Chapter 2 chemistry part 1 inorganic compounds
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
matter
has mass and takes up space
atom
smallest stable unit of matter
molecules
when atoms combine (two or more held by chemical means)
element
all of the same type of atom, cannot be broken down into simpler substances by ordinary means. (note: periodic table of elements, 118 known)
compound
2 or more elements held by chemical bonds
Example: (glucose molecule) C6H12O6 is a compound, O2 is not
II. Energy (E)
the capacity to do work
potential
the E an object has because of its position in relation to other objects.
(i.e. an object has the capability to do work) = “stored energy”
kinetic
the E associated with a moving object (the form of E that is actually doing work) = “energy of motion”
III. Atomic structure
Fundamental particles: protons
(+) in nucleus and one mass unit *protons and neutrons similar in size and mass
neutrons
(no charge) in nucleus and one mass unit *protons and neutrons similar in size and mass
electrons
(-), smaller (1/1800th the size),
considered weightless
located in orbitals/clouds surrounding the nucleus
IV atomic measurements
periodic table
lists measurements, appendix E (94 naturally occurring)
1.atomic number
the number of protons in nucleus is written to the left of the symbol *(Note: since the number of protons in a NEUTRAL atom always equals the number of electrons, the atomic number indirectly tells the number of electrons.)
2.mass number
sum of the masses of its protons and neutrons (mass of electrons is so small that it is ignored)
atomic weight
derived from an average mass, determined for any individual element.
V isotopes
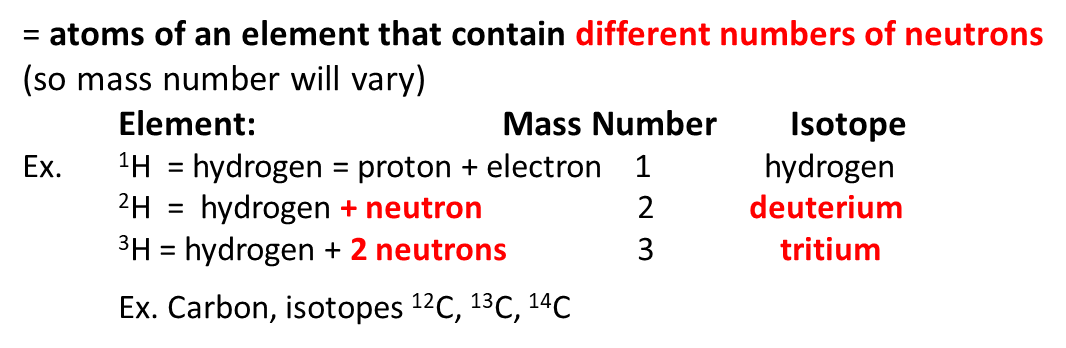
radioisotopes
elements with unstable nuclei that emit subatomic particles in measurable amounts. (alpha, beta, or gamma particles)
Half life
time required for 50% reduction in radioactivity
(seconds/hours to tens of thousands of years)
-Used in Medical Imaging; measures vitamin absorption, blood volume, organ functioning…etc. (uses isotopes with short half-lives)
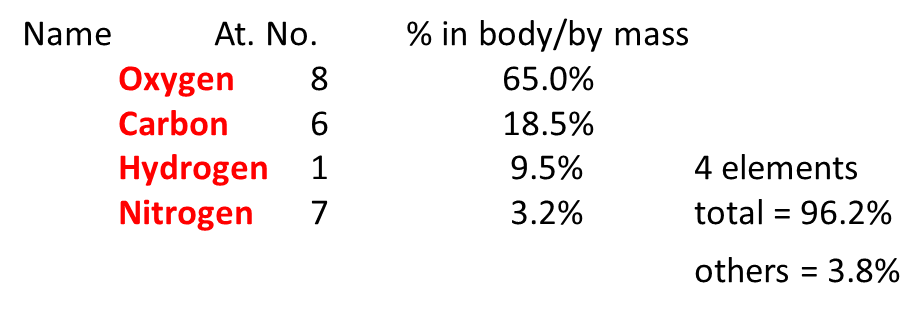
VI. 4 Elements of the human body:
VII. Matter in Combination
A, mixture
: 2 or more substances physically intermixed (not bonded)
Three types
1.solution
when two or more substance are EQUALLY mixed
“homogeneous”
solvent
dissolving compound (largest quantity)
solute
compound being dissolved (smallest quant.)
colloid – (ex. jello, cytosol in cells)
when two or more substances are UNEQUALLY
mixed, “heterogeneous”
- particles do not settle out, solutes generally larger.
3.suspension (ex. blood)
Also, a heterogeneous mixture
- Large, sometimes visible solutes
- Solutes will settle out if left long enough
(Ex. antibiotic suspension, sand and water)
Principles of chemical bonds
I. Atomic Energy Levels
Electrons occupy specific areas in the atom, the amount of E that can be contained in any one space (level) determines the number of electrons that will be present at the specific level.
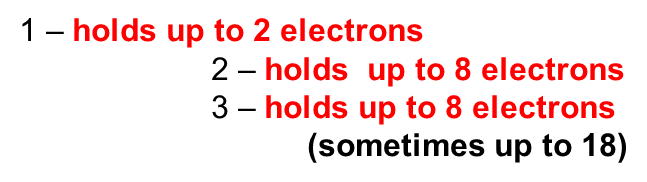
Energy Levels (shells):
Rule of 8 will govern reactivity
“octet rule”
Ex. Ne, an inert (Noble) gas, has a stable configuration of electrons in its “outer shell”, also called its “valence” shell.
Ex. Na, a highly reactive element, will tend to lose the outer electron to get down to the most stable configuration (8 in the outer shell= “valence shell”)
II. atomic interactions
A. Two Rules Govern Interactions:
1. An unfilled electron shell is “unstable”
Atoms tend to interact to “fill” the electron shell via gaining, sharing, or losing electrons. (full shells: #1 = 2 electrons, #2 & 3 = 8 electrons)
2. Number of electrons in outer shell determines properties of an element
(Periodic Table helps predict reactivity)
Ions defined
atoms or molecules that have a + or - charge
cation
positive chg. ion (loss of electrons)
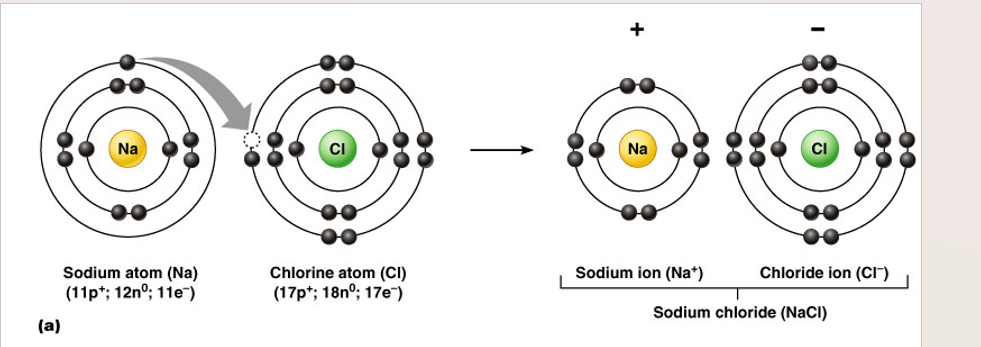
anion
negative chg. ion (gain of electrons)
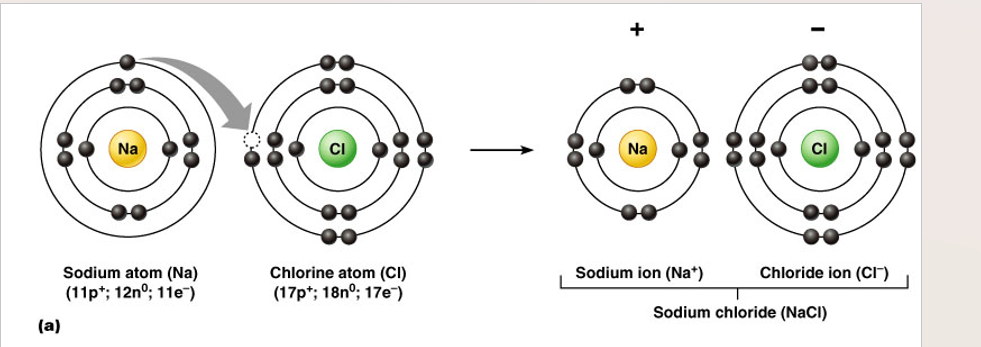
Ionic bonds
•Atoms in this type of bond donate or take on electrons
•Results in a stable outer shell
•Occurs between particles that are charged (ions)
b. Types of molecular bonds:
1.Ionic bonds
2.non polar covalent bonds
Ex. H2, Hydrogen molecule, single covalent bond
Goal: to attain a stable electron configuration (i.e. to fill the energy level requirements)
2.Non-polar Covalent Bonds
Goal: to attain a stable electron configuration (i.e. to fill the energy level requirements)
Ex. O2, Oxygen molecule, double bond
Ex. CO2, Carbon dioxide, double bond
Ex. N2, Nitrogen molecule, triple bond
Characteristics of covalent bonds
strong
often electrically neutral (equal sharing of electrons)
If non-polar indicates no “poles” to molecule
Polar covalent bonds
Sharing of electrons is UNEQUAL
Ex. water H2O
Neg. charge predominates at oxygen
“Separation of charges exists”
Hydrogen Bonds (attraction)
After forming polar or ionic bonds with other elements (oxygen and nitrogen frequently)…
The weakly positive hydrogen atoms can be attracted to nearby atoms/ions that carry a negative charge.
Important for inter-molecular attractions - Ex. 3-D
surface tension in water, or structures of proteins
III. chemical notation
Used to describe events in a precise and concise way.
A. Rules
Element symbol indicates one atom (know these):
Ex. H, C, N, O, Fe, Mg, Zn, Ca, Na, K, P, CI, S, I
The number preceding the symbol indicates more than one atom/molecules.
Ex. 2 He, there are two helium atoms
Ex. 4 H20, there are four water molecules
Number (subscript) written following symbol indicates the number of that specific element in a molecule.
Ex. H2 = one hydrogen molecule (2 H atoms bound together
Ex. 3 H2 = 3 hydrogen molecules (6 H atoms)
Note: NO subscript means one atom present in molecule
Ex. Water, H2O
writing reactions (rxn), (shorthand)
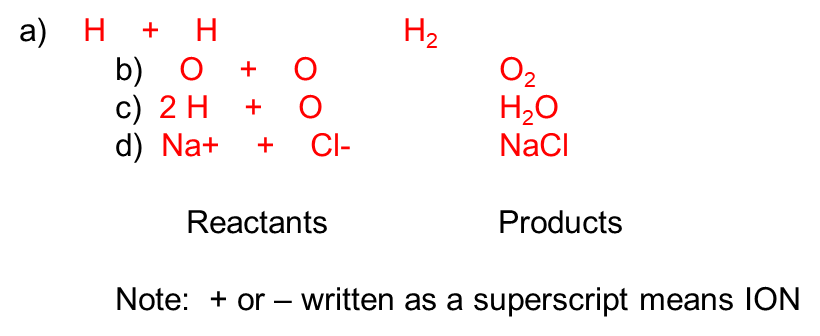
5. All RXNs must be balanced-
Reactants must equal the Products
(No. of atoms on one side of the arrow equal the no. of atoms on the other side of the arrow)
2 H + O —) H2O , balanced but Hydrogen and Oxygen atoms don’t exist in nature by themselves, so must change the equation.
2 H2 + O2 —) H2O , formulae are accurate, but equation is not balanced
2 H2 + O2 —) 2 H2O , Balanced with correct formulae

IV. chemical reaction
A. characteristics of chemical bonds
contain energy
stronger bond means more E: (see textbook)
STRONGEST ^ covalent bonds (nonpolar)
Covalent bonds (polar)
Ionic bonds
WEAKEST v Hydrogen bonds
IV. chemical categories
B. categories of reactions
decomposition -
molecules broken down into smaller fragments
AB —) A + B + (E) * Used by organisms to do work.
E = energy available

synthesis
smaller fragments are assembled to make larger molecules.
(E) + A + B —) AB E = energy used

IV. chemical reactions
B. categories of reactions
exchange -
reactants and products contain the same components in different combinations (aka displacement reactions)
AB + CD —) AC + BD

a. Exergonic rxn –
when E released from the decomposition rxn is more than the E required for synthesis rxn.
b. Endergonic rxn
when E required for synthesis is more than E produced from decomposition rxn.
IV. chemical reactions
C. characteristics of chemical reactions
reversible (theoretically)
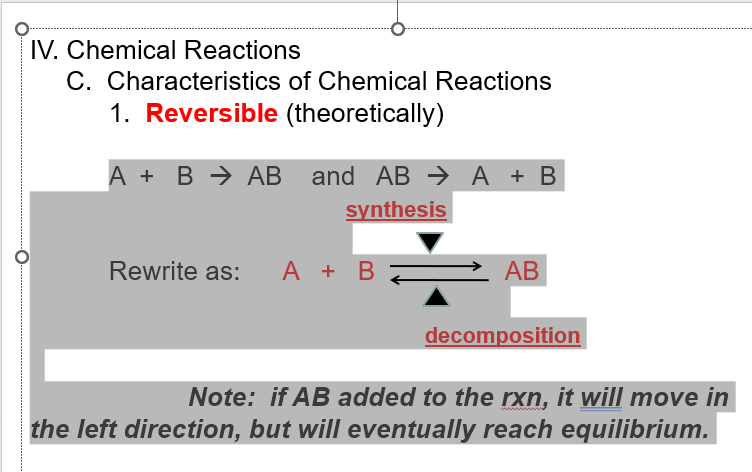
IV. Chemical Reactions
C. Characteristics of Chemical Reactions
2. Chemical Equilibrium–
at some point the product AB is synthesized at the same rate AB is
decomposed to A + B.
“AT EQUILIBRIUM”
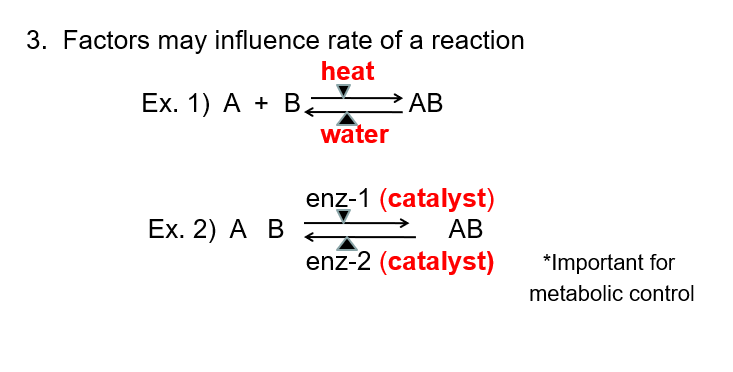
3. Factors may influence rate of a reaction