Inorganic ions, water and ATP
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Give two roles of phosphate ions in cells
1. (In) DNA;
2. (In) RNA; If neither DNA or RNA named allow 1 mark for nucleotide/nucleic acid/phosphodiester bond/sugar-phosphate backbone
3. (In) ATP/ADP; Accept any other correct biological compound containing phosphate; eg (in)RuBP or GP or triose phosphate or NAD
4. Phosphorylation; Accept binds with substance to make it more reactive
5. (In) phospholipids;
Diseased lungs can cause carbon dioxide to build up in the blood plasma. This leads to an increase in hydrogen ion concentration in the plasma. Describe the effect this increase in hydrogen ion concentration has on the plasma and on the proteins in the plasma. Do not refer to the Bohr effect.
1. Increased (plasma) acidity OR Decreased (plasma) pH;
2. Denatures (protein) OR Changes (protein) tertiary structure OR Changes active site (shape) OR Changes antigen-binding site (shape);
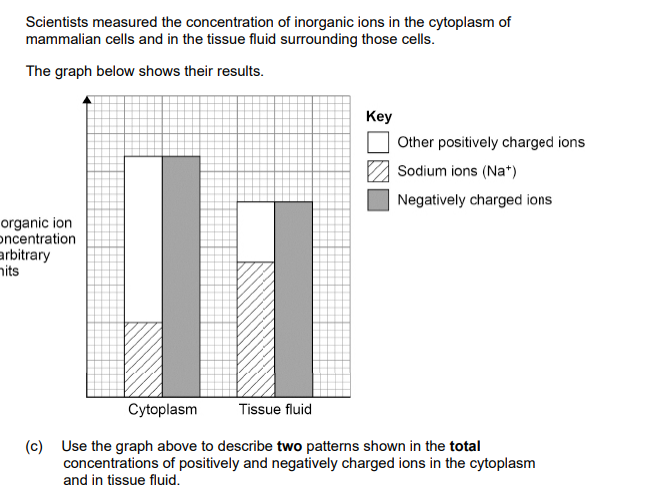
1. Equal positive and negative (in both); Accept answers in either order Ignore reference to sodium ions Accept no overall charge or no net charge
2. Higher (ion concentration) in cytoplasm; Ignore more ions in cytoplasm
(d) In these mammalian cells, the: • cell-surface membrane is permeable to sodium ions • sodium ion concentration does not increase in the cytoplasm over time. Use this information and the graph above to suggest and explain the ion transport mechanisms involved in the transport of sodium ions.
1. (Sodium) ions move in (to cells) by facilitated diffusion down a concentration gradient; Accept a description of the concentration gradient, eg from high (concentration) to low (concentration)
2. (Sodium) ions move out (of cells) by active transport against a concentration gradient; Accept a description of transport against a gradient, eg from low (concentration) to high (concentration)
Explain a property of iron ions that enables these ions to carry out their role in red blood cells.
(Is) charged/polar OR (Is) part of haem(oglobin); Accept Fe2+ OR Fe3+ for ‘charged’
2. (So) binds/associates/loads (with) oxygen OR (So) forms oxyhaemoglobin OR (So) transports oxygen; Accept ‘carries̵for transports
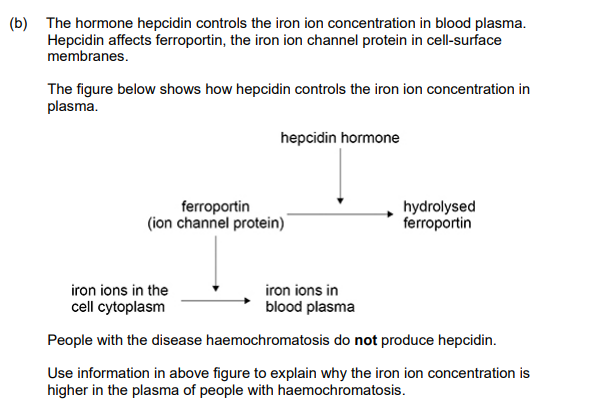
1. Less/no ferroportin hydrolysis/breakdown; Accept ‘channel protein’ for ferroportin 2. (So) more ferroportin (in cell-surface membranes); Accept ‘channel protein’ for ferroportin Accept ‘many’ for more 3. (So) more iron (ion) transport from cytoplasm/cell; Accept ‘many’ for more
Describe how an ATP molecule is formed from its component molecules.
(a) 1. and 2. Accept for 2 marks correct names of three components adenine, ribose/pentose, three phosphates;; Accept for 1 mark, correct name of two components Accept for 1 mark, ADP and phosphate/Pi Ignore adenosine Accept suitably labelled diagram 3. Condensation (reaction); Ignore phosphodiester 4. ATP synthase; Reject ATPase
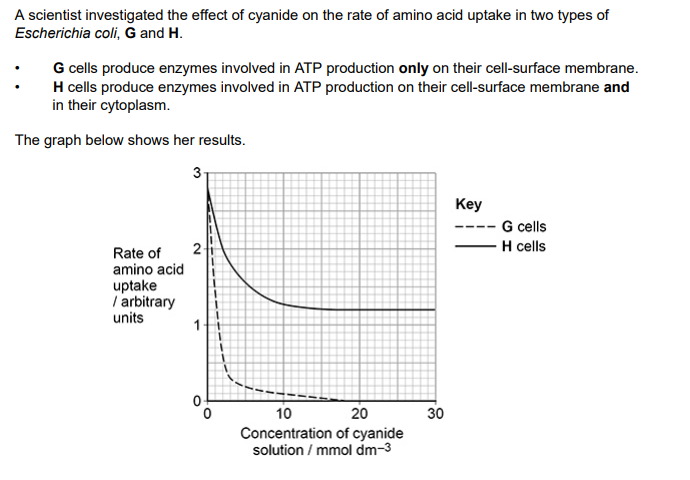
(b) Use the graph above to calculate the percentage decrease in the rate of amino acid absorption by H cells in 30 mmol dm–3 cyanide solution.
(c) Using the graph above and the information provided, what can you conclude about amino acid uptake by G cells and by H cells?
(b) Correct answer for 1 mark = 57/57.1; 1 (c) 1. (Amino acid uptake by) active transport; Accept for ‘transport’, process 2. Cyanide reduces/stops amino acid uptake; 3. ATP production stops on membranes OR Enzymes not working on membranes; 4. ATP production continues in cytoplasm OR Enzymes active in cytoplasm
State and explain the property of water that can help to buffer changes in temperature.
(a) 1. (water has a relatively) high (specific) heat capacity; Ignore numbers relating to heat capacity 2. Can gain / lose a lot of heat / energy without changing temperature; OR Takes a lot of heat / energy to change temperature; Accept due to H bonding between water molecules
Hydrolysis of ATP is catalysed by the enzyme ATP hydrolase. A student investigated the effect of ATP concentration on the activity of ATP hydrolase. She used shortening of strips of muscle tissue caused by contraction as evidence that ATP was being hydrolysed. • She took four slides A, B, C and D, and added strips of muscle tissue of the same length to each slide. • She then added the same volume of ATP solutions of different concentrations to the four slides and left each slide for five minutes. • She then recorded the final length of each strip of muscle tissue. Her results can be seen in the table.
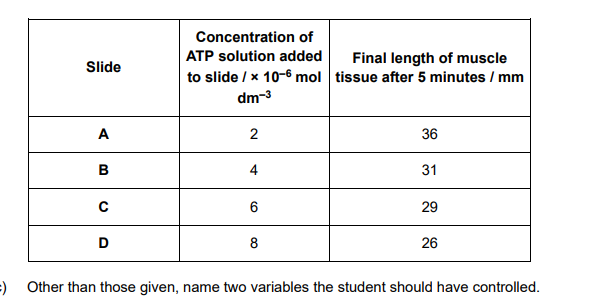
1. Species / organism the muscle tissue came from; OR Thickness / type / source of the muscle tissue; Ignore surface area of muscle tissue 2. Temperature of the muscle tissue / ATP solution / slides; Need to be qualified 3. pH of the ATP solution; Need to be qualified Reject concentration / volume of ATP hydrolase
Describe and explain the pattern shown by the data in the table.
Description 1. As concentration of ATP increases, length of muscle decreases; Accept negative correlation
Explanation 2. More ATP (hydrolysed by ATP hydrolase), so more energy released, so more muscle contraction / shortening of muscle; Accept more ATP available for correct/named aspect of muscle contraction Idea of more is required once. Reject energy produced
(a) Describe the roles of iron ions, sodium ions, and phosphate ions in cells.
Iron ions
1. Haemoglobin binds/associates with oxygen OR Haemoglobin transports/loads oxygen; Ignore reference to 2+ or 3+ in Fe2+ or Fe3+ 4.
Sodium ions
2. Co-transport of glucose/amino acids (into cells); 3. (Because) sodium moved out by active transport/Na – K pump; 4. Creates a sodium concentration/diffusion gradient; 5. Affects osmosis/water potential;
Phosphate ions
6. Affects osmosis/water potential; Accept 5. OR 6. – not both 7. Joins nucleotides/in phosphodiester bond/in backbone of DNA/RNA/in nucleotides; 8. Used in/to produce ATP; Reject ‘energy produced’ 9. Phosphorylates other compounds (usually) making them more reactive; 10. Hydrophilic/water soluble part of phospholipid bilayer/membrane
The movement of substances across cell membranes is affected by membrane structure. Describe how.
1. Phospholipid (bilayer) allows movement/diffusion of non-polar/lipid-soluble substances
2. Phospholipid (bilayer) prevents movement/diffusion of polar/ charged/lipidinsoluble substances OR (Membrane) proteins allow polar/charged substances to cross the membrane/bilayer; Accept water-soluble 3. Carrier proteins allow active transport; 4. Channel/carrier proteins allow facilitated diffusion/co-transport; Accept aquaporins allow osmosis 5. Shape/charge of channel / carrier determines which substances move; 6. Number of channels/carriers determines how much movement; 7. Membrane surface area determines how much diffusion/movement;
8. Cholesterol affects fluidity/rigidity/permeability;
Explain five properties that make water important for organisms.
(a) 1. A metabolite in condensation/hydrolysis/ photosynthesis/respiration; 2. A solvent so (metabolic) reactions can occur OR A solvent so allowing transport of substances; 3. High heat capacity so buffers changes in temperature; For ‘buffer’ accept ‘resist’. 4. Large latent heat of vaporisation so provides a cooling effect (through evaporation); 5. Cohesion (between water molecules) so supports columns of water (in plants); For ‘columns of water’ accept ‘transpiration stream’. Do not credit ‘transpiration’ alone but accept description of ‘stream’. For ‘columns of water’ accept ‘cohesion-tension (theory)’. For cohesion accept hydrogen bonding 6. Cohesion (between water molecules) so produces surface tension supporting (small) organisms;