A Level Chemistry - Dr Starkey
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
Moles formula for pure substances
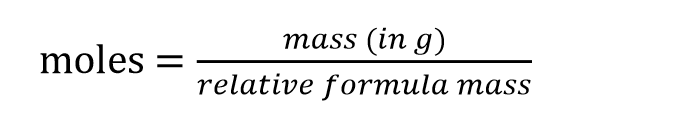
Moles formula for gases
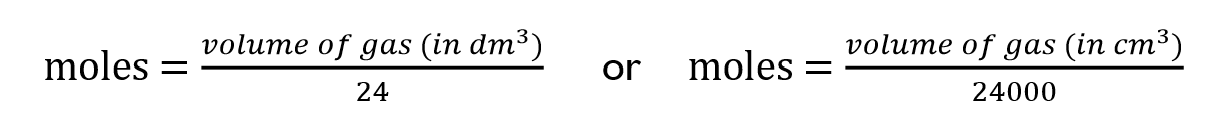
Moles formula for solutions
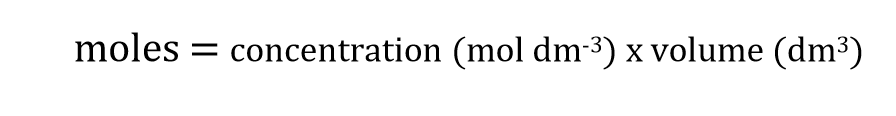
Moles formula using Avogadros constant
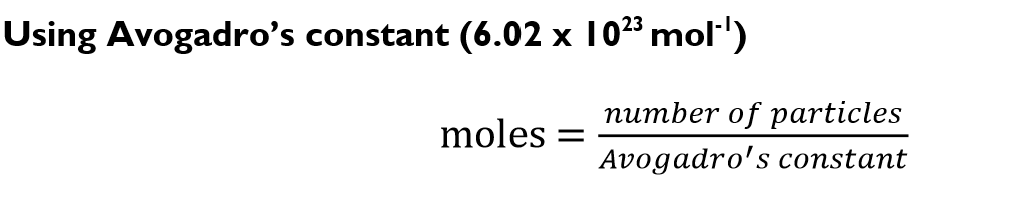
Percentage yield formula
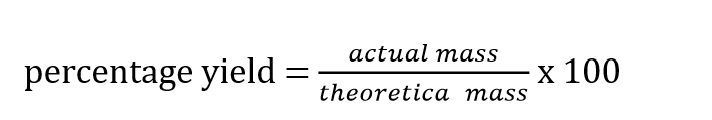
Atom economy formula
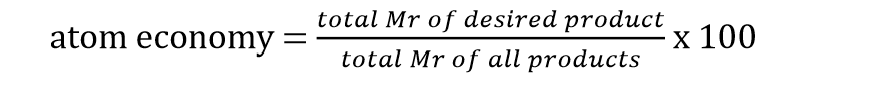
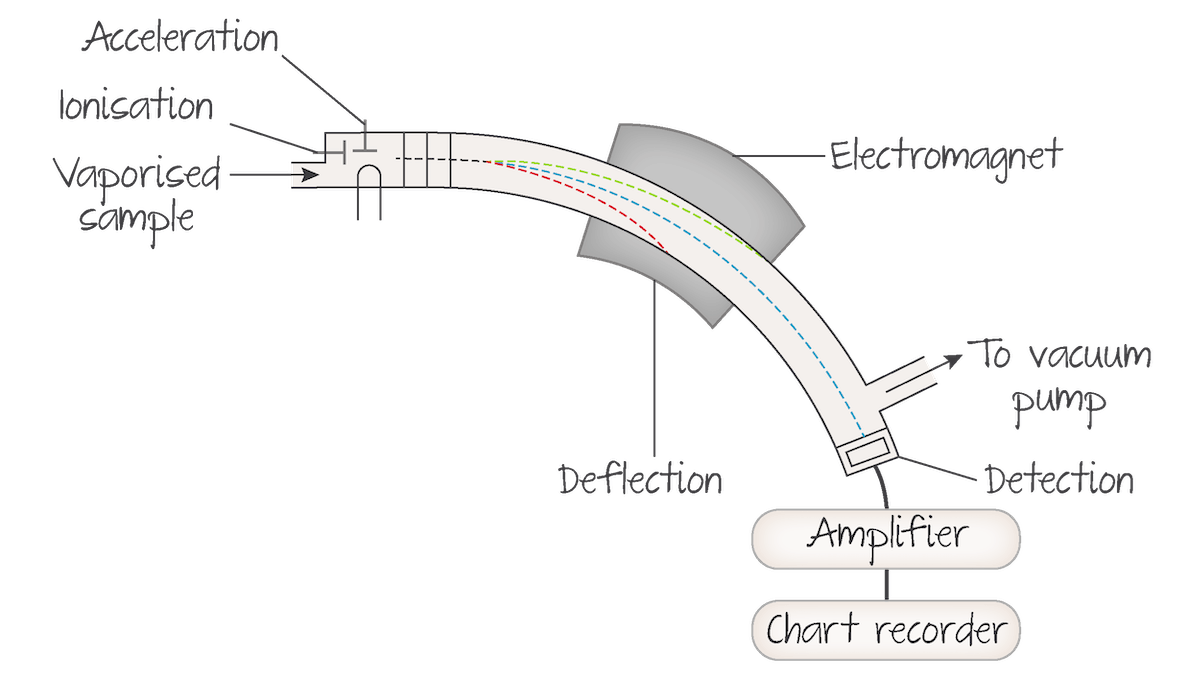
What is a mass spectrometer used for?
machine used to determine the RAM of elements and the structure of organic compounds
Stages of a sample in a mass spectrometer
The sample to be analysed is:
first vaporised to form a gas
it is bombarded by high-energy electrons, producing positive ions, which are accelerated in an electric field. aims to produce ions with a one positive (1+) charge.
The positive ions are deflected in a magnetic field depending on their mass to charge ratio (m/z). Ions with a higher mass to charge ratio are deflected less in the magnetic field than ions with a lower mass to charge ratio.
Finally, the positive ions reach the detector, where they produce a mass spectrum.
Mass spectrometer peak charts
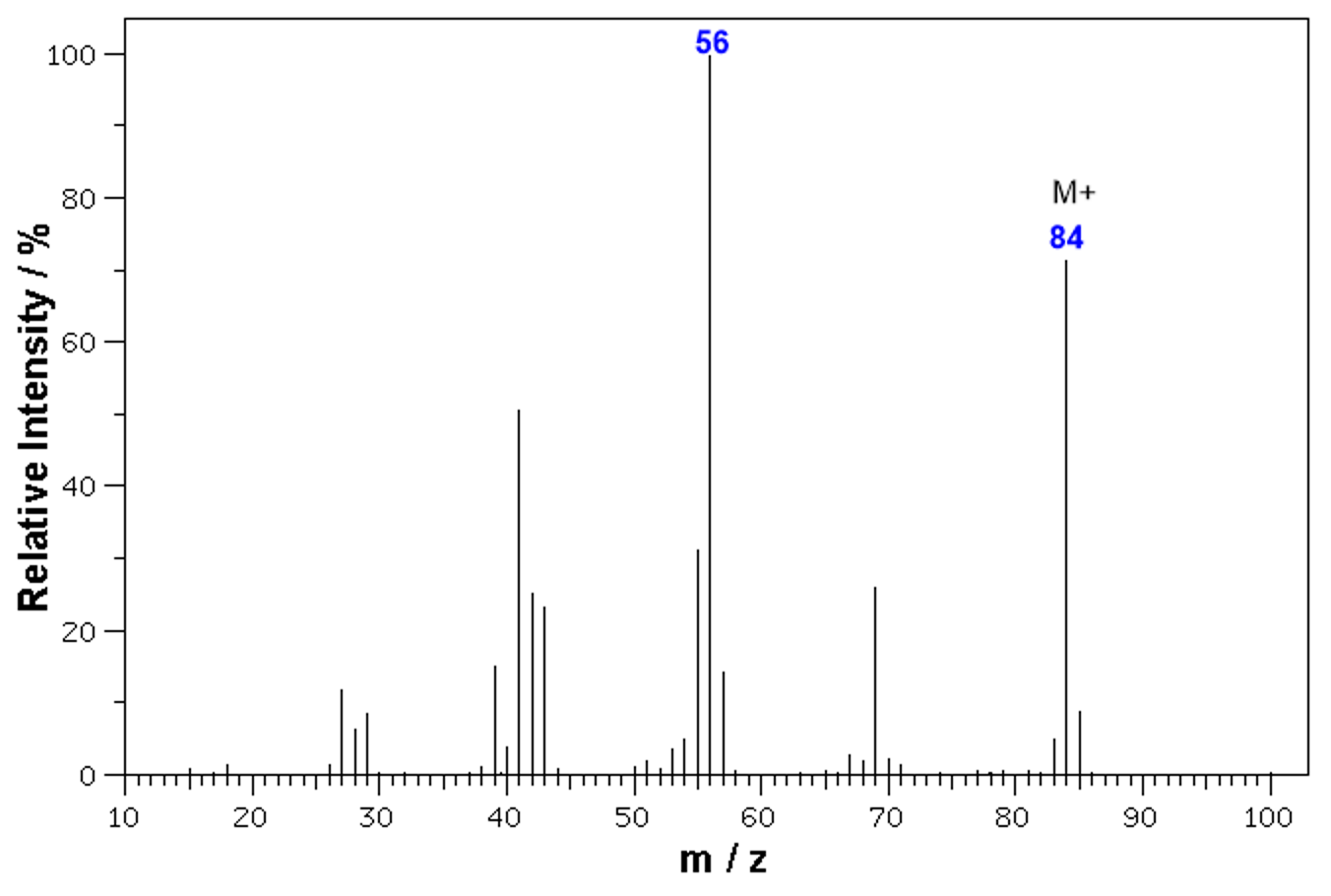
How to work out mass to charge ratio (m/z)?
mass of isotope/charge on ion
EM spectrum
radio, microwave, infrared, visible light, ultraviolet, x-ray, gamma
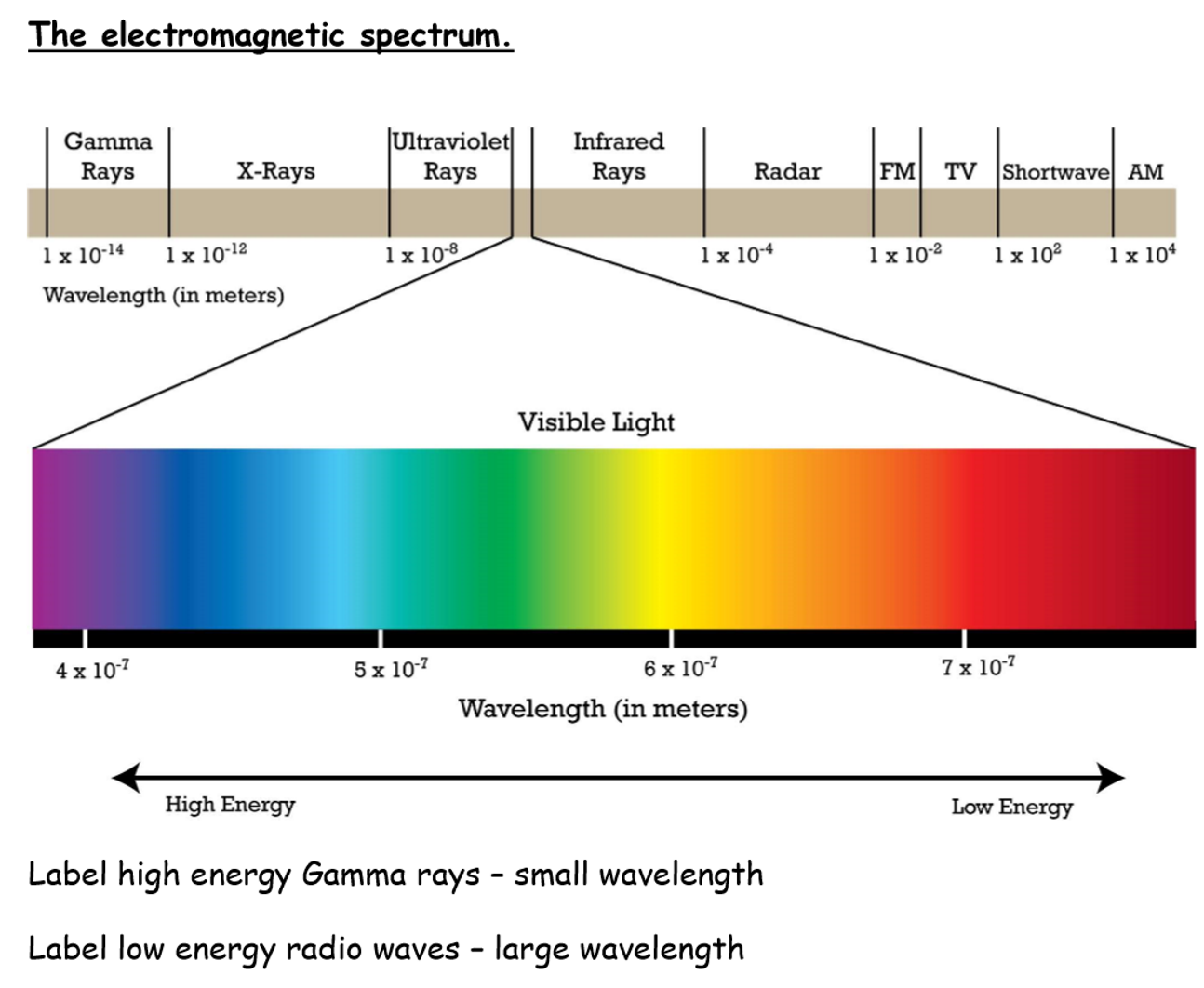
What is the relationship between wavelength, frequency and speed
speed = frequency x wavelength
wavelength (y lamda)- distance between 2 crests or 2 troughs
frequency (f) - number of waves that pass a point in 1s
speed of light ( c)- all em radiation travels at the same speed
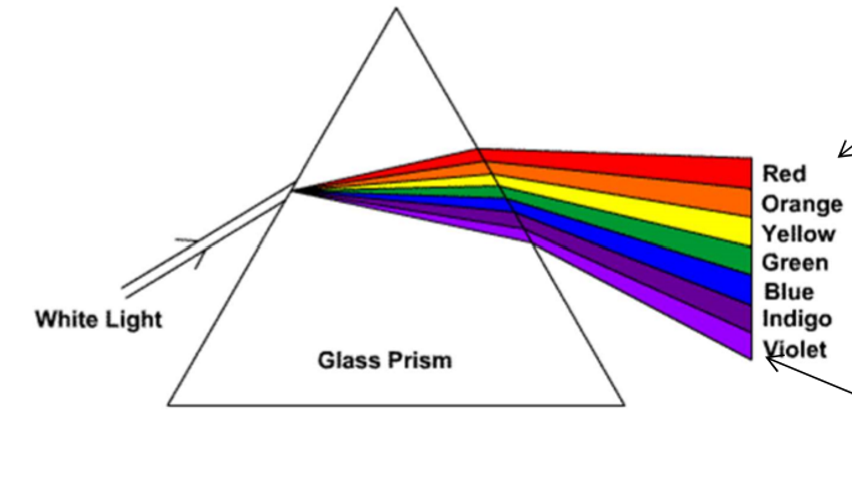
What is a continuous spectrum
when white light is passed through a prism a continuous spectrum is produced
ROYGBIV
red - lower energy and lower frequency
violet - high energy and higher frequency
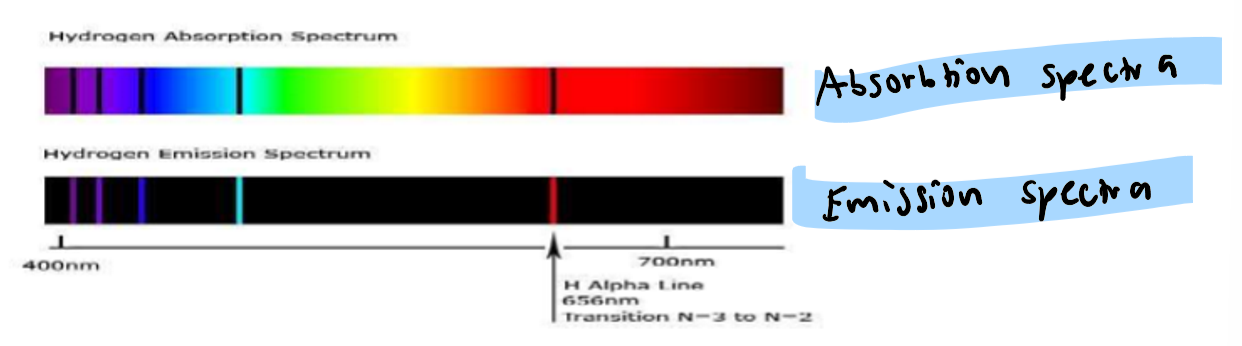
What is are the 2 types of line spectra?
when white light is passed through a gas, an absorption spectrum is produced: this is a line spectrum with some colour of the continuous spectrum missing
if high voltage is applied to a gas, a corresponding emission line spectrum is produced.
all elements have different line spectra that provide info about the arrangement of electrons in an atom
How to calculate Ar from mass spectra charts?
Ar = (m/z x abundance)/ total abundance
What is ionisation energy?
the amount of energy required to remove an electron from an atom or ion. measured in kJ/mol.
Always endothermic as energy must be taken in to remove an electron because the strong attraction between the negative electron and the positive nucleus must be overcome
What is first ionisation energy?
the energy required to remove one mole of electrons from one mole of atoms in the gas phase
X (g) → X+ (g) + e-
What is second ionisation energy?
the energy required to remove one mole of electrons from one mole of positive ions in the gas phase
X+ (g) → X 2+ + e-
Why do successive ionisation energies always increase?
as the ion becomes increasingly positive with each electron removal, the attraction between the nucleus and the remaining electrons becomes stronger
therefore more energy is required to overcome this attraction and remove the next electron
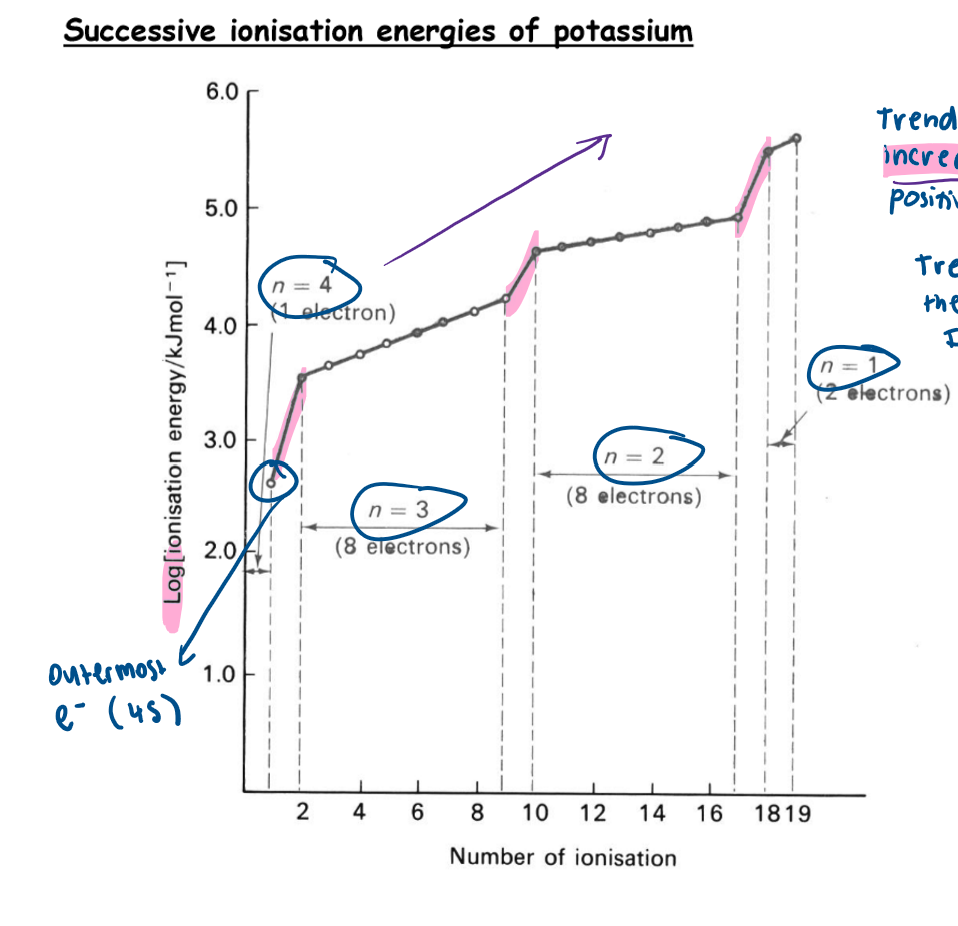
What are the trends for successive ionisation energies of an element?
successive ionisation energies increase
occasionally there is a jump in the ionisation energy
these jumps represent an electron taken from a new shell closer to the nucleus
What is the general trend for first ionisation energies across a period?
first ionisation energies increase due to:
nuclear charge increasing due to increasing number of protons in nucleus
atomic radius decreases across a period due to the increasing electrostatic attraction between the nucleus and outer electrons
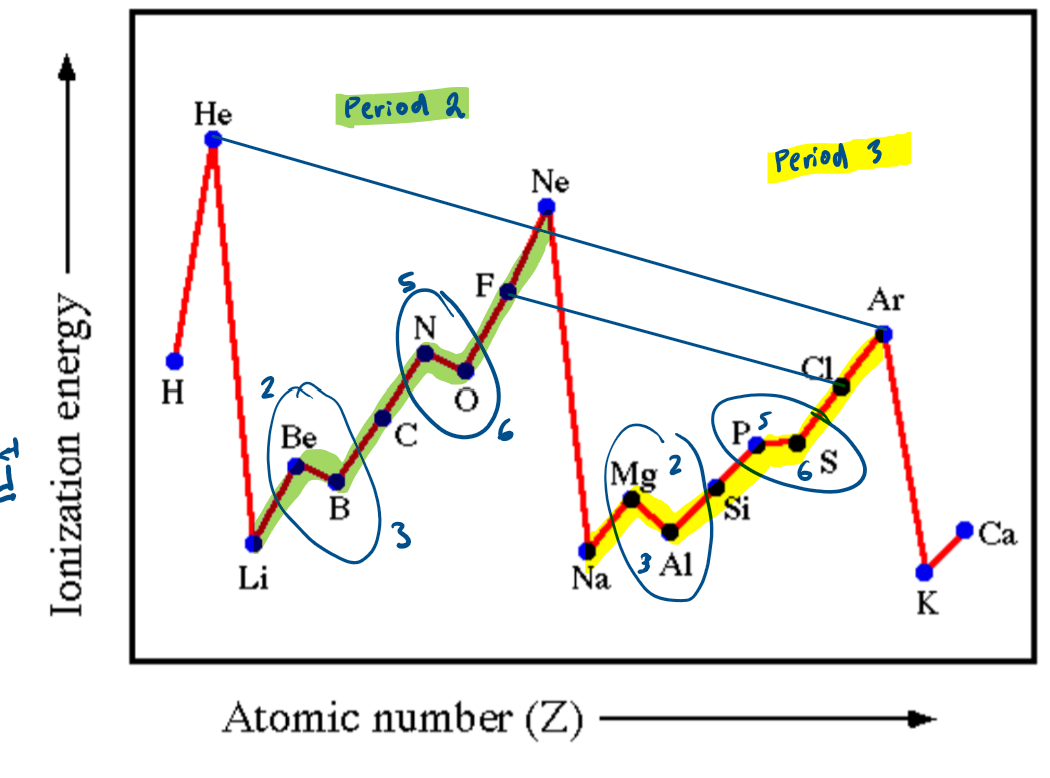
Why are there dips in the first ionisation energy across a period?
dips between groups 2 and 3:
ionisation energy is lower for group 3 than 2 as it is easier to remove an electron from a higher energy p-orbital than from a lower energy s-orbital
dips between groups 5 and 6:
ionisation energy is lower for group 6 than 5 as there is one electron is each p-orbital in group 5, whilst in group 6 one of the p-orbitals has 2 electrons
the repulsion between these electrons in the paired orbital makes one easier to remove
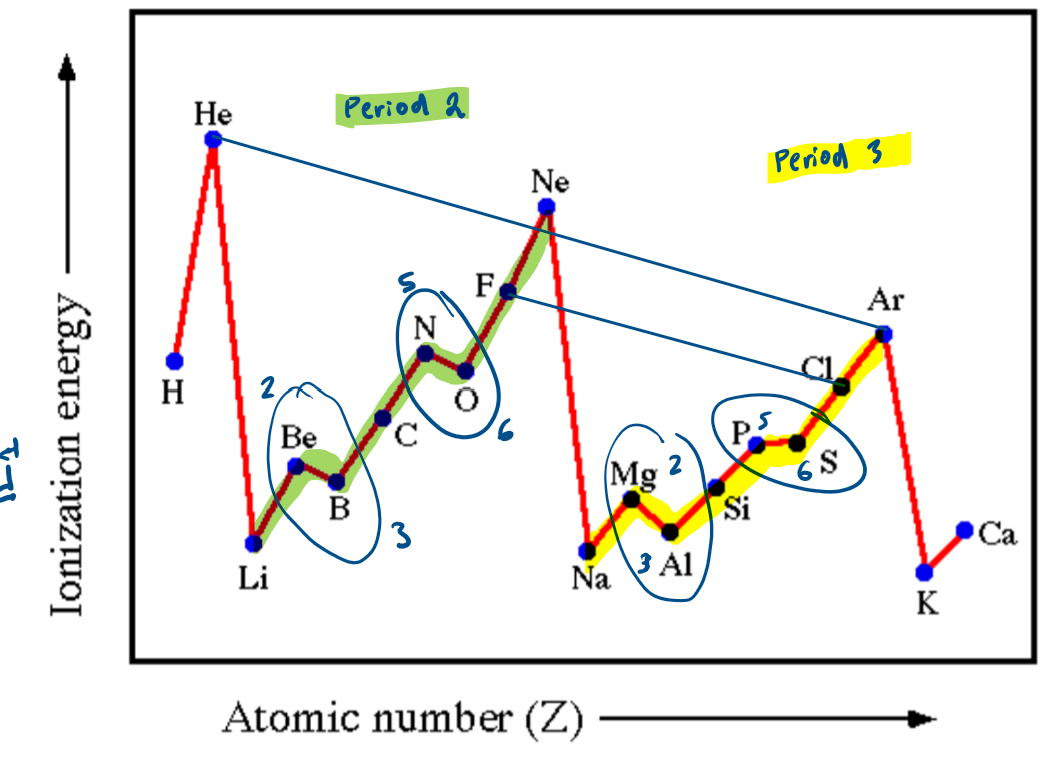
What is the general trend for first ionisation energies down a group?
the first ionisation energies decrease as you go down the group
this is because the outer electron is in a higher energy shell that is further from the nucleus and therefore is less attracted to the nucleus
electron shielding also increases which reduces the effect of the electrostatic forces of attraction
What is electron affinity?
the neutral atom's likelihood of gaining an electron
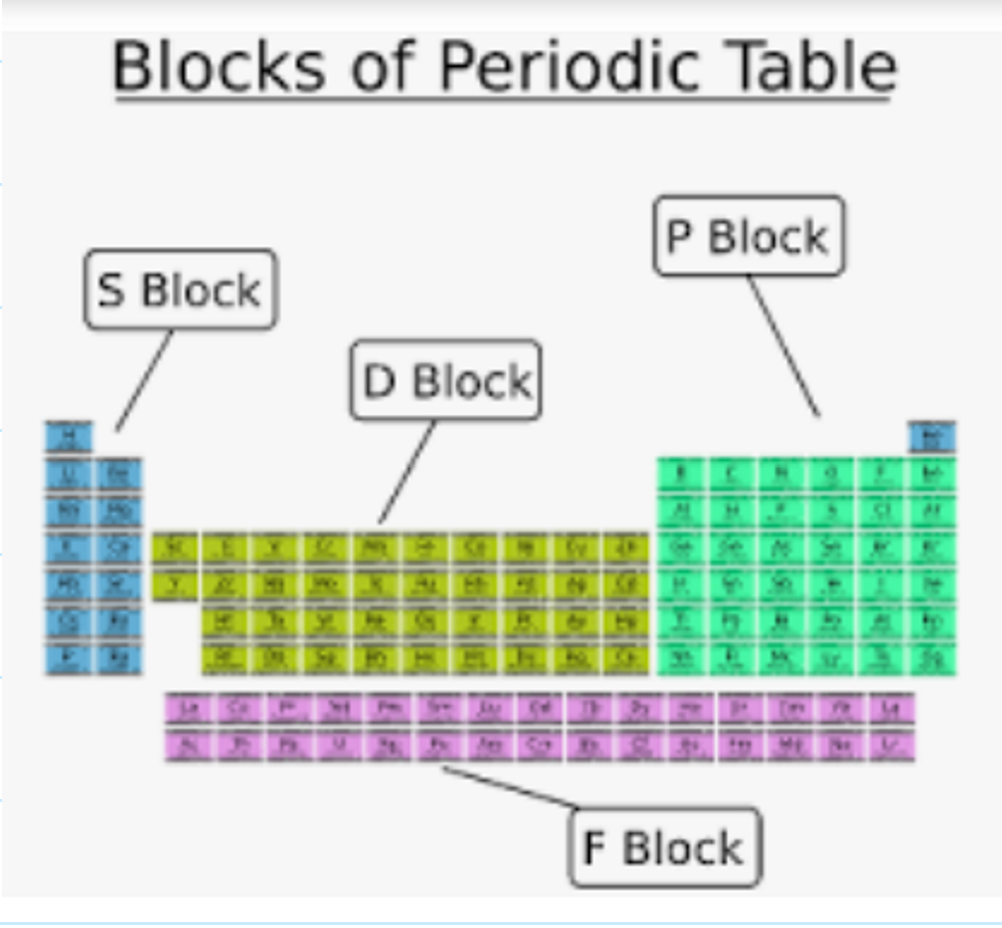
What are the 4 blocks within the periodic table?
s-block = groups 1 + 2
p-block = groups 3-0
d-block = transition metals
f-block = radioactive elements
What happens to atomic radius across a period?
atomic radius decreases across a period as:
increasing nuclear charge - outer electrons are pulled in closer to the nucleus as the increased charge produces a greater attraction
What happens to atomic radius down a group?
atomic radius increases down a group as:
an electron shell is added each time, increasing the distance between the outer electrons and the nucleus, reducing the power of attraction
more shells also increase electron shielding, reducing the power of attraction
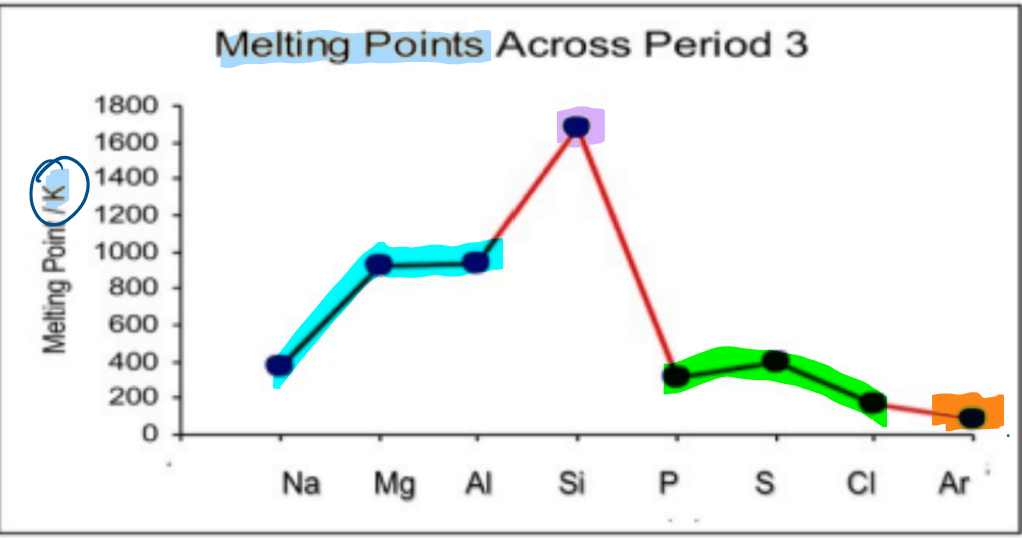
What happens to melting points across a period?
elements in group 1-3 are metallic elements:
the melting point increases as the metallic bond gets stronger due to stronger attraction between positive ions and delocalised electrons
element in group 4 has a giant covalent structure:
has a very high melting point as lots of heat energy is needed to break the many covalent bonds
elements in group 5-7 are simple covalent molecules:
have low melting points as weak intermolecular forces between molecules don’t require lots of heat energy to overcome them
element in group 8/0 is monatomic:
has the lowest melting point as it has the weakest forces between atoms that don’t require much heat energy to overcome them
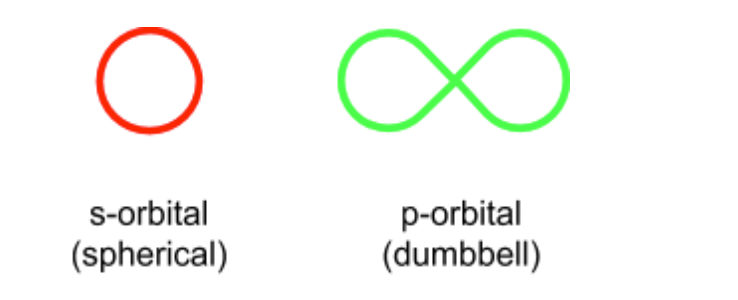
What is an orbital and what are the types?
clouds of negative charge
s, p, d and f
the energy of the orbitals increases from s to d meaning the orbitals are filled in this order
Each can hold up to two electrons with opposite spins
What is a subshell?
subshells contain orbitals and therefore contain different numbers of electrons
s subshell - 1 orbital, 2 electrons
p subshell - 3 orbitals, 6 electrons
d subshell - 5 orbitals, 10 electrons
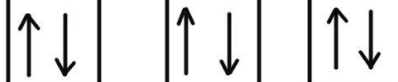
What is spin?
within an orbital electrons pair up with the opposite spin so that the tom ios as stable as possible
Represented by opposite arrows
Rules for writing electron configuration?
lowest energy orbital is filled first
electrons with the same spin fill up an orbital first before pairing begins
no orbital can hold more than 2 electrons
4s comes before 3d
exceptions to the rules: copper and chromium
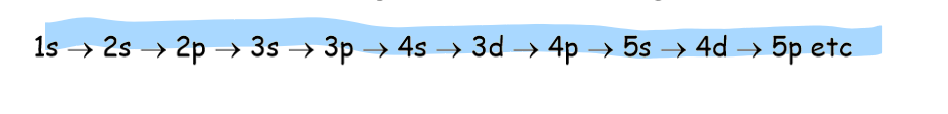
What is ionic bonding?
Electrostatic forces of attraction between oppositely charged ions following electron transfer
What determines the strength of the electrostatic attraction between ion?
charge - the higher the charger the stronger the electrostatic attraction
size - smaller ions form stronger ionic bonds as they can be closer together, increasing electrostatic attraction
How can we measure the strength of an ions electrostatic attraction?
melting point
Properties of ionic compounds
brittle, soluble in polar solvents, and conduct electricity when molten or in aqueous solution
Why are ionic compounds brittle?
when force is applied, layers slide so similarly charged ions line up in the lattice, ions repel and lattice breaks apart
Why are ionic compounds soluble in polar solvents?
polar: part of the molecule is slightly positive and part is slightly negatively charged
positive ion attracted to negative
negative ion attracted to positive
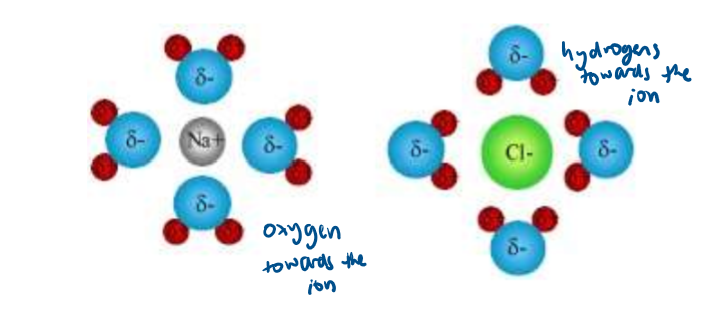
Why do ionic compounds only conduct electricity when molten or aqueous?
ionic compounds have charged particles that move and carry charge
particles are unable to move in a solid, only vibrate
Migration of ions in electrolysis
cations (+) go to cathode (-)
anions (-) go to anode (+)
What happens to ionic radii as you go down a group?
ionic radii decrease down the group
as you go down the group an extra shell is added each time
distance from nucleus to electrons increases
forces of electrostatic attraction decrease meaning ion gets larger
What are isoelectronic ions?
ions with the same electronic configuration
Trend in ionic radii in isoelectronic ions?
ionic radius decreases as the nuclear charge increases
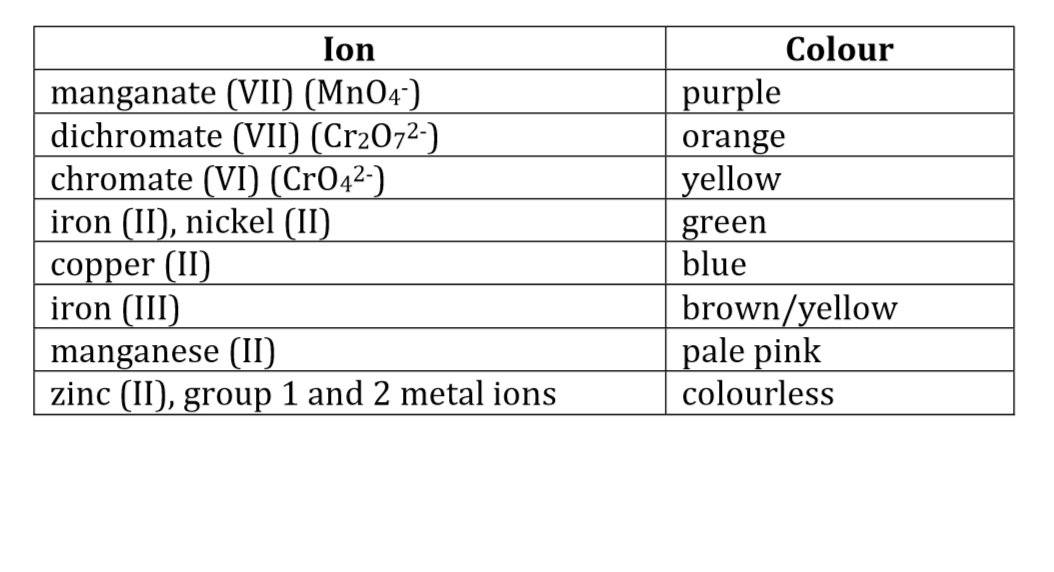
Colour of ions of transition metals
How can we calculate the radii of ions?
x-ray diffraction is used to measure the spacing between ions in a crystal
from the diffraction patterns you can calculate the radii of individual ions
Ionic radius vs atomic radius?
cations have a smaller ionic radius than atomic radius as the metal ion has lost its outer shell so there is increased attraction between protons and electrons
anions have a larger ionic radius than atomic radius as the ratio of protons to electrons has decreased, therefore each electron experiences a weaker electrostatic force of attraction. electrons also experience increased repulsion.
What is a covalent bond?
strong electrostatic attraction between the nuclei of two non-metal atoms and the electrons shared between them
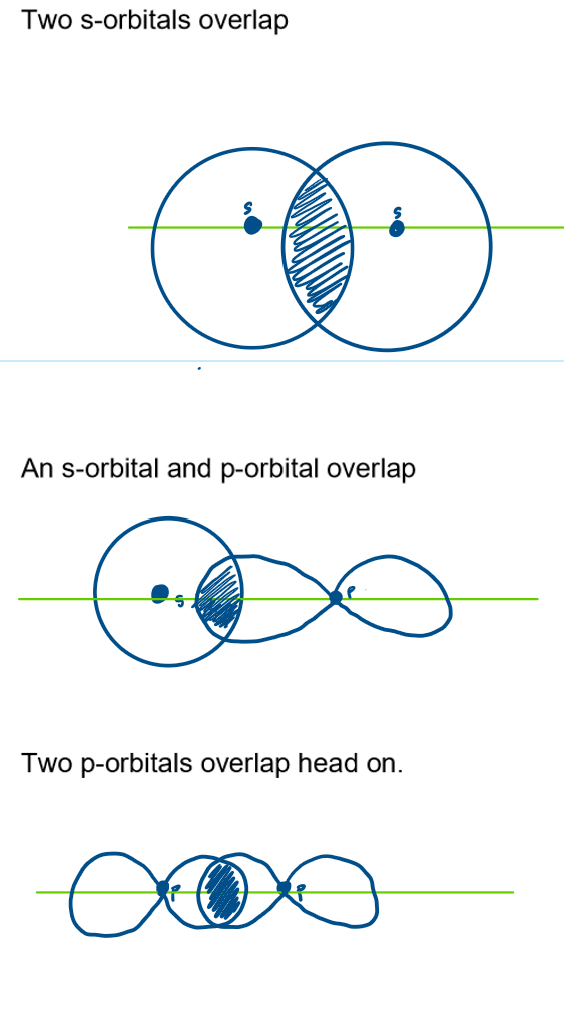
How can a sigma bond be formed and diagrams?
sharing of 2 electrons
either:
2 s-orbitals overlap
an s-orbital and p-orbital overlap
two p-orbitals overlap head on
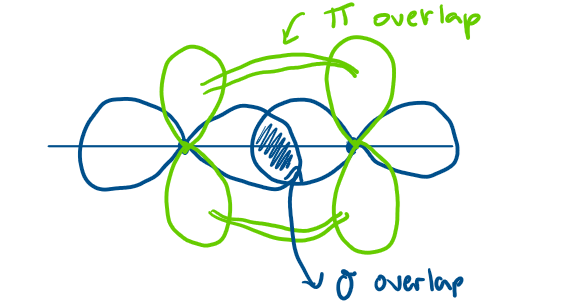
What is a pi bond?
when two p-orbitals overlap above and below the line of the nucleus
not as efficient as a sigma bond
How is a double bond formed and diagram?
sharing of 4 electrons
sigma + pi bond
How is a triple bond formed?
sharing of 6 electrons
sigma + 2 pi bonds
What is a dative bond?
when both the electrons in the shared pair come from the same atom
indicated using an arrow from the lone pair electron
When can electrons be promoted?
period 2 elements
if there are empty orbitals in the outer shell electrons in paired orbitals can be promoted to create more one electron orbitals and therefore more covalent bonds
What does it mean when elements expand their octet?
all elements except period 1 and 2 elements are able to have more than 8 electrons in their outer shell
How does the bond length affect the strength of the covalent bond?
as the bond length increases the bond strength decreases
How do you draw shapes of molecules (single bonds)?
draw dot and cross diagram
count bond pairs and lone pairs
assign to correct shape
striped line into page and shaded block out of page
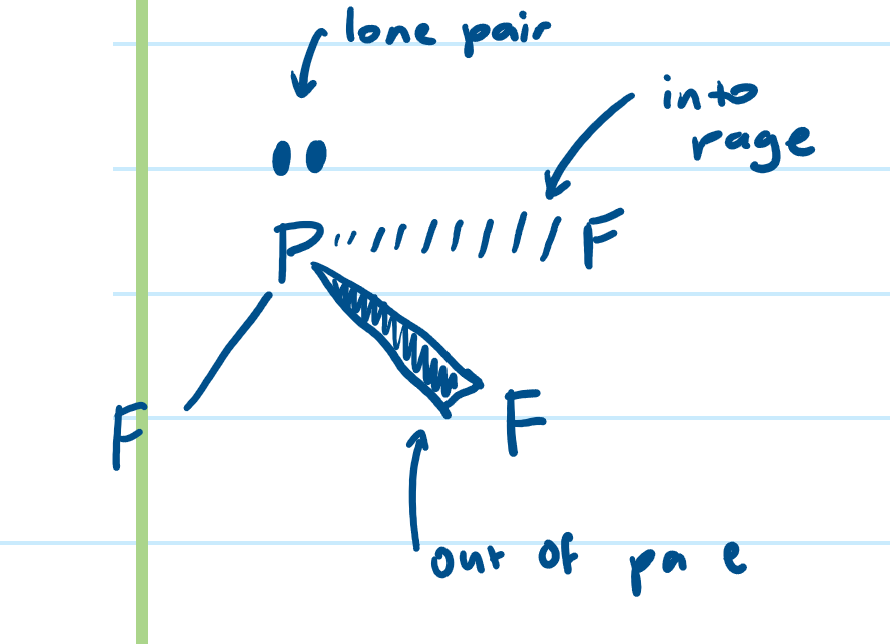
How do you determine shapes of molecules with double bonds?
count the number of sigma bonds (each double has 1)
count number of lone pairs
assign to correct shape
essentially treat sigma bond like bond pair
Why do molecules take the shapes they do perfect paragraph:
the molecule X has X bonding pairs and X lone pairs
electrons repel to positions of minimum repulsion and maximum separation
the shape is based on X
choice of:
bp repel equally
repulsion between lp bp > bp bp
repulsion between lp lp > lp bp > bp bp
so X has bond angles of X therefore is X shape
What shape do molecules with 2 electron pairs take?
2 bp 0 lp - linear 180
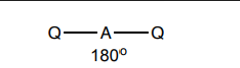
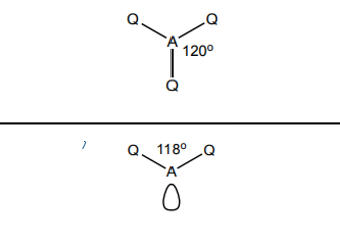
What shape do molecules with 3 electron pairs take?
3 bp 0 lp - trigonal planar 120
2 bp 1 lp - v shape 118
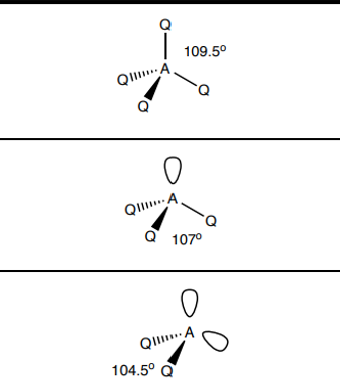
What shape do molecules with 4 electron pairs take?
4 bp 0 lp - tetrahedral 109.5
3 bp 1 lp - trigonal pyramidal 107
2 bp 2 lp - v shape 104.5
What shape do molecules with 5 electron pairs take?
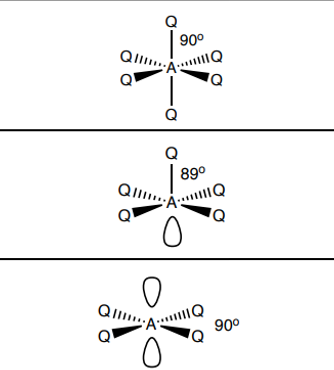
5 bp 0 lp - trigonal bipyramidal 120, 90
4bp 1 lp - see saw 119, 89
3 bp 2 lp - t shape 89
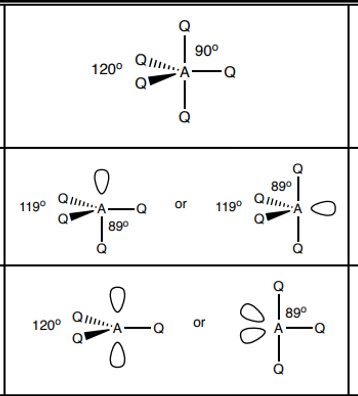
What shape do molecules with 6 electron pairs take?
6 bp 0 lp - octahedral 90
5 bp 1 lp - square pyramid 89
4 bp 2 lp - square planar 90
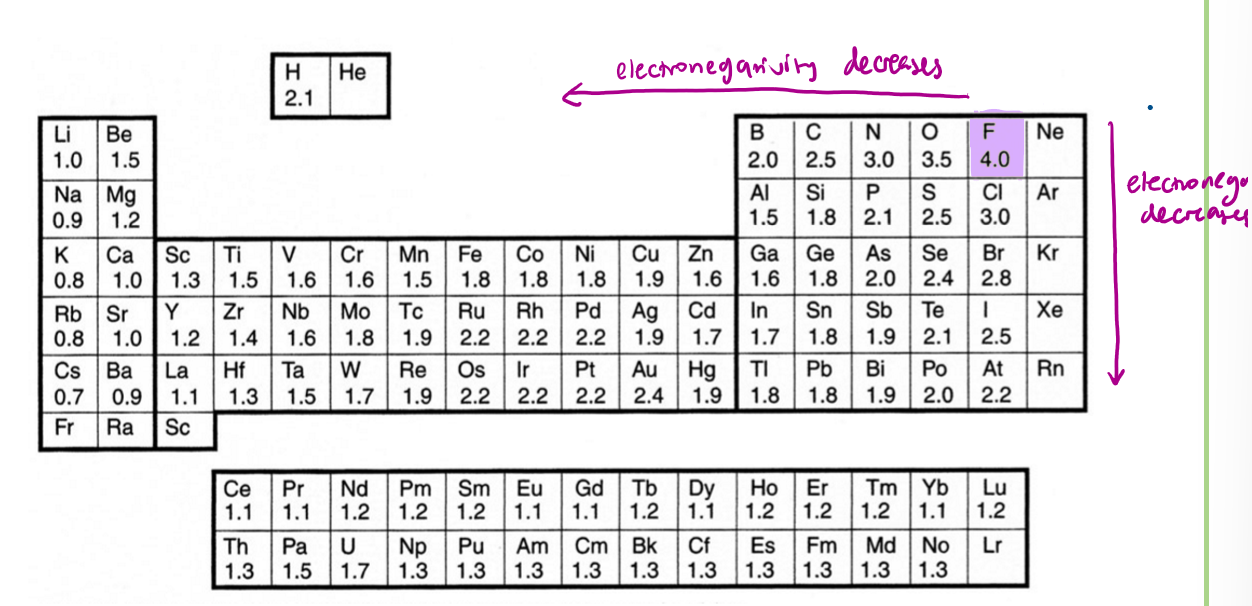
What is electronegativity and what scale is used?
the ability of an atom in a covalent bond to attract an electron pair towards itself
Pauling scale most commonly used, electronegativity decreases down and left from F (F most electronegative element at 4.0)
What happens to electronegativity across a period?
electronegativity increases across a period
atomic radius decreases across a period
distance between nucleus and bonding electrons decreases
electrostatic attraction increases
What happens to electronegativity down a group?
electronegativity decreases down a group
atomic radius and shielding increase down a group
distance between nucleus and electrons increases
electrostatic attraction decreases
What is a polar bond?
When two elements in a covalent bond have different electronegativities the bond is polar
Greater difference in electronegativity = more polar bond
The more polar the bond the more bond ionic character the molecule has
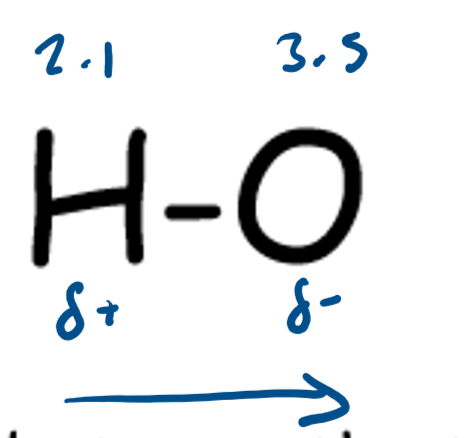
What is a polar molecule?
Contains polar bonds which are asymmetric meaning the dipoles do not cancel out
(e.g. H2O and CH3Cl)
Results in an overall dipole moment so experience permanent dipole-dipole interactions
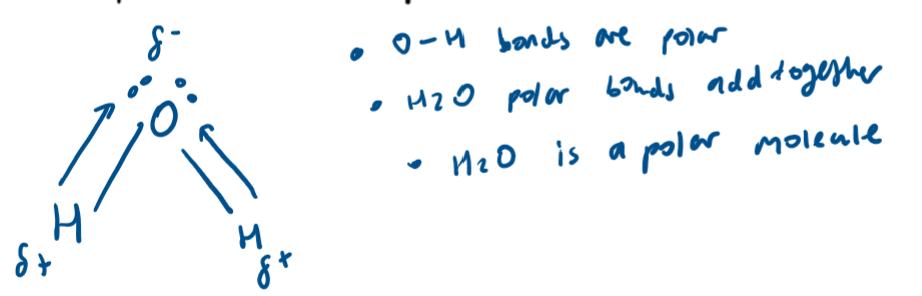
Deflecting jets experiment
Charge up a plastic rod by rubbing it with a cloth
Set up a burette and a beaker underneath to catch the liquid
Bring the charged rod towards the jet of liquid
Observe how much of the jet is deflected
Polar molecules are deflected, non-polar molecules are not
The charged rod induces a dipole in the molecule causing the jet of polar liquid to be attracted to the rod
What are the three types of intermolecular force?
hydrogen bonds
London forces (Van der Waals)
dipole-dipole
What is hydrogen bonding?
strongest type of intermolecular force
only occurs if there is a H bonded to N, O or F
caused by electrostatic force of attraction between electropositive H bonded to electronegative N/O/F and a lone pair on an adjacent molecule
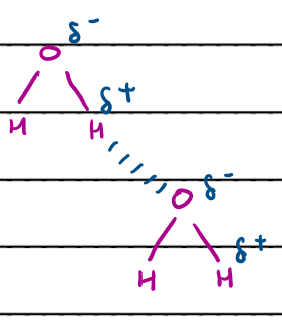
Hydrogen bonding in water?
hydrogen bonds are longer than covalent O-H bonds
ice - water molecules held together in a lattice by hydrogen bonds
water - water molecules moving so H bonds being continually broken and formed, water molecules are more spread out
so ice is less dense than water
What are London forces?
exist between all covalent molecules
electrons move around a molecule
at a given moment more electrons will be in place
this makes one part of the molecule delta negative
instantaneous dipole will induce a dipole in a neighbouring molecule
there is a force of attraction between instantaneous and induced dipole
What does strength of London forces depend on?
the more electrons a molecule has the stronger the London forces
the larger the surface area the stronger the London forces
What are permanent dipole dipole forces?
exist between polar molecules
the delta positive on molecule attracts the delta negative on another
weakest intermolecular forces
What is meant by the idea of “like dissolving like”?
solvents only allow solutes to dissolve that have similar intermolecular forces to those of the solvent
eg propanol which has H bonds can easily dissolve in water, but iodine which only has London forces cannot
therefore there is a scale of solvent polarity as polar compounds do not dissolve in non-polar solvents and vice versa
What is metallic bonding?
electrostatic forces of attraction between metal ions and a sea of delocalised electrons
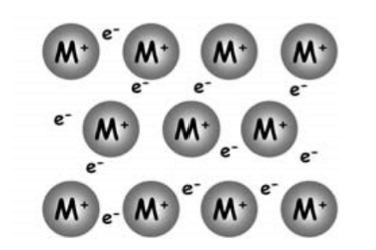
How do you find metallic radius?
half the distance between the nuclei of adjacent atoms
How does size and charge of metallic ions affect the melting point?
the smaller the radius, the stronger the metallic bonding, and the higher the melting point
the larger the charge on the metal ion, the stronger the metallic bonding, and the higher the melting point
Why are metals able to conduct electricity?
delocalised electrons are able to move through the structure and carry charge
the more delocalised electrons there are per metal cation the better it is at conducting
(eg Al 3+ is better than Li+)
Why are metals malleable and ductile?
pressed into shape (malleable) or stretched into a wire (ductile) as the layers of positive ions can be forced to slide across each other
What are ionisation energies and electronegativities of metals like?
metals have lower ionisation energy than non-metals in the same period as they have fewer protons
metals have lower electronegativity than non-metals so tend not to attract electrons
Metal reaction word equations?
metal + acid → salt + hydrogen
metal + water → metal hydroxide + hydrogen
What are the different types of particles?
Atoms → covalent structures
Ions → ionic compounds
Molecules → simple covalent
Electrons → metallic bonding
What are the different types of structure?
ionic
simple molecular
giant covalent
metallic
Giant ionic lattices
Regular 3D structure of alternating positive and negative ions
Strong electrostatic forces between oppositely charged ions
High melting point
Soluble in water
Conducts electricity when molten
e.g. NaCl
Simple covalent molecules
Covalently bonded substances like iodine (I₂) and ice (H₂O)
Exist as simple molecules
Held together by weak intermolecular forces (e.g. London forces or hydrogen bonding)
Low melting and boiling points
e.g. CH4 or I2
Giant covalent lattices
Examples: diamond, graphite, silicon(IV) oxide (SiO₂)
Atoms bonded by strong covalent bonds throughout structure
High melting point
Diamond
Each carbon bonded to 4 others (tetrahedral)
Giant covalent structure, very hard, high melting point, non-conductor
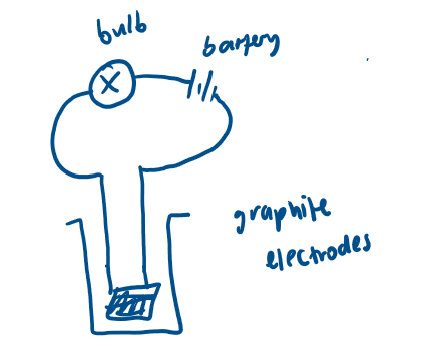
Graphite
nanotubes
Each carbon bonded to 3 others, forms layers
Delocalised electrons between layers → conducts electricity
Layers held by weak forces → slippery
Graphene
Single layer of graphite
Very strong, excellent conductor
Fullerenes
Simple experiment to test conductivity?