Biol 3140 Exam 1 - Iowa State University
1/58
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
Cell Theory
All organisms are made up of one or more cells
All cells come from pre-existing cells
The cell is the basic unit of structure and organization that performs life functions
Prokaryotic Cells
Single-celled organism
Has no nucleus
Lacks some membrane-bound organelles
Has a cell membrane, cytoplasm, and genetic material (ex., ribosomes)
Has circular DNA
Eukaryotic Cells
Multi-celled organisms
Has a nucleus
Has membrane-bound organelles
Has a cell membrane, cytoplasm, and genetic material (ex., ribosomes)
Has linear DNA
How are Cells Studied?
Microscopes
Light Microscopy
Uses light as an illuminating source
Has a 1000x maximum magnification
Uses live or dead specimens
Uses glass as a lens material
Has a 0.25um-0.3um resolving power
Produces colored images
Used to study internal structure
Electron Microscopy
Uses electrons as an illuminating source
Has a 2000000x maximum magnification
Uses dead or dried specimens
Uses electromagnets as a lens material
Has a 0.001um resolving power
Produces black and white images
Used to study external surfaces, cell structure, and small organisms
Advantage and Disadvantage of Electron Microscopy
Advantage: higher magnification and resolution that allows for small details to be analyzed
Disadvantage: cells have to be dead and thinly sliced for the transmission electron microscopes
Advantage and Disadvantage of Light Microscopy
Advantage: see live cells in their natural colors
Disadvantage: details are not as clear as electron microscopy
Nucleus
Largest organelle
Information and DNA storage of the cell
Enclosed in a nuclear envelope (two concentric membranes)
Endoplasmic Reticulum
Considered an “irregular maze”
Major site for synthesizing proteins, secreted proteins, and membrane lipids
Golgi Apparatus
Flattened membrane enclosed sacs
Helps with synthesis of proteins, modifications, and sorting proteins/lipids during secretion
Mitochondria
Believed to be evolved from engulfed bacteria
Contains it’s own DNA
Reproduce by dividing
Generate energy from food molecules
Chloroplasts
Only present in plants and algae
Carry out photosynthesis
Make all foods we consume directly OR indirectly
Believed to be evolved from photosynthetic bacteria
What is a Major Function of Cytoskeletons?
Maintain shape and facilitate organelle movement
Why do Scientists Study Model Organisms?
Understanding them allows us to understand other organisms because we share similar genes with them. It allows us to practice “ethics” by not using humans to do certain experiments. Since we all descended from a common ancestor, these model organisms are more like us than not.
How Do Cells Exploit the Laws of Chemistry and Physics to Survive, Thrive, and Reproduce?
Use energy from the environment through chemical reactions
Use thermodynamics for metabolism and replication
Use a continuous input of energy to maintain themselves
Based on carbon compounds, so they’re very regulated
How Do Different Atoms Interact to Form Molecules?
By sharing or transferring electrons and creating chemical bonds
Why Do Carbon, Hydrogen, Oxygen, and Nitrogen Prevalent in Living Cells?
They can bond easily to the carbon. Carbon is considered a backbone, because of all the bonds it can form
Covalent Bonds
Form by the sharing of electrons
Spatial arrangement of them can be formed by oxygen, nitrogen, carbon
These molecules have precise 3D structures defined by covalent linkage bond angles and lengths
The polarity depends on the relative electronegativities of the participant atoms
Noncovalent Bonds
Weaker interactions than covalent bonds
Are ionic bonds, hydrophobic bonds, electrostatic interactions, and Van der Waals
Electromagnetic interactions instead of electron sharing
What is a Unique Property of a Noncovalent Bond?
The electron jumps from one atom to another. These are called salts instead of molecules. They are formed by gain or the loss of electrons (ex., NaCl)
Ionic Bonds
Formed by the gain or loss of electrons
Form salts instead of molecules
Hydrogen Bonds
Gives water special properties
High boiling point
The slight positive charge association with the hydrogen atoms is electrically attracted to the slight negative charge of the oxygen atom
Broken by random thermal motions
What are the Four Major Carbon-Based Molecules Found in All Organisms?
Sugar
Fatty acid
Amino acid
Nucleotide
What are Sugar’s (Glucose) Major Properties and Functions in Living Cells?
Can be made into larger molecules
Can be broken down into smaller subunits
Common examples are starches, glycogens, and cellulose
Readily available energy source that can be stored for later
Pack densely and form polysaccharides
What are Fatty Acid’s Major Properties and Functions in Living Cells?
Both hydrophobic and hydrophilic (amphipathic)
The head of them loves water, the tail doesn’t
Helps to form cell membranes
What are Nucleic Acid’s Major Properties and Functions (DNA/RNA) in Living Cells?
DNA: held by phosphodiester bonds
Are subunits of DNA/RNA
Energy currency
What are Amino Acid’s Major Properties and Functions in Living Cells?
Subunits of proteins
20 different AA in 1 protein
Held together via peptide bonds in proteins
How are Different Macromolecules Built-In Cells?
They are added to one end of a chain via condensation reactions, which releases water
How do Cells Use Energy?
All cells get energy from the sun, and they use it for chemical reactions and processes
How do Cells Obtain and Store Energy?
Photosynthetic cells obtain energy from the sun and then create organic molecules. Animal cells get energy from food, using chemical bond energy
What are Catabolic Reactions?
The breakdown of large molecules into smaller components (ex., digestion of food; breakdown of glucose)
What are Anabolic Reactions?
Chemical reactions create complex molecules from smaller ones, using ATP as a main energy source for these reactions (ex., protein synthesis; building muscle mass)
How do Enzymes Catalyze Chemical Reactions in Cells?
They reduce the energy needed to initiate spontaneous reactions
What are the Major Differences Among Different Activated Carriers?
ATP is the more widely used carrier
The two outermost phosphate groups are held by high energy phosphoanhydride bonds
Interconverting ATP to ADP occurs in a cycle
ADP —→ ATP releases H2O and ATP —→ ADP requires H2O
The terminal phosphate of ATP can be transferred to other molecules, occurring in coupled reactions
CoA is another carrier
Has a thioester bond with high energy
Releases a lot of energy when hydrolyzed
Activated carriers are important because they store and transfer energy for cells to use
How are Different Activated Carriers Used by Cells?
They transfer energy, electrons, or chemical groups in order to power different reactions. They store energy in an easily accessible/exchangeable form
How are Biological Polymers Synthesized in Cells?
They are synthesized by joining small building blocks together through covalent bonds, releasing water molecules in the process. Dehydration synthesis is key. Driven by ATP hydrolysis
What Determines the Shape of a Protein?
Folding via noncovalent bonds inside of the protein
Hydrophobic forces help fold into compact structures
Stability is determined by combined strength of the bonds in the protein
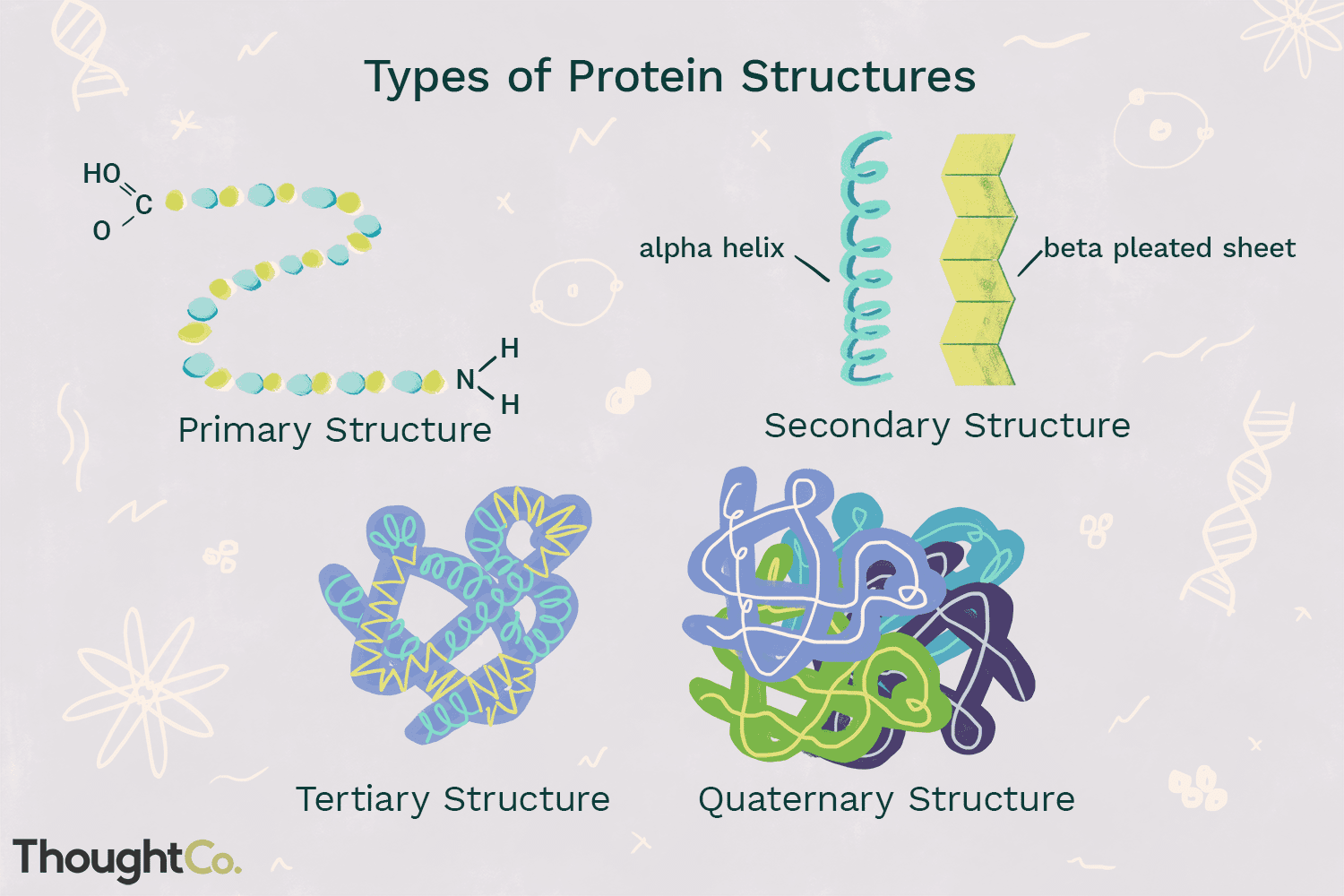
What are the Differences of Primary, Secondary, Tertiary, and Quaternary Protein Structures?
Primary structure: the linear sequence of amino acids in a polypeptide chain
Secondary structure: local folding patterns like alpha helices and beta sheets formed by hydrogen bonds
Tertiary structure: overall 3D shape of a single polypeptide chain due to interactions between side chains, the noncovalent bonding that occurs
Quaternary structure: arrangement of multiple polypeptide chains to form a functional protein complex
What are Enzymes?
Proteins that speed up chemical reactions in living things. They are responsible for building some substances and breaking down others. All living things have enzymes and our bodies naturally produce them. They convert substrates into products
How do Proteins Bind to Other Molecules?
Binds through a specific region on their surface called a binding site
The binding site interacts with the target molecule using a combination of weak, noncovalent bonds
ex., hydrogen bonds, ionic bonds, Van der Waals, and hydrophobic interactiojns
There is a highly specific “lock and key” fit between the protein and molecule
What Determines the Specific Binding of Proteins to Other Molecules?
Determined by the 3D structure of the protein. This is also determined by the amino acid sequence
What Domain of an Antibody is Responsible for the Specific Binding of an Antigen?
The amino-terminal variable or V domains of the heavy and light chains
Antibody
Y-shaped with two identical antigen-binding sites
Held via disulfide bonds
Produced by the immune system in response to foreign molecules
Condensation
Builds a larger molecule (polymer) from smaller ones (monomers) by removing a water molecule
Hydrolysis
Breaks down large molecules into smaller ones by adding a water molecule
Spontaneous Reaction
Occurs naturally
ΔG < 0
Releases energy into surroundings
Products have a lower energy than reactants
Nonspontaneous Reaction
Does not occurs naturally
ΔG > 0
Needs an external energy source
Products have a higher energy than reactants
How Do Enzymes Lower Activation Energy?
Positioning of substrates to favor the reaction
Straining bonds within the bound substrate
Rearrangement of electrons in the substrate
Formation of covalent bond between substrate and enzyme
Allosteric Regulation of Enzymes
Enzymes have 2 binding sites on the surface
Active site - binds substrate (not this one)
Regulatory site - binds regulatory molecule
Binding on the regulator conformationally changes protein
Increases activity (activator) or inhibits activity (inhibitor)
Ion-Exchange Chromatography
Separates molecules based on their charge. Often used to purify proteins or nucleic acids
Gel-Filtration Chromatography
Separates molecules based on their size
Affinity Chromatography
Separates molecules based on their specific binding interactions
SDS-Page
Separates proteins based on their molecule weight using denaturing
2D-Page
Separates proteins based on their isoelectric (pH at which a molecule has no net charge) point
Mass Spectrometry
Used to identify and sequence protein by determining the price masses of peptide derived from them
What is an Advantage and Disadvantage of X-Ray Crystallography?
Advantage: can provide high-resolution, detailed three-dimensional images of the arrangement of atoms within a crystal
Disadvantage: it relies on the formation of high-quality protein crystal
What is an Advantage and Disadvantage of NMR Spectroscopy?
Advantage: can solve dynamic protein structures in solution
Disadvantage: NMR becomes increasingly challenging as the size of the protein complex increases
What is an Advantage and Disadvantage of Cryo-Electron Microscopy?
Advantage: can visualize biological macromolecules and complexes at high resolution without the need for crystallization
Disadvantage: can not visualize smaller proteins