Molecular Orbital Theory and Bonding Concepts
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Molecular Orbital Theory
Describes electron distribution and energy in molecules.
Valence Bond Theory Failure
Inadequate for explaining molecular properties like O2.
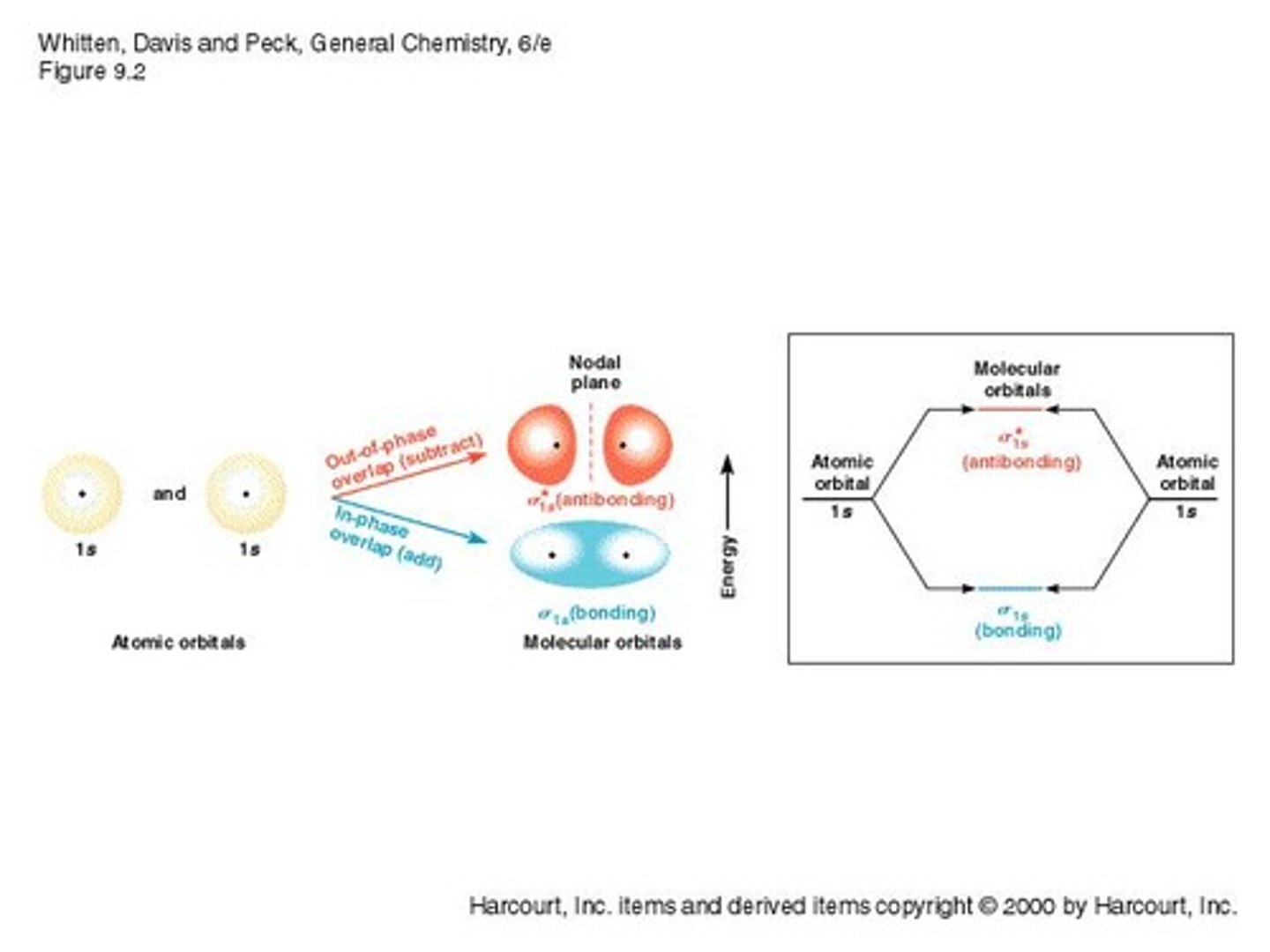
Bonding Orbitals
Lower energy orbitals formed from in-phase overlap.
Antibonding Orbitals
Higher energy orbitals from out-of-phase overlap.
Nonbonding Orbitals
Orbitals with energy equal to atomic orbitals.
Wave Function
Mathematical description of electron behavior in orbitals.
Schrödinger Wave Equation
Equation used to describe wave functions of electrons.
Constructive Interference
In-phase wave functions combine to lower energy.
Destructive Interference
Out-of-phase wave functions combine to higher energy.
Sigma Bonding Orbital
Formed by head-on overlap of atomic orbitals.
Nodal Plane
Region where probability of finding electron is zero.
Pi Molecular Orbitals
Formed by side-on overlap of p atomic orbitals.
Bond Order
Indicates number of bonds; higher means more stability.
Pauli Exclusion Principle
No two electrons can have identical quantum states.
Hund's Rule
Electrons occupy degenerate orbitals singly before pairing.
Aufbau Principle
Electrons fill lowest energy orbitals first.
Bond Energy
Energy required to break one mole of bonds.
Paramagnetic Molecules
Contain unpaired electrons in molecular orbitals.
Diamagnetic Species
Have no unpaired electrons in molecular orbitals.
Homonuclear Diatomic Molecules
Molecules composed of two identical atoms.
Energy Level Diagram
Visual representation of molecular orbital energies.
Overlap of 1s Orbitals
Produces bonding and antibonding molecular orbitals.
Overlap of 2pz Orbitals
Head-on overlap forms sigma orbitals.
Overlap of 2py Orbitals
Side-on overlap forms pi molecular orbitals.
Overlap of 2px Orbitals
Similar to 2py, produces bonding and antibonding p orbitals.