nuclear chemistry vocab + notes
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
a process that changes the nucleus of an atom by releasing/absorbing energy
nuclear reaction
atoms of the same element with the same number of protons but a different number of neutrons
isotope
a nuclear reaction in which 2 light atomic nuclei combine to form a heavier nucleus, releasing large amounts of energy
fusion
high speed electron emitted from decaying nucleus and has a medium penetration
beta particle
the time it takes for half of a sample of a radioactive substance to decay into a stable form
half-life
process of changing energy from one form to another
energy transformation
high-energy electromagnetic waves released from a radioactive nucleus and has no mass or charge; has a high penetration power
gamma radiation
the central core of an atom and made of protons and neutrons; contains most of the atoms mass
nucleus
series of nuclear fission reactions in which the neutrons released by reaction reaction cause additional fission reactions
chain reaction
nuclear reaction in which a heavy nucleus splits into smaller nuclei, releasing energy
fission
a type of radiation made of 2 protons and 2 neutrons
alpha particle
process by which an unstable atomic nucleus loses energy by emitting radiation
radioactive decay
is the sun/stars an example of fission or fusion?
fusion
is the chain reaction in U-235 (the atomic bomb) an example fission or fusion
fission
what are the conditions needed for nuclear fusion to occur?
extremely high pressure and temperature (greater than 40 million degrees celsius)
what are the conditions needed for nuclear fission to occur?
a neutron needs to be released from a previous U-235 nuclei that went through fission
what happens to the energy in a nuclear fission reaction
the energy comes from kinetic energy from fission fragments; emitted neutrons turn to heat as they collide with surrounding atoms
what happens to the energy in a nuclear fusion reaction
the mass is transferred into thermal energy, releasing large amounts of energy greater than fission
how many protons and neutrons are in Strontium - 90?
38 protons and 52 neutrons
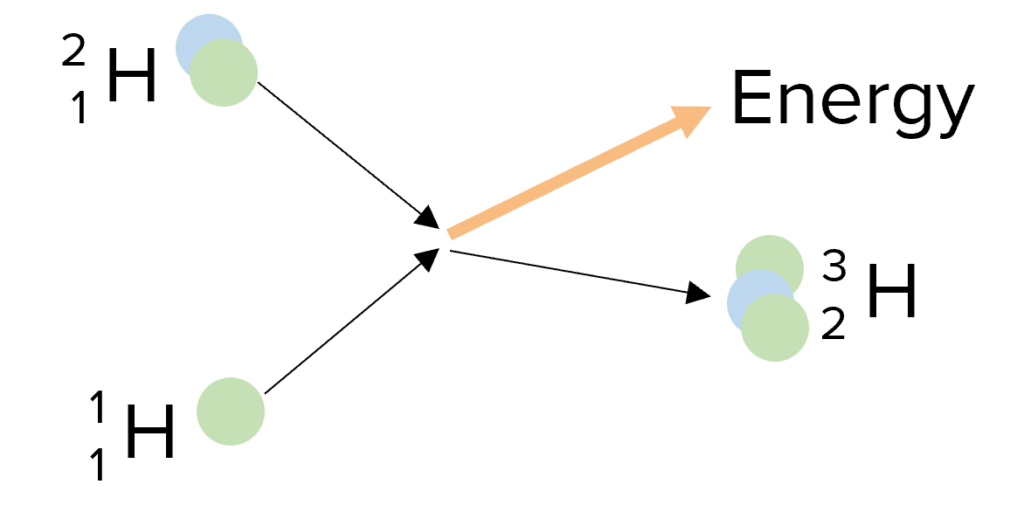
does this diagram show nuclear fission or nuclear fusion?
fusion
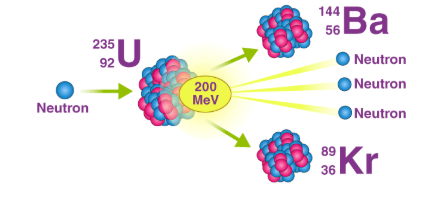
does this diagram show nuclear fission or nuclear fusion?
fission
name the family of radioactive elements
Actinide family
find the subatomic particles of 14C (carbon-14).
6 protons, 8 neutrons, 6, electrons
who discovered radioactive decay
Henri becquerel, marie curie and her husband
what are the 3 types of radiation and what are their penetration powers
alpha (weak), beta (medium), and gamma (strong)
what are the advantages of radiation
medical treatment, energy generation, and industrial use
what are the disadvantages of radiation
illness, nuclear accidents, difficult disposal
which elements are more likely to be radioactive
elements with a high atomic number are unstable (83)
what is the process of creating energy in a nuclear power plant
fission occurs in the reactor core, releasing heat →heats water → produces steam → steam spins turbine → turbine drives generator → ELECTRICITY → steam cools back to water to be reused
what does the boiling water reactor (BWR) do
water boils inside the reactor and turns directly into steam
what does the pressurized water reactor (PWR) do
water is kept under pressure so it does not boil, but steam is still produced