WJEC AS Chemistry Unit 1
1/161
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
162 Terms
What is oxidation?
The loss of electrons
What is reduction?
The gain of electrons
If something has been reduced, what is it?
Oxidising agent (it has gained electrons, causing a different species to lose electrons)
If something has been oxidised, what is it?
Reducing agent (it has lost electrons, causing a different species to gain electrons)
How do metals react in terms of electrons?
React by losing electrons
- oxidised
- reducing agent
How to non-metals react?
React by gaining electrons
- reduced
- oxidising agent
What are the rules for oxidation state numbers?
F is always -1
O is always -2
H is always +1
What are the exceptions of the rules of oxidation numbers?
Hydrogen peroxide (oxygen in -1)
Lithium hydride (hydrogen is -1)
What has happened if the oxidation number decreases?
It has been reduced so is an oxidising agent
What has happened if the oxidation number has increased?
It has been oxidised so is a reducing agent
Atomic Number
No. of protons in the nucleus
Mass Number
No. of protons + no. of neutron
Isotopes
Atoms which have the same number of proton, but a different number of neutrons
Exam Tips
Atomic Number = Number of protons
Number of protons = Number of electrons
Number of neutrons = Mass no. - Atomic no.
Representing atoms key positions
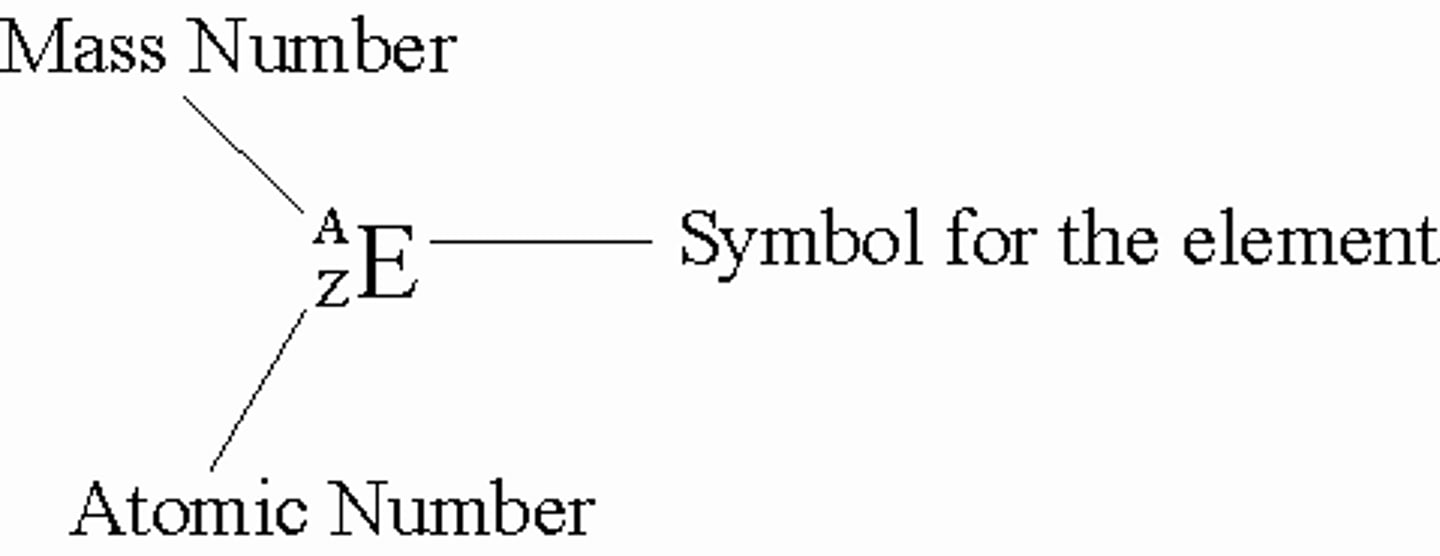
Radioactivity
Isotopes are unstable and split up to form smaller atoms. The nucleus divides, causing protons, neutrons and electrons to fly out.
Types of emmission
Alpha, Beta and gamma are types of radiation given off from unstable isotopes.
Alpha Particles
Consist of two protons and neutrons and are helium nuclei. Least penetrating and are stopped by paper or even air. Highly ionising.
Beta Particles
Consists of streams of high energy electrons and are more penetrating. Stopped by 5mm of aluminium. weak ionising.
Beta particles are electrons from nucleus not electrons
Gamma Particles
High energy electromagnetic waves and most penetrating, pass through many centre metres of lead. Least ionising to none.
Alpha Emission
Mass number decrease by 4 and the atomic number by 2
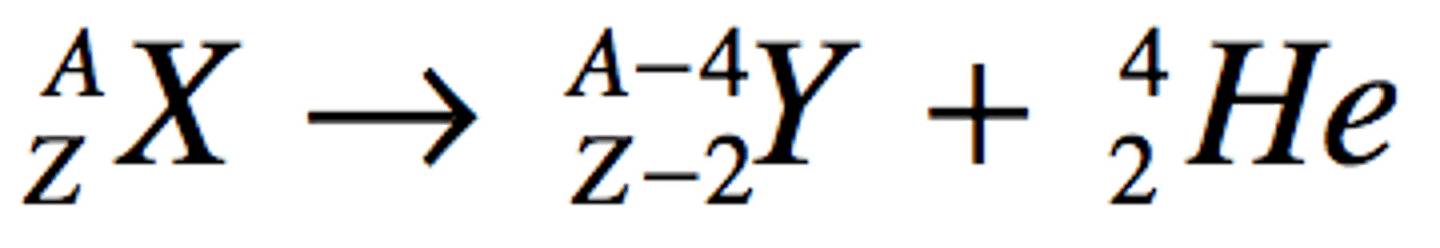
Beta Emission
Mass number stays the same and atomic number gains 1
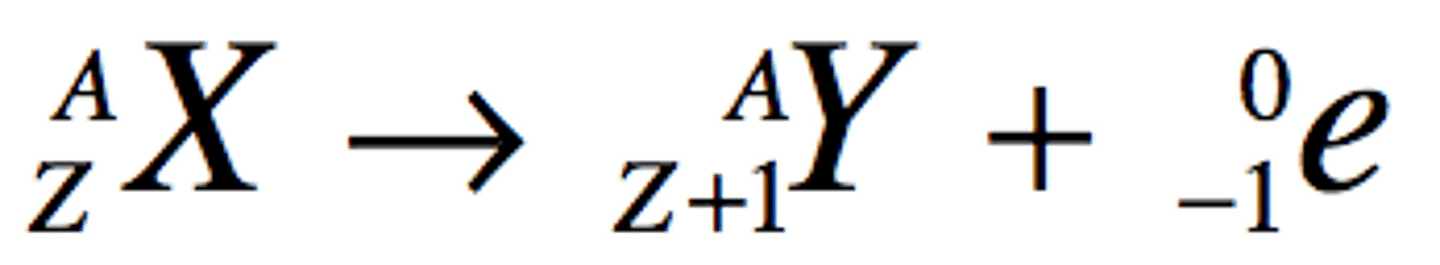
Beta Decay
Normal: moves one place to the right in periodic table
Electron: Opposite: Atomic number loses 1, as a positively charged electron i used. (positron) One place to the left.
Gamma Emissions
No change
Radiation on living cells
Everyone receives background radiation, but it isn't harmful unless it taken in by big doses. High level radiation causes serious damage to cells and organs
Radiodating
Half life can be used to determine the age of either organisms or rocks. Carbon-12 is used to measure.
Radiation in medicine
Cobalt-14 is used in radiotherapy for the treatment of cancer. High energy is used to kill cancer cells.
Radiation in industry and analysis
Beta is used to measure the thickness of metal sheets
Half Life
The time taken for half of the nuclei to decay.
Half life importance
Indicated how quickly isotopes decay
Not effected by the original mass or the temperature
Different from one radioisotope to another
Inversely proportional to the rate of decay
The half life of ¹³³Xe is 5 days. How long does it take 64g of ¹³³Xe to disintegrate to 1g
64→32→16→8→4→2→1
6*5 = 30 days
S atomic orbital
e.g 1s
P orbital
Measure at right angels within the shell
Determination of greater ionising ennergy
Nuclear charge - No. protons
Atomic radius - Distance from electron to nucleus
The shielding effects - Number of full outer shells outer shell and nucleus
Describe the line emission spectrum of hydrogen.
A series of dark lines of light of an exact frequency on a black background. The lines get closer together as frequency increases. (Balmer series - visible region - n=2)
What are the two equations needed when dealing with frequencies and wavelengths etc...?
Speed of light = frequency x wavelength
C = f x λ
Energy = Plancks constant x frenquency
E = h x f
What happens to wavelength as you increase frequency?
Decrease wavelength
What happens to energy as you increase frequency?
Increase energy
Explain the line emission spectrum of hydrogen.
An electrical discharge is passed through atomic hydrogen.
An electron in a lower energy level will about an exact amount of energy that is EQUAL to the energy difference between two energy levels.
This electron is promoted to a higher energy level.
In the higher energy level, the electron is unstable so will fall back into the lower energy level. As the electron falls, it releases energy as an exact frequency of light.
This is why we see lines of exact frequency.
What do the exact lines of light tell us about electrons?
Electrons can only exist in exact energy levels and these energy levels must get closer together as their energy increases
What would you see if electrons could exist anywhere?
A spectrum of continuous light
Explain why the lines of light become closer together at the high frequency end of the spectrum?
E can only exist in exact energy levels, which get closer and closer together as energy and frequency increases
Describe the Balmer series.
Visible region
Lower energy
Falling back into n=2
Describe the Lyman series
U.V region
Higher energy
Falling back into n=1
Why do we use the Lyman series for the ionisation energy and not the Balmer?
The electron is lost from the groundstate in the Lyman series whereas in the Balmer series it is not lost from the groundstate
What is the convergence limit?
This is where separate lines can no longer be seen as they converge. At this point, the e no longer falls back into a lower energy level, therefore the electron has been lost
Explain the absorption spectra.
An electron will absorb an exact frequency of light that is DIRECTLY PROPORTIONAL to the energy gap between two energy levels.
This will cause the e to 'jump' int the higher energy even. However, this electron is unstable so 'falls' back down. As it falls, it releases the energy as an exact frequency of light.
Explain why hydrogen emits only certain definite frequencies of visible light.
The e can only exist in exact energy levels so when they fall back down from a higher energy levels, they emit light at exact frequencies
Mᵣ
Abreviation for Molecular Mass
Aᵣ
Abreviation for Atomic Mass
Atomic mass
The average mass of one atom of the element relative to ¹/₁₂ the mass of an atom of carbon-12
Formula Mass
The average mass of a molecule/formula unit relative to ¹/₁₂ the mass of an atom of carbon-12
Isotopic Mass
The mass of an atom to an isotope relative to the ¹/₁₂ of the mass of an atom of carbon-12
Molecular Mass
Total mass of an empirical formula
Empirical Formula
A formula showing the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.
Molecular Formula
A formula showing the numbers and types of atoms in a molecule.
Molar mass
The mass of a mole of a substance
Mole
6 × 10²³ of something
Atom economy
A way to measure the atoms wasted when making a chemical .
Percent yield
A measure of the efficiency of a chemical reaction using percentage.
Theoretical yield
The amount of product predicted to be made based its reaction formula.
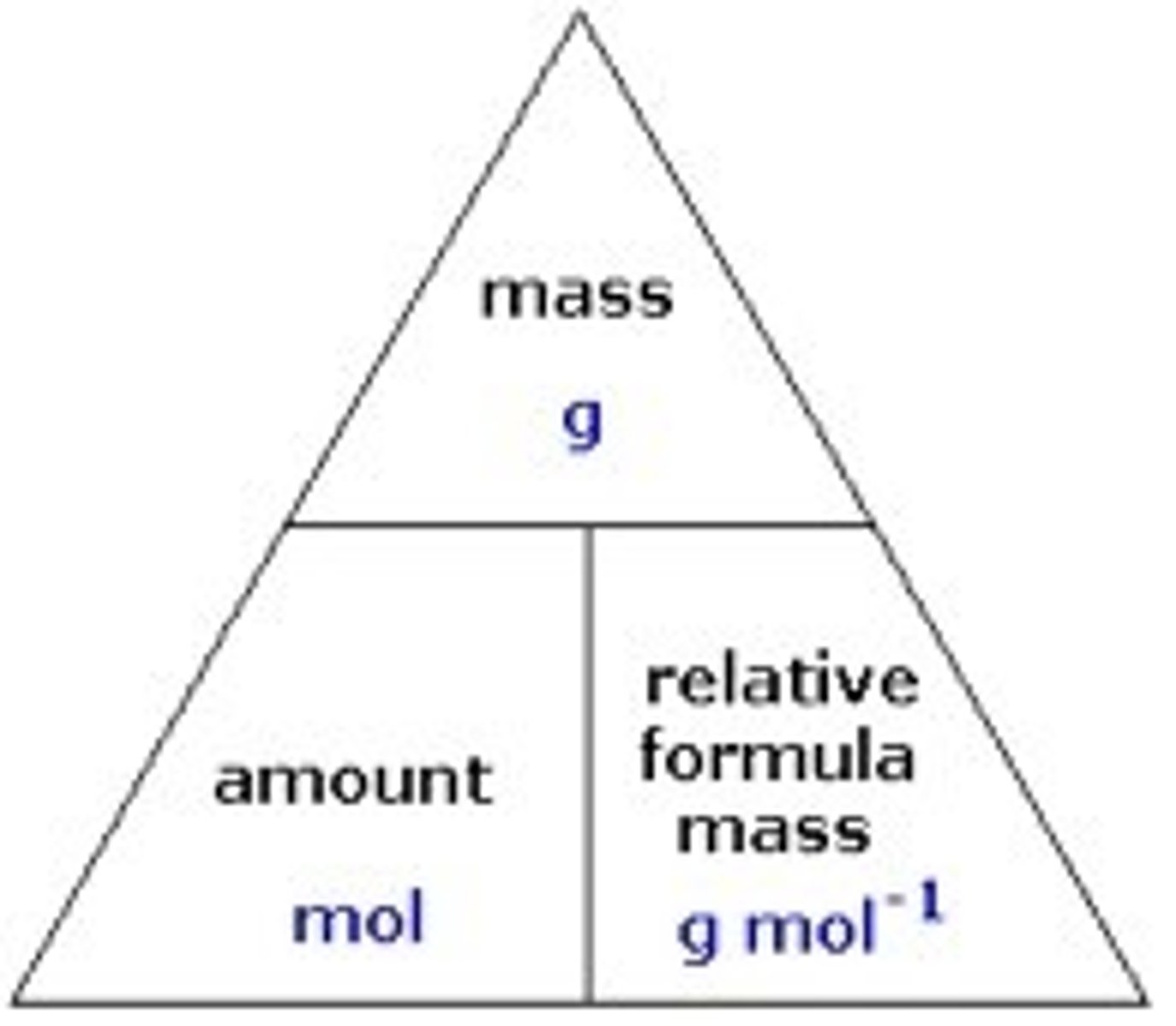
Mole formula
n=m/M
Mole = Mass / Molar mass
No. of particles
N=nL
No. particles = Moles * Avogadro number
Volume of gas formula
Mole = Volume / Molar Volume
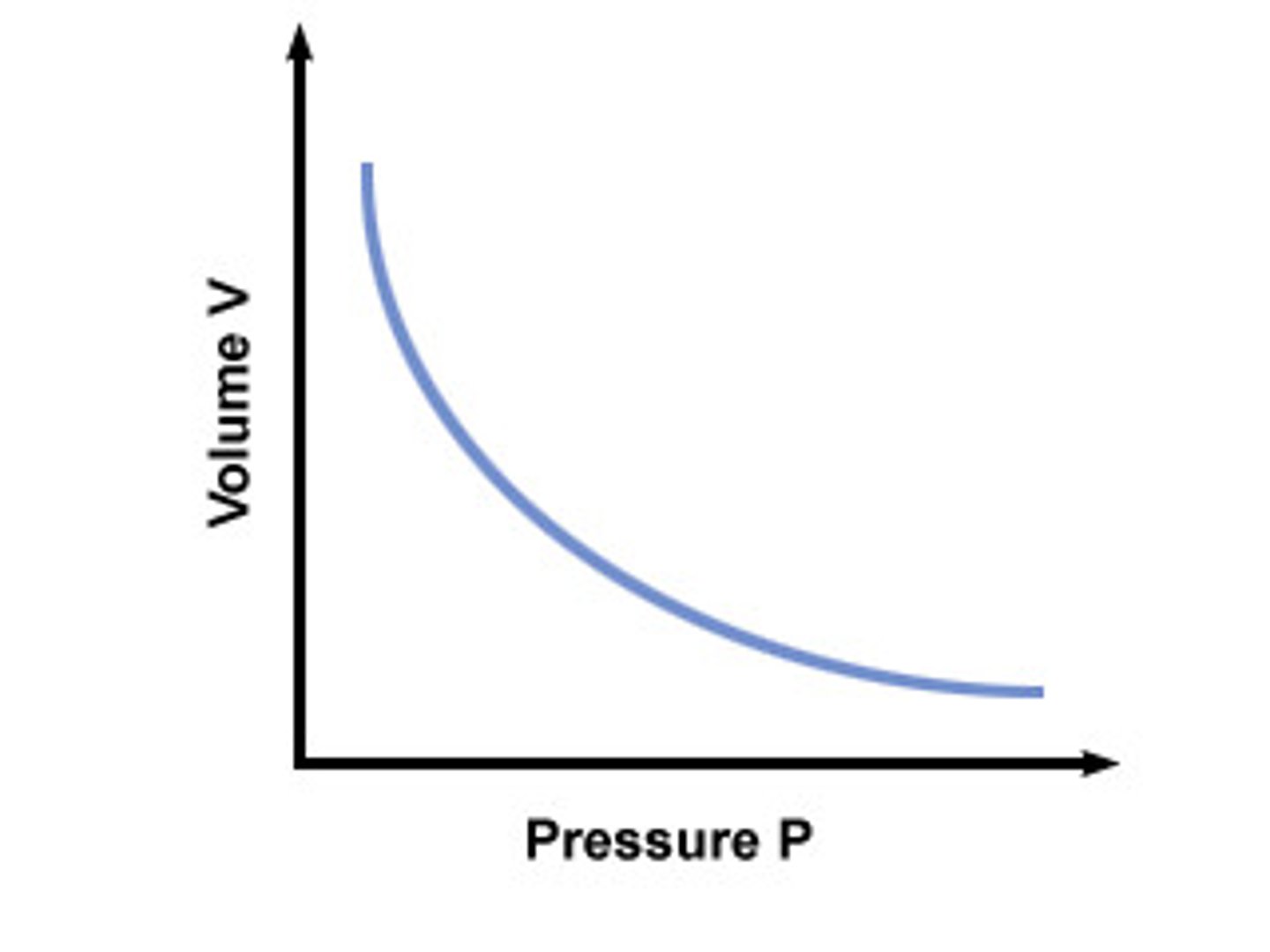
Boyles Law
Pressure * volume = Constant
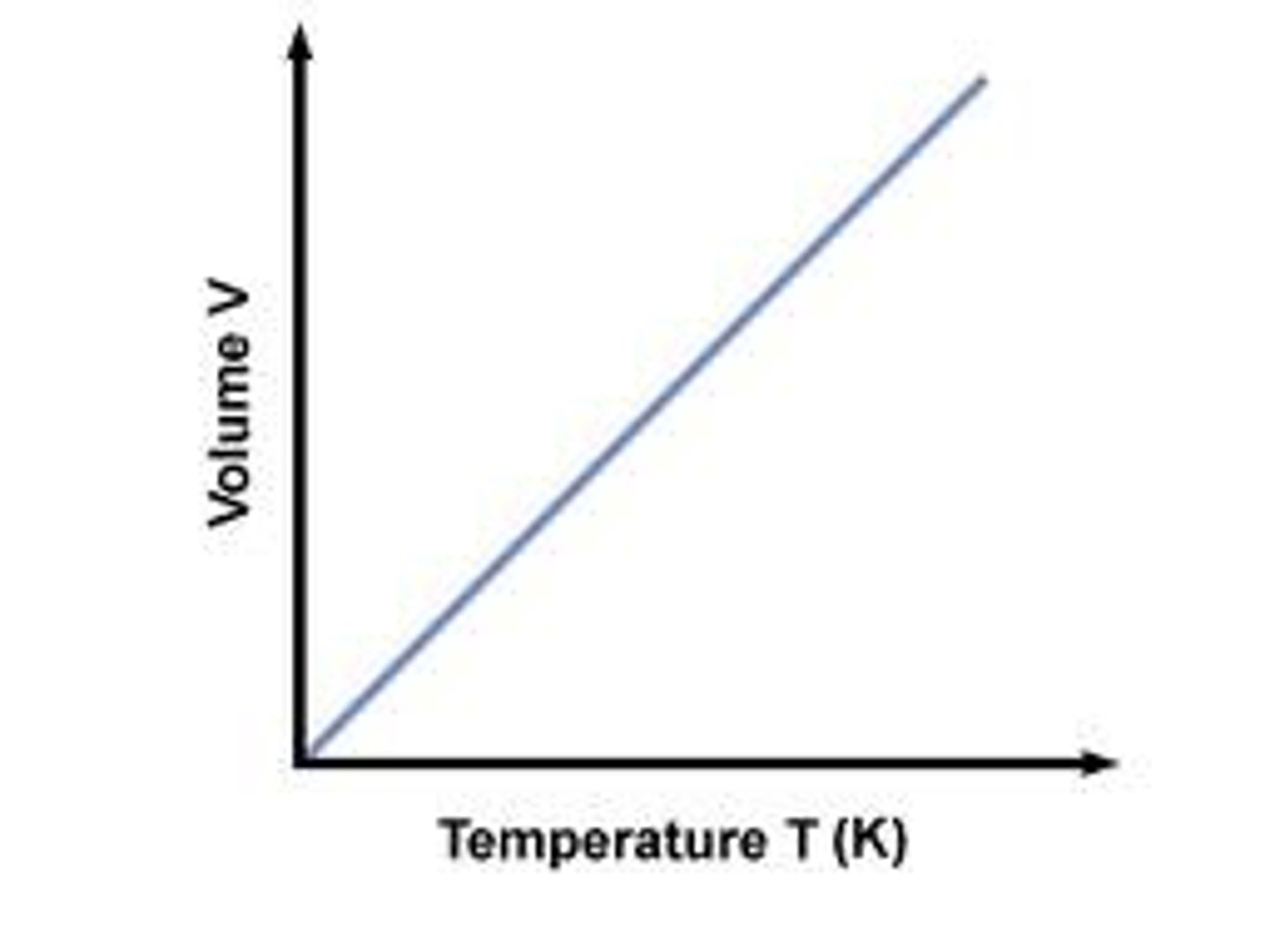
Charles Law
Volume / Pressure = Constant
Temperature - Kelvin (K)
Avogadro Principle
Equal volume of different gases contain the same number of particles
Gas laws

Ideal gases Calculation
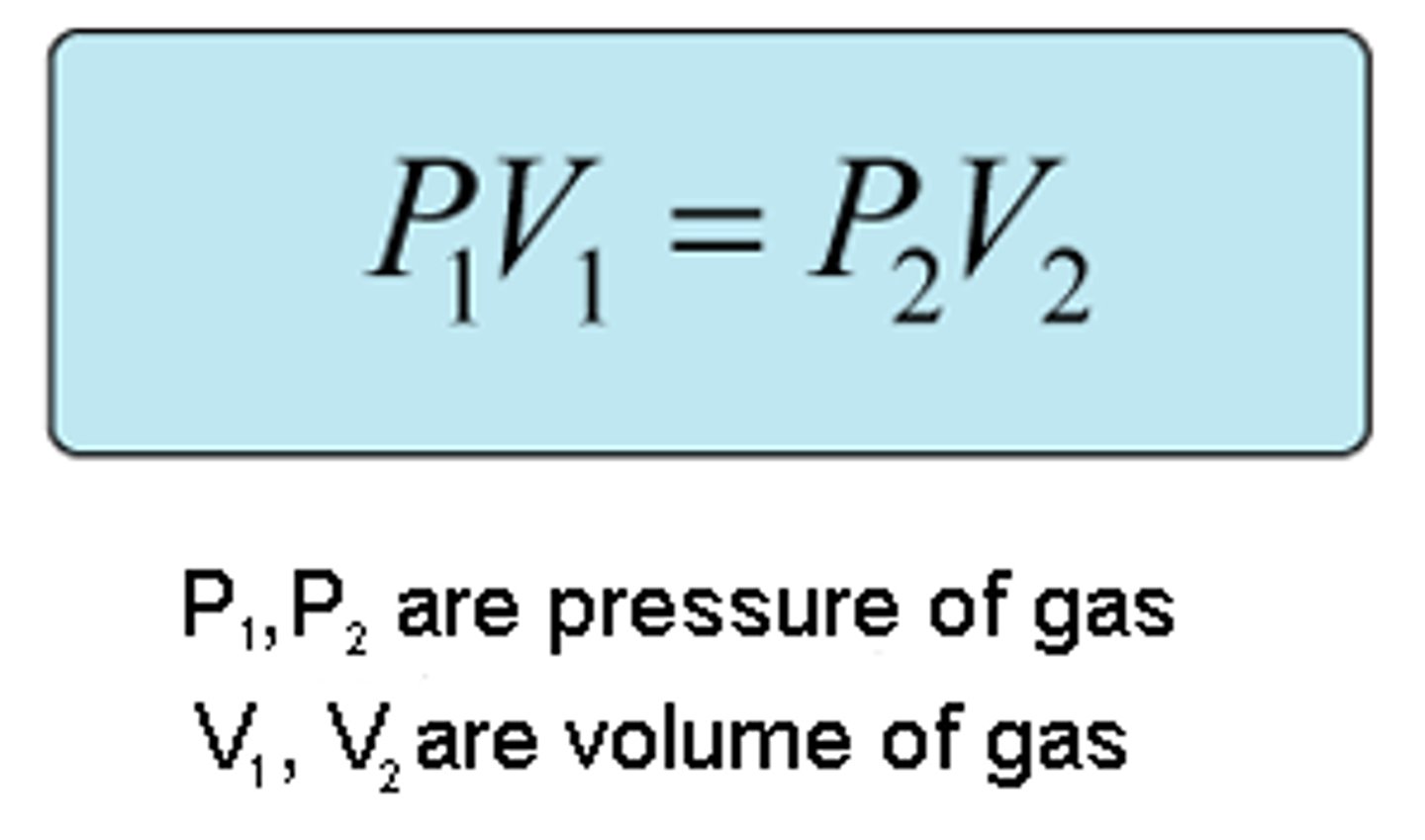
Mass Spectrometer
Use to measure to a great accuracy , measures the Aᵣ of an element and the Mᵣ of a compound.
Vapourising
A sample of the element is placed inside as a vapour, solids provide heating, some substances thermally decompose and can't be used.
Ionising
Element is in an evacuated chamber under a vacuum. Bombarded by high energy electrons, this results in electron loss and causing positive ions.
Ionising example
E + e⁻ → E⁺ + e⁻ + e⁻
atom + high energy electron → E⁺ + e⁻ + e⁻
Acceleration
Positive ions accelerated by the electrostatic field, ions pass through slits which carry the field
Deflect
Different ions are deflected by magnetic fields by different amounts. It depends on:
Mass of ion - Light ions deflected more the heavier.
Ion charge - Ions with two or more positive deflected more than one positive ion.
Detection
Only ions with the correct with the correct mass. The mass and the relative abundance % are indicated by a peak charge. A vacuum is needed to make sure there are no air particles in the way, to give a clean run to the chamber.
Vacuum
Gets rid of air particles or any impurities within the chamber, so the ion has a clean run to the detector, so it doesn't effect the result.
Describe the mass spec of chlorine.
Because chlorine is a molecule, it can be ionised straightaway or undergo fragmentation then ionisation. If it stays as a molecule, the mass number will be a lot higher due to a molecular ion:
- 35 Cl2 +
- 35 Cl 37 Cl +
- 37 Cl2 +
The chlorine molecule can also be broken down and then ionised, resulting in the Cl being a single ion so it has a lower mass number:
- 35 Cl +
- 37 Cl +
Explain why on a mass spectrum the peak heights of 35 Cl and 37 Cl are not the same height.
These isotopes occur naturally in different abundance percentages. The ratio is 3:1 (75%:25%)
What is the natural abundance ratio of the molecular ions of chlorine?
35Cl 2 + : 35Cl 37Cl + : 37Cl 2 +
9:6:1
Explain why there are three different molecular ion peaks for Chlorine.
Chlorine has two isotopes, 35 Cl and 37 Cl so there are 3 different combinations for a molecular ion
Ions
Ions are charged particles formed when atoms lose or gain electrons.
Ions form as they are more stable (less energy) as a result forming full shells
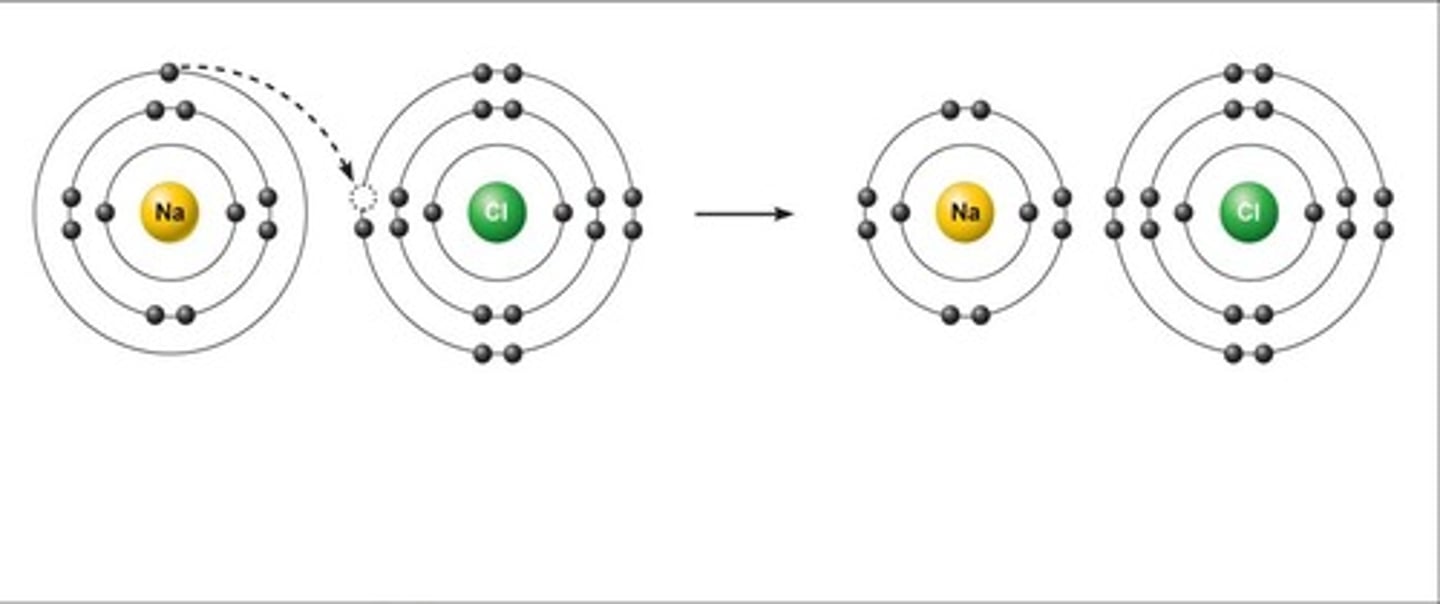
Ionic Bonding
The strong electrostatic attraction between oppositely charged ions ( Cation and Anion)
Between a metal and non metal
Large electronegativity values
Covalent Bonds
A strong attraction between a shared pars of electrons between two nuclei
Between two non metals
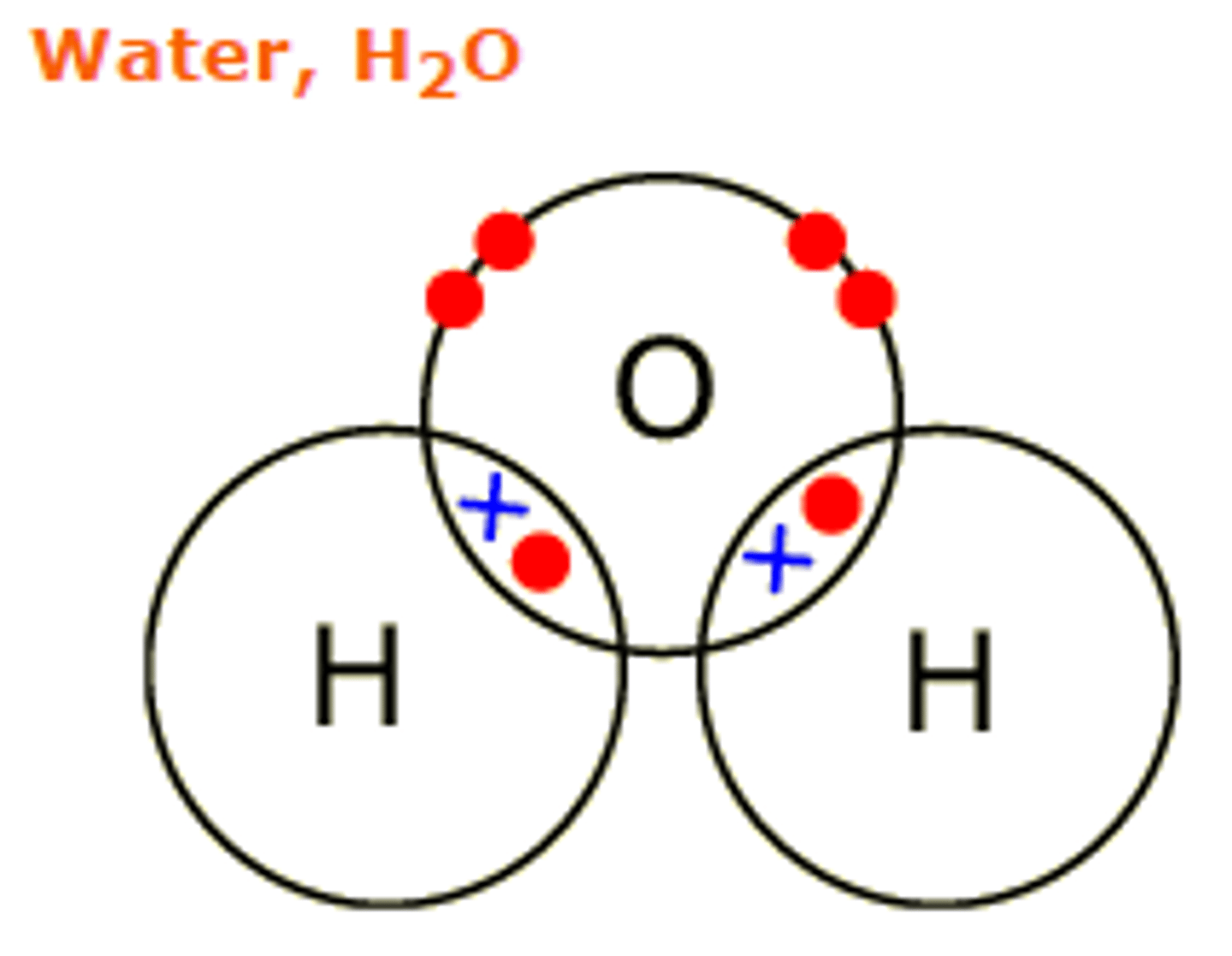
Inside a covalent bond
Electrons are within an orbital, electrons move around this orbitals randomly. The orbital exists between two nuclei.
Why does silicon have a high melting temperature?
Is a giant covalent structure therefore takes more energy to break the many strong covalent bonds
Coordinate bonds
A covalent bond in which both electrons come from one of the atoms
Metallic bonding
Within all metals, there is a regular arrangement of positive metals ions surrounded by a sea of delocalised valance shell electrons. The force of attraction between these electrons and the positive ions is the metallic bond
How are metals able to conduct electricity when a voltage is applied?
Happens because the delocalised electrons are able to move
Electronegativity
The ability of an atom to attract the electron density in a covalent bond towards itself

5 Electronegative atoms
Fluorine, Oxygen, Nitrogen, Bromine, Chlorine
Why do the electrons in each orbital spin in opposite directions?
In order to minimise repulsion
Atomic Radius
Distance from nucleus to outer electrons, Bigger atomic radius the holder to hold
Shielding
The number of full shells in between the nucleus and the outer electron
Effective Nuclear Charge
The amount of protons in the nucleus and they hold on to the outer electrons.
Atomic radius vs Effective nuclear charge
AS atomic radius increase effective nuclear charge decrease. Visa versa
What is the definition of the first molar ionisation energy?
The enthalpy change to lose 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of gaseous +1 ions
Explain why there is a difference in coordinate number for NaCl and CsCl.
NaCl is 6,6 and CsCl is 8,8. This is because Cs+ ion is a larger ion than Na+ ion so more Cl- ions can be packed around a Cs+ ion compared to an Na+ ion
Type of Bonding
0 = Non-polar covalent
>0 <1.7 Polar Covalent
>1.7 Ionic
Intermolecular Bonding
Weak bonding holding the molecules together