics
energy can be transferred and transformed but cannot be created or destroyed
principle of conservation of energy
second law of thermodynamics
every energy transfer or transformation increases the entropy of the universe
during every energy transfer, some energy is unusable (often lost as heat)
role of ATP
Captures and transfers free energy
Can be hydrolysed to ADP and Pi -> releases a lot of energy for endogonic reactions
Can also phosphorylate other molecules -> molecules gain some energy
structure of ATP
three phosphate groups bonded to a carbon if a ribose molecules → nucleotide
end bond in triphosphate group can by hydrolysed to produce an inorganic phosohate → ADP, inorganic phosphate, free energy
exergonic reaction w example
release of energy
hydrolysis of ATP produces ADP, inorganic phosphate and energy
endergonic reactions w example
input of energy
condensation of ADP and inorganic phosphate produces ATP
ATP for chemical work example
Glutamic acid to glutamine = endergonic → not spontaneous
conversion reaction coupled with ATP hydrolysis → phosphorylated intermediate
ATP phosphorylates glutamic acid → less stable with more free energy
ammonia displaces phosphate group → glutamine formed
overall delta G = negative
ATP for kinetic work example - transport work
ATP hydrolysis causes shape and binding affinity change in some proteins embedded in cellular membrane
allows transport of solutes
ATP for kinetic work example - mechanical work
ATP binds noncovalently to motor proteins → hydrolysed
causes shape change that ‘walks’ the motor protein forward along cytoskeletal track
coupling reactions with ATP
ATP hydrolysis = exergonic and provides the input of energy for endergonic reactions to proceed
change in free energy always negative → more energy has to be released than consume
biological order and disorder
cells create ordered structures from less ordered materials
organisms also replace ordered forms of matter with energy with less ordered forms
energy flows into an ecosystem in the form of life and exits in the form of heat
How does the evolution of more complex organisms not violate the second law of thermodynamics
entropy may decrease in an organism but the total entropy of the universe increases → the more we try to order cells or the inside of living organisms → the more energy is released into the universe, increasing chaos
what is free energy
the amount of energy available to do work
all chemical reactions affect free energy
measures the instability of a system → tendency to change to a more stable state
equilibrium and energy
equilibrium =state of max stability
spontaneous reactions can perform only when it is moving towards equilibrium
ATP for chemical work → glutamic acid to glutamine (no atp)
endergonic reaction → not spontaneous
glutamic acid + ammoni → glutamine delta G = +ve kJ/mol
ATP for chemical work → glutamic acid to glutamine coupled with ATP hydrolysis steps
2 steps coupled by phosphorylation intermediate → ATp phosphorylates glutamic acid → less stable with more free energy → ammonia displaces phosphate group → glutamine, ADP, Pi
ATP for chemical work → glutamic acid to glutamine coupled with ATP hydrolysis free energy change
exergonic reaction → spontaneous
delta G (Glu) + delta G (ATP) = delta G (net) => -ve kJ/mol
ATP for kinetic work key example (2)
ATP hydrolysis can cause changes in the shapes and binding affinity of proteins
transport work
mechanical work
ATP for kinetic work - transport work
can change the shape of a protein embedded in the cellular membrane by phosphorylating it → shape change allows transport oof solutes
ATP for kinetic work - mechanical work
ATP binds non-covalently to motor proteins → hydrolysed
causes shape change that walks the motor protein forward
coupling of reactions
endergonic reactions often coupled with exergonic reactions so that energy produced from hydrolysis of ATP can push the endergonic reaction
delta G is always negative as overall reaction has to be exergonic
catabolic reactions w example
may break down an ordered structure into smaller more randomly distributed products
Those that release energy = exergonic
hydrolysis of sucrose into glucose and fructose
anabolic reactions w example
may make a single highly ordered product out of many smaller less ordered reactants
those that absorb free energy = endergonic
synthesis of sucrose from glucose and fructose
equilibrium and metabolism (closed v open systems)
reactions in a closed system eventually reach equilibrium and then do no work
cells are not in equilibrium → open systems experience constant flow of materials
metabolism is never at equilibrium
define cellular metabolism
a series of numerous chemical reactions that are occurring to enable cells to exist
occurs within the cell
enzymes are the only biological catalysts
False
enzymes as biological catalysts
act as a framework where reactions can take place by reducing activation energy → more unstable forms with higher free energy
increases proportion of reactants in transition state
process of enzyme catalysts
substrate approaches enzyme’s active site → active site R groups are specific for the substrate and allow it to enter
substrate binds to the enzyme’s active site forming enzyme-substrate complex → R groups of amino acids in active site react with bonds int he substrate → environment of isntabiity → easier to break apart
products are released and enzyme returns to its original shape, ready to catalyse a new reaction
enzymes alter the change in free energy of a reaction
false.
enzyme catalyse reactions have a lower activation energy than uncatalysed reactions but do not influence the overall change in delta G
enzyme substrate interaction types - overview (3)
orientation
physical strain
chemical charge
enzyme substrate interaction types - orientation
active site allows substrates to be orientated in a way that the bonds between molecules can occur → single molecule released
enzyme substrate interaction types - physical strain
position of R groups in the active site allow pressure to be placed ont he substrate → less stable → easier to change
enzyme substrate interaction types - chemical charge
enzyme adds charges, change or manipulate polarity of bonds → change molecule state
how is reaction rate measured (2)
decrease of concentration of the substrate
increase of concentration of product
rate of change = amount of products produced or reactants consumed in a defined time
define kinetic measurements
measurement of change in a defined time interval
rate of catalysed reactions depend on…
substrate concentration until all enzymes are saturated and max rate is achieved
define reaction rate in terms of enzyme kinetics
measure and effects of varying conditions of the reaction
what does the michaelis-menten model explain
how the rate of reaction varies with substrate concentration
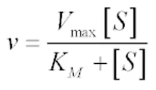
explain the variables int he equation
v = michaelis menten constant → always the same for each particular enzyme but varies from one enzyme to another
measure of affinity of the enzyme for its substrate → low constant = high affinity
v max = max velocity achieved by a system at max substrate concentration
KM = substrate concentration where reaction velocity is 50% of Vmax
s = substrate
how does temperature influence enzyme activity
Optimum temp of most enzymes in humans is usually 37
Little activity at low temps
Lose activity at temps above 50 -> denaturation -> loss of active site conformation and catalytic activity
Thermophilic bacteria have higher optimal temp than humans
how does pH influence enzyme activity
Optimum pH of most enzymes in humans is usually 7.4
Depends on where enzyme is located
Enzymes in stomach will usually have lower pH
Enzymes in liver will usually have higher pH
Enzymes contain R groups of amino acids with proper charges at optimum pH -> lose activity in low or high pH (tertiary structure disrupted)
cofactors overview (3)
non-rotein enzyme helpers that may be inorganic or organic
activators
co-enzymes
prosthetic groups
activators as cofactors
increase rate of enzymatic catalysed reactions by promoting formation of active site or other reactants
eg. magnesium ion, calcium ion, potassium ion
coenzymes as cofactors
complex non-protein organic molecules that transfer chemicals from active site of one enzyme to the active site of another
eg. NAD+ and CoA
prosthetic groups as cofactors
non-protein organic molecules that are attached to the enzme and act like built-in coenzymes
eg. vitamin b6
types of enzyme inhibitors (overview)
competitive
non-competitive
permanent
competitive inhibitors
similar shape to the substrate → bind to active site of an enzyme to compete with the substrate
can be reversed by increasing concentration of the susbtrate
non-competitive inhibitors
bind to allosteric sites of enzymes → causes enzyme to change shape, making active site less effective
cannot be reversed by increasing concentration of substrate
permanent inhibitors
forms covalent bonds with amino acid R groups that prevent catalytic activity
eg. toxins and poisons → nerve gas
allosteric regulation
non-substrate molecules bind an enzyme at a site away from the active site → changes shape
active in inactive forms can be interconverted depending on substrate molecules binding away from the active site
inhibitors and activators bind other polypeptides at regulatory sites to regulate metabolic processes
allosteric enzyme structure
typically have quaternary structure with active site on the catalytic subunit
Some allosteric enzymes have mutliple subunits with acitve sites
substrate binding at one site has an allosteric effect and increases rate of reaction
non-allosteric enzymes with one active site have different reaction rates at low substrate concentration
describe allosteric activators and inhibitors
active form oscillates with inactive form
activator binds away from the active site and stabilises the active form
inhibitor binds way from the active site and stabilises the inactive form
even if substrate is around → will not bind to active site
describe cooperativity, the type of allosteric activation
relies on enzyme being in an inactive form
once substrate binds to the active site → active form is stabilised
will oscillate between active and inactive form until stabilised when substrate binds to one active site
how do allosteric enzymes regulate metabolism w example
Allosteric enzymes catalyse certain points in the respiratory pathway to regulate pace of glycolysis and the citric acid cycle
Phosphofructokinase catalyses the commitment step in glycolysis and is inhibited by ATP and citrate → feedback inhibition + negative feedback
principles of metabolic pathways (5)
complex transformation occur in a series of separate reactions
each reaction is catalysed by a specific enzyme
many metabolic pathways are similar in all organisms
in eukaryotes, metabolic pathways are compartmentalised in specific organelles
key enzymes can be inhibited or activated to alter the rate of the pathway
catabolic processes that harvest energy from glucose (overview)
glycolysis
cellular respiration
fermentation
where does glycolysis take place and what are the inputs and outputs
in the cytosol
6-carbon glucose -> 2 3-carbon sugars -> oxidised -> remaining atoms rearranged to form 2 pyruvate
produces net 2 ATP and 2 NADH
stages of glycolysis
first 5 steps = energy investment stage
2 ATP is needed and steps 1-3 are endergonic
last 5 steps = energy payoff stage
4 ATP and 2 NADH produced
regulation of glycolysis
phosphofructokinase catalyses commitment step of glycolysis → inhibited by ATP and citrate produced in citric acid cycle → activated by ADP
define anaerobic respiration
the production of ATP using a molecule other than oxygen as the final acceptor in the ETC
define fermentation
the production of energy in the absence of oxygen and without the ETC
different end products depend on what the starting compound was
consists of glycolysis and reactions that regenerate NAD+ by transferring electrons from NADh to pyruvate or derivatives of pyruvate
explain the key difference between anaerobic respiration and fermentation
Anaerobic respiration uses a molecule other than oxygen as the final electron acceptor whilst fermentation does not involve the ETC and uses pyruvate or its derivates as the final electron acceptor
lactic acid fermentation
pyruvate is electron acceptor
lactate = product
occurs in microorganisms and some complex organisms
lactate dehydrogenase catalyses fermentation
in the presence of O2 → catalyses oxidation of lactate to pyruvate
during intensive exercise → oxygen cannot be delivered to cells fast enough for aerobic respiration → muscle cells break down glycogen for lactic acid fermentation
when lactic acid builds up → increase in hydrogen ions lowers pH → can cause muscle pain
alcohol fermentation
occurs in yeast and some plant cells
requires 2 enzymes to metabolise pyruvate to ethanol
reactions are reversible and used to produce alcoholic beverages
downfalls of fermentation
cellular respiration yields more energy than fermentation
glucose is only partially oxidised in fermentation → more energy remains in the product than in CO2
alternative names to citric acid cycle
krebs cycle
TCA cycle
outputs of citric acid cycle per glucose molecule
6 NADH, 2 GTP, 2FADH2, 2 oxaloacetate, 4 CO2, 2 H2O
GTP and ATP
GTP can easily transfer the phosphate group and energy to ADP to produce ATP
role of oxygen in the citric acid cycle
citric acid cycle does not directly use O2 but it has to be present as the final electron acceptor in the ETC so that NAD+ and FAD+ can be regenerated to be reduced in the cycle
oxidative phosphorylation involves what 2 processes
the electron transport chain and chemiosmosis
explain oxidative phosphorylation
the synthesis of ATP by reoxidation of electron carriers in the presence of oxygen → relies on proton gradient between hydrogen ion concentration in the mitochondria matrix and inter membrane space
explain the electron transport chain
the transfer of hdyrogen ions and electrons from NADH and FADH2 from one electron carrier to the next, coming it with oxygen to make H2O
occurs across the inner mitochondrial membrane
consists of a series of protein complexes
Q mvoes between I and III
Cytochrome C moves between I and IV
whilst electrons are passed through complexes, protons are pumped into the intermembrane space,e creating a highly positive environment and an electrochemical gradient
explain chemiosmosis
the movement of hydrogen ions back across the membrane to a relatively more neutral environment and electron movement through the ETC provides the energy need for synthesis of ATP from ADP
oxygenic photosynthesis is a ____ and an ______ reaction
redox; endergonic
photosynthesis reaction overall
6CO2 + 6H2O → C6H12O6 +6O2
structure of chloroplast (photosynthesis)
2 membranes surrounding dense fluid → stroma
thylakoids = sacs that form third membrane system suspended within stroma
chlorophyll in thylakoid membranes = green pigment
light absorbed by chlorophyll drives synthesis of organic molecules in the chloroplast
explain light as a form of energy
light = electromagnetic radiation
propagated as waves, energy is inversely proportional to wavelength
light behaves as particles
when a photon of light hits a molecule, it can… (3)
bounce off → scattered or reflected
pass through → transmitted
be absorbed → adding energy to molecule = excited s
when a photon of light hits a chlorophyll molecule…
absorbs blue and red light but scatters green
major pigment of photosynthesis
chlorophyll a
how does chlorophyll anchor itself in the thylakoid
chlorophyll has a hydrocarbon tail that anchors it in a protein complex in the thylakoid
light reactions overview
light energy comes in as a photon to drive electron flow along the electron chain
water is oxidised and electrons move through photosystem II then I to rpdoceu ATP and NADPH
describe photosystem
process by which heterotrophs use energy from sunlight to drive the synthesis of organic molecules from CO2 and water
what comes first, photosystem I or II
photosystem II
photosystem process: step 1 (photon hits)
photon of light strikes one of the pigment molecules in light-harvesting complexes → boosts one of the electrons to a higher energy level
as electron falls back to ground state, released energy transferred to a nearby pigment molecule → process continues until it is transferred to P680 pair in reaction centre
photosystem process: step 2 (P680+)
excited electrons transferred to priamry electron acceptor via redox reaction → leaves P680 missing its negative charge → P680+
P680+ is strongest biological oxidising agent known
photosystem process: step 3 (photolysis)
enzyme catalyses tge splitting of water into 2 hydrogen ions and 2 electrons
electrons replace on that was transferred to priamry electron acceptor one by one
hydrogen ions released to thylakoid space
oxygen atom immediately binds with another to form oxygen gas
photosystem process: step 4 (II to I)
excited electron passes from primary electron acceptor to photosystem I via electron transport chain
movement of electrons pumps protons into thylakoid membrane
photosystem process: step 5 (chemiosmosis)
protons move through ATP synthase to generate ATP
proton gradient stores potential energy
components of electron transport one
Pq, cytochrome complex, Pc