Topic 2 - states of matter and mixtures
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
describe the arrangement, movement and the relative energy of particles in each of the three states of matter
the three states of matter are solid, liquid and gas
melting and freezing take place at the melting point
solid → liquid: melting
liquid → solid: freezing
boiling and condensing take place at the boiling point
liquid → gas: boiling
gas → liquid: condensing
they can be represented by the simple model below, particles are represented by small solid spheres
gas: particles have the most energy, as the particels are the most spread apart
liquid: particles have more energy than those in a solid, but less than those in a gas and solid have the least energy - particles vibrate in a fixed position
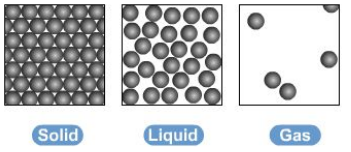
recall the names used for the interconversions between the three states of matter
state changes (melting, boiling, freezing and condensing) are physical changes - they involve the forces between the particles of the substances but the particles themselves don’t change
chemical changes are where a new product has been formed
explain the changes in arrangement, movement and energy of particles during these interconversions
particle theory can help to explain melting, boiling, freezing and condensing…
the amount of energy needed to change state from solid to liquid and from liquid to gas depends on the strength of the forces between the particles of the substance
the nature of the particles involved depends on the type of bonding and the structure of the substance
the stronger the forces between the particles the higher the melting point and boiling point of the substance
predict the physical state of a substance under specified conditions, given suitable data
if you are given the melting point and boiling point of a substance:
at temperature below the melting point, the substance will be solid
at temperatures above the melting point but below the boiling point, the substance will be liquid
at temperatures above the boiling point, the substance will be a gas
what is a mixture
consists of 2 or more elements or compounds not chemically combined together
chemical properties of each substance in the mixture are unchanged
what is a pure substance
a pure substance = a single element or compound, not mixed with any other substance
in everyday language, a pure substance = substance that has had nothing added to it, so it is unadulterated and in its natural state, eg. pure milk
interpret melting point data to distinguish between pure substances
pure substances melt and boil at specific/exact temperatures, mixtures do not:
this means melting and boiling points data can be used to distinguish pure substances from mixtures (which melt over a range of temperatures due to them consisting of 2 or more elements or compounds)
explain the experimental technique for simple distilation
simple distilation is used to seperate a solvent from a solution, it is useful for producing water from salt solution
simple distilation works because the dissolved solute has a much higher boiling point than the solvent
when the solution is heated, solvent vapour evaporates from the solution. the gas moves away and is cooled and condensed
the remaining solution becomes more concentrated in solute as the amount of solvent in it decreases
explain the experimental technique of fractional distilation for seperation of mixtures
used to seperate a pure liquid from a mixture of liquids
works when the liquids have different boiling points
commonly used to seperate ethanol from water
(taking the example of ethanol) ethanol has a lower boiling point than water so it evaporates first. the ethanol vapour is the cooled and condensed inside the condenser to form a pure liquid
sequence of events in distilation is as follows: heating → evaporating → cooling → condensing
fractional distilation is also used to seperate oil. the oil is heated in the fractionating column and the oil evaporates and condenses at a number of different temperatures
the many hydrocarbons in crude oil can be seperated into fractions each of which contains molecules with a similar number of carbon atoms
the fractionating column works continuously, heated crude oil is piped in at the bottom. the vaporised oil rises up the column and the various fractions are constantly tapped off at the different levels where they condense
the fractions can be processed to produce fuels and feedstock for the petrochemical industry
explain the experimental technique of filtration for seperation of mixtures
if you have produced eg. a precipitate (which is an insoluble salt), you would want to seperate the salt/precipitate from the salt soluton
you would do this by filtering the solution, leaving behind the precipitate on the filter paper
explain the experimental technique of crystallisation for seperation of mixtures
if you were to have produced a soluble salt and you wanted to seperate this salt from the solution that it was dissolved in
you would first warm the solution in an open container, allowing the solvent to evaporate, leaving a saturated solution
allow this solution to cool
the solid will come out of the solution and crystals will start to grow, these can then be collected and allowed to dry
explain the experimental technique of paper chromatography for seperation of mixtures
chromatography..
used to seperate mixtures and give information to help identify substances
involves a stationary phase and a mobile phase
seperation depends on the distribution of substances between the phases
Rf value = distance moved by substance/distance moved by solvent
different compounds have different Rf values in different solvents, which can be used to help identify the compounds
compounds in a mixture may seperate into different spots depending on the solvent but a pure compound will produce a single spot in all solvents
paper chromatography
analytical technique separating compounds by their relative speeds in a solvent as it spreads through paper
the more soluble a substance is, the further up the paper it travels
seperates different pigments in a coloured substance
pigment
solid, coloured substance
describe paper chromatography
the seperation of mixtures of soluble substances by running a solvent (mobile phase) through the mixture on the paper (the paper contains the stationary phase), which causes the substances to move at different rates over the paper
interpret a paper chromatogram: to distinguish between pure and impure substances, to identify substances by comparison with known substances and to identify substances by calculation and use of Rf value
pure substances: should only have one spot on a chromatogram
impure substances/mixtures: will show up with more than one spot on a chromatogram
to identify by comparing with known substances: carry out paper chromatography with both the known substance and substance you’re testing on the same paper. if both spots are at the same height up the paper at the end then you know the substance you’re testing is the same as the known substance
to identify by calculation of Rf values: you can calculate Rf values and then compare them to known values for different substances
Core practical: Investigate the composition of inks using simple distillation and paper chromatography
Aims:
to investigate the composition of inks using simple distillation and chromatography
Simple distillation
method:
add a small volume of ink to a flask. connect the flask to the fractionating column and secure it with a stand, boss and clamp
attach a condenser to the top of the fractionating column, connect it to a cold water tap and sink, secure it over a beaker
heat the flask using a Bunsen burner, reducing the flame as necessary to achieve gentle simmering
collect a small sample of the distilled solvent, then turn the Bunsen burner off
results
describe the appearance of the distilled solvent
if your apparatus included a thermometer at the top of the column, record the maximum temperature reaches as the solvent was collected
analysis
explain any difference in the appearance of the solvent and ink
if you measured the maximum temperature, compare this to the boiling points of possible solvents. these could include water, ethanol and propanol
Paper chromatography
method:
draw a pencil line across the chromatography paper, 1-2 cm from the bottom
use a pipette or capillary tube to add small spots of each ink to the line on the paper
place the paper into a container with a suitable solvent in the bottom
allow the solvent to move through the paper, but remove the chromatogram before it reaches the top
allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent
results
record your results in a suitable table
analysis
calculate the Rf value of each spot
compare the Rf values and colours of each spot in the inks. describe their similarities and differences
how can waste and ground water can be made potable, including the need for sedimentation, filtration and chlorination, sea water can be made potable by using distillation and water used in analysis must not contain any dissolved salts
potable water: it is suitable for drinking so much have:
low levels of microbes
low levels of containing substances
it is not the same as pure water but is still safe
making waste and ground water potable:
sedimentation: large insoluble particles will sink to the bottom of the water
filtration: water is filtered through beds of sand which removes small insoluble particles
chlorination: chlorine gas is put through water to kill microbes
making sea water potable using distillation
filter the seawater
boil it
water vapour is cooled and condensed
water used in analysis:
must be pure because any dissolved salts could react with the substances you are analysing, leaving you with a false result