Inorganic Chemistry
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
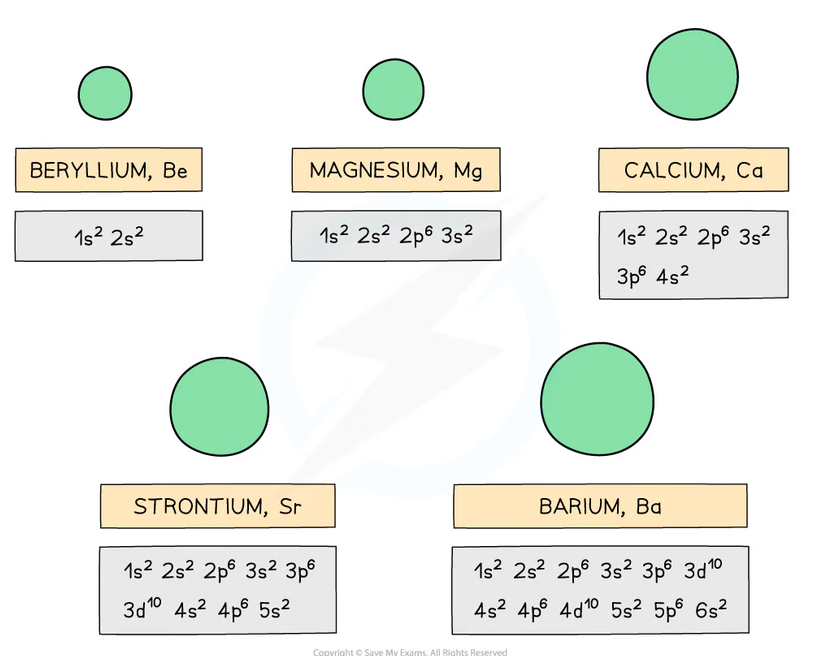
How and why does the atomic radius vary down group 2?
As you go down the group, the elements have more electron shells
So the atom radii increase
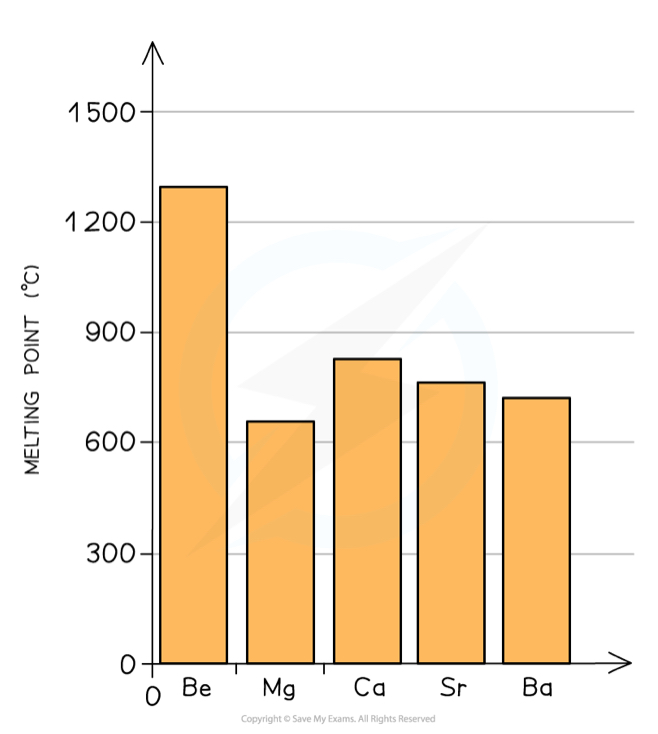
How and why do the melting points vary down group 2?
As you go down the group, the elements have more shells, so the atomic radii increase
This means that the outer shell is further away from the nucleus, so there is a weaker force of attraction towards the delocalised electrons in metallic bonding
So the melting points decrease
(Magnesium is an anomaly)
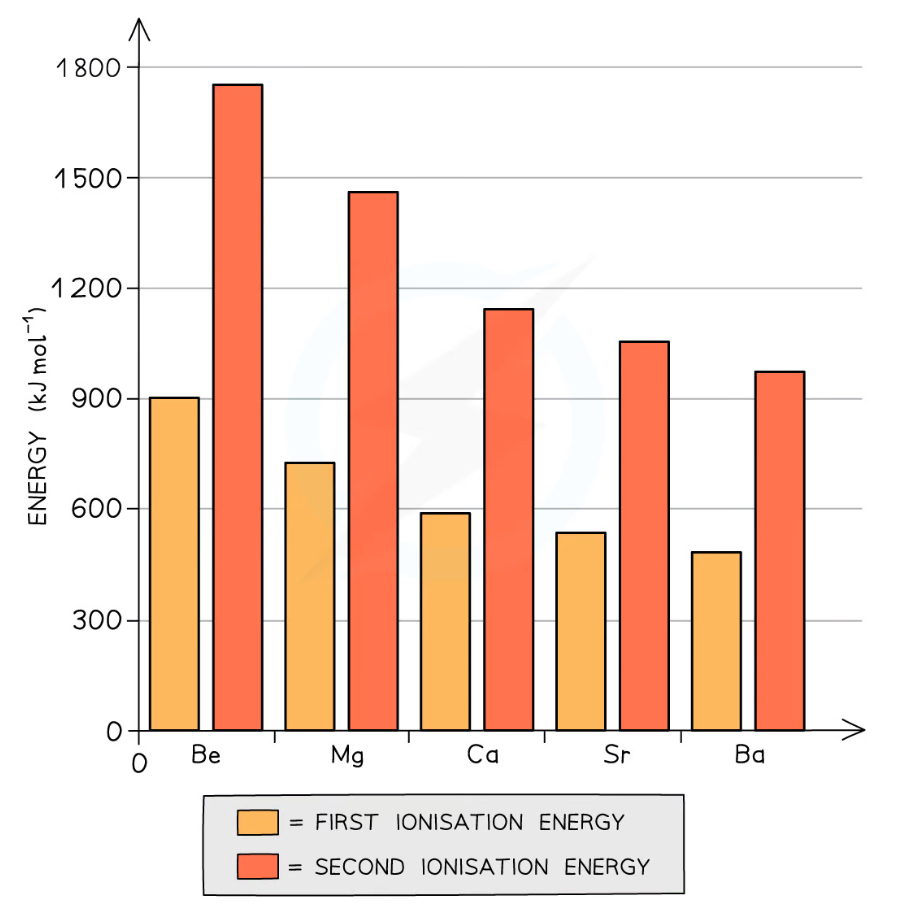
How and why does the ionisation energy vary down group 2? How does this affect the trend in reactivity?
As you go down the group, the elements have more shells, so the atomic radii increase
This means that the outer shell is further away from the nucleus, and the electrons experience more shielding (like charges repelled by the inner electron shells)
So the electrons are easier to lose and the ionisation energies decrease
Therefore the reactivity increases down the group
Compare the reactions of Mg-Ba with water, including the equations
Mg reacts very slowly with water, but reacts vigorously with steam
(Mg(s) + 2H₂O(l) → Mg(OH)₂(s) + H₂(g))
(Mg(s) + H₂O(g) → MgO(s) + H₂(g))
The rest react with water with increasing speed down the group
(X(s) + 2H₂O(l) → X(OH)₂ + H₂(g))
Magnesium oxide and all group 2 hydroxides are white solids
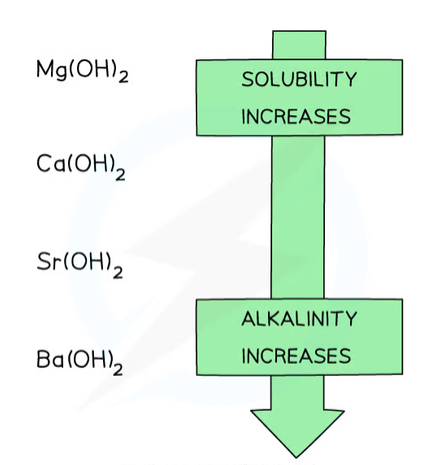
What is the trend in solubility of group 2 hydroxides down the group? How does this affect the pH trend down the group?
The solubility of metal hydroxides increase down the group
Magnesium hydroxide is almost completely insoluble, while barium hydroxide is very soluble
The pH increases down the group, as the metal hydroxides become more soluble and more OH⁻ ions are dissociated in solution
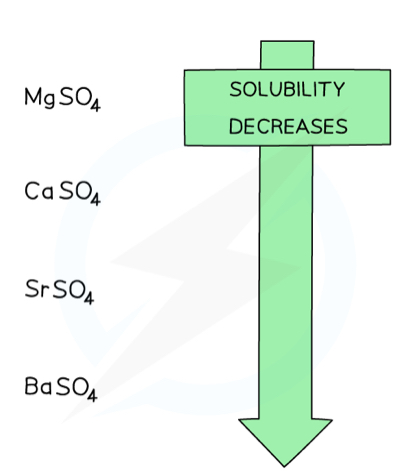
What is the trend in solubility of group 2 sulphates down the group?
The solubility of metal sulphates decrease down the group
Magnesium sulphate is very soluble, while barium sulphate is almost completely insoluble
How are magnesium and it’s compounds used?
Magnesium is used in the extraction of titanium from its ore (TiO2)
TiO2 (s) + 2C (s) + 2Cl2 (g) → TiCl4 (g) + 2CO (g)
TiCl4 (g) + 2Mg (l) → Ti (s) + 2MgCl2 (l)
Magnesium hydroxide is used in medicine to neutralise excess stomach acid. This is safe to do because magnesium hydroxide is only partially soluble, so it doesn’t dissociate very well, making the pH about 10
Mg(OH)2 (s) + 2HCl (aq) → MgCl2 (aq) + 2H2O (l)
How are calcium compounds used?
Calcium hydroxide is used in agriculture to raise the pH of soil by neutralising hydrogen ions
Ca(OH)2 (s) + 2H+ (aq) → Ca2+ (aq) + 2H2O (l)
Calcium oxide and calcium carbonate are used to remove sulfur dioxide from flue gases in coal power stations
How are barium compounds used?
Barium chloride is used in chemistry to test for sulfate ions, as barium ions would react to form insoluble barium sulphate as a precipitate. The solution is first acidified with nitric or hydrochloric acid in order to remove carbonate ions (if barium carbonate was formed, it would also give a white precipitate)
Ba2+(aq) + SO42–(aq) → BaSO4(s)
Barium sulphate is used in medicine to create clear X-rays of the intestines. This is safe to do because barium sulfate is insoluble so the toxic barium isn’t absorbed into the blood
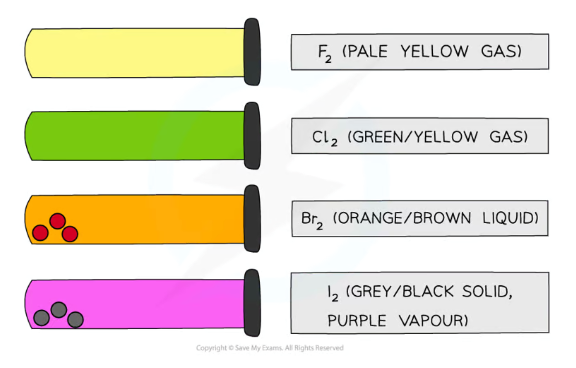
What are the colours and states of the halogens?
Fluorine- pale yellow gas
Chlorine- pale green gas
Bromine- brown liquid/orange vapour
Iodine- grey solid/purple vapour
How and why does the atomic radius vary down group 7?
As you go down the group, the elements have more electron shells
So the atom radii increase
How do melting and boiling points change down group 7?
Increase
Atoms have more electrons, meaning they can form greater temporary and induced dipoles (greater van der Waals forces)
How and why does the electronegativity vary down group 7? How does this affect the trend in reactivity?
As you go down the group, the elements have more shells, so the atomic radii increase
This means that the outer shell is further away from the nucleus, and experiences more shielding (like charges repelled by the inner electron shells)
So the bonding pair of electrons is less attracted, and electronegativity decreases going down the group
Because of this, reactivity of halogens decreases down the group
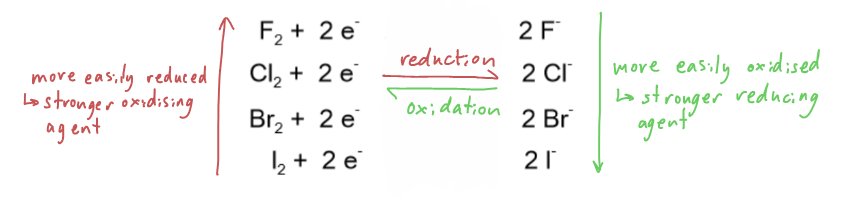
How can halogens and halides act as oxidising agents and reducing agents?
Halogens- oxidising agents:
Halogens can oxidise atoms by removing an electron
They gain the electron, so are themselves reduced
Halogens are electron acceptors, so they are oxidising agents
Halide ions- reducing agents:
Halides can reduce atoms by donating an electron
They lose the electron, so are themselves oxidised
Halide ions are electron donors, so they are reducing agents
What are the trends in oxidising and reducing power down group 7?
Halogens- oxidising power:
Down the group, the atomic radii increase
The outer shell electrons are further from the nucleus and experience more shielding
This means that it is harder for the atoms to accept (gain) an electron
Oxidising power decreases down the group
Halide ions- reducing power:
Down the group, the ionic radii increase
The outer shell electrons are further from the nucleus and experience more shielding
This means that it is easier for the ions to donate (lose) aan electron
Reducing power increases down the group
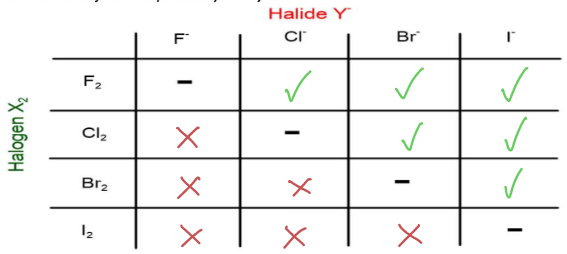
Explain the displacement reactions of halogens in terms of oxidising power
Oxidising power decreases down the group as the halogens are less easily reduced (harder for bigger atoms to gain electrons)
This means that the halogens at the top of the group are stronger oxidising agents, so they can oxidise a lower halide, displacing it from its metal halide compound
Eg. chlorine is higher up than bromine, so it is a stronger oxidising agent and can oxidise bromide ions, meaning it will displace bromine from sodium bromide
Cl2 + 2NaBr → 2NaCl + Br2
Ionic equation= Cl2 + 2Br- → 2Cl- + Br2
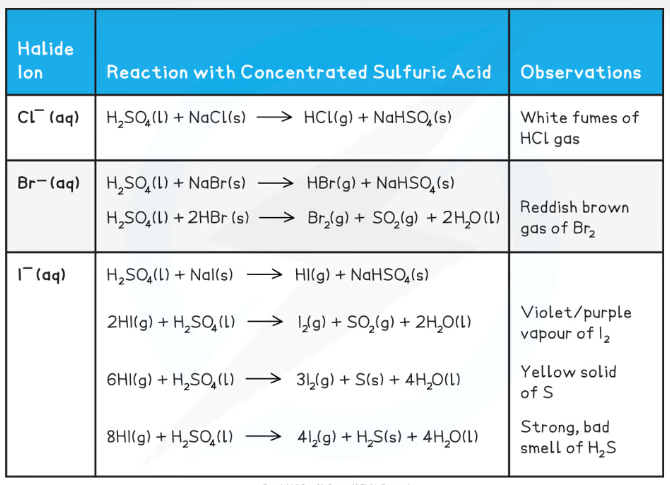
Describe the reaction of sodium chloride with sulfuric acid
An acid-base reaction occurs: H2SO4(l) + NaCl(s) → HCl(g) + NaHSO4(s)
So white misty fumes produced (hydrogen chloride), and damp blue indiciator paper turns red (acidic)
The chloride ions are too weak of a reducing agent to reduce the sulfuric acid
Describe the reaction of sodium bromide with sulfuric acid
An acid-base reaction occurs: H2SO4(l) + NaBr(s) → HBr(g) + NaHSO4(s)
So, white misty fumes produced (hydrogen bromide), and damp blue indicator paper turns red (acidic)
The bromide ions produced are strong enough reducing agents to reduce the sulfuric acid to sulfur dioxide: 2HBr(g) + H2SO4(l) → Br2(g) + SO2(g) + 2H2O(l)
So orange fumes produced (bromine gas), and orange dichromate paper turns green (sulfur dioxide present)
Describe the reaction of sodium iodide with sulfuric acid
An acid-base reaction occurs: H2SO4 (l) + NaI (s) → HI (g) + NaHSO4 (s)
So white misty fumes produced (hydrogen iodide), and damp blue indicator turns red (acidic)
The iodide ions are strong enough reducing agents to reduce sulfuric acid to sulfur dioxide: 2HI (g) + H2SO4 (l) → I2 (g) + SO2 (g) + 2H2O (l)
So purple fumes produced (iodine gas), and orange dichromate paper turns green (sulfur dioxide present)
They are also strong enough to reduce sulfuric acid to solid sulfur: 6HI (g) + H2SO4 (l) → 3I2 (g) + S (s) + 4H2O (l)
So yellow solid produced (solid sulfur)
They are also strong enough to reduce sulfuric acid to hydrogen sulfide: 8HI (g) + H2SO4 (l) → 4I2 (g) + H2S (s) + 4H2O (l)
So white lead ethanoate paper turns black (hydrogen sulfide present)
Explain the reactions of sodium halides with sulfuric acid in terms of reducing power
Reducing power increases down the group as the halides are more easily oxidised (easier for bigger atoms to lose electrons)
This means that the halide ions are better at reducing sulfuric acid as you go down the group, and can reduce it to a lower oxidation state
Chloride can’t reduce H2SO4 (sulfur stays +6)
Bromide can reduce H2SO4 to SO2 (+4)
Iodide can reduce H2SO4 to SO2 (+4), to S (0), and to H2S (-2)
How can we test for the halides?
Add nitric acid and silver nitrate solution
If a precipitate is formed, a halide ion is present
Chloride = white precipitate
Bromide = cream precipitate
Iodidie = yellow precipiate
Often it is hard to distinguish between these colours, so adding ammonia solution is used as a follow-up test, by observing whether the precipitate dissolves
Chloride = dissolves in dilute ammonia
Bromide = dissolves only in concentrated ammonia
Iodidde = doesn’t dissolve in concentrated ammonia
Why is silver nitrate solution used to test for halides?
Silver chloride, silver bromide and silver iodide are insoluble, with different coloured precipitates
Ag+ (aq) + X- (aq) → AgX (s)
Why is silver nitrate acidified in the halide ion test?
Nitric acid will react with any carbonate or hydroxide ions, as silver carbonate and silver hydroxide are insoluble, so they would also give a positive test
This works because:
OH-(aq) + H+(aq) → H2O(l)
CO₃²⁻(aq) + H⁺(aq) → CO₂(g) + H2O(l)
How does chlorine react with water? Why is this useful?
In a disproportionation reaction, chlorine (oxidation state 0) is both reduced to -1 in hydrochloric acid, and oxidised to +1 in chloric (I) acid
Cl2 (g) + H2O (l) → HCl (aq) + HClO (aq)
We use this reaction to sterilise drinking water and pools because HClO dissociates to ClO- (chlorate (I) ion), which is a dissinfectant
Why does chlorine have to be regularly topped up in pools?
Remaining chlorine is depleted in sunlight to form HCl, and no longer acts as a dissinfectant
2Cl2 (g) + 2H2O (l) → 4HCl (aq) + O2 (g)
How is it safe to add chlorine to drinking water?
Though chlorine is toxic, the benefits of adding it in small quanitities to water to sterilise it outweigh the risks
How do we use chlorine to produce bleach?
Chlorine reacts with cold, dilute sodium hydroxide in a disproportionation reaction to form sodium chlorate (I), NaClO, the active ingredient in bleach
Cl2 (aq) + 2NaOH (aq) → NaCl (aq) + NaClO (aq) + H2O (l)
What is the trend in atomic radii across period 3?
As you go along the group, the nuclear charge increases and electrons are added to the same shell
This means that the nucleus attracts the electron shells more and pulls them closer
So the atomic radius decreases
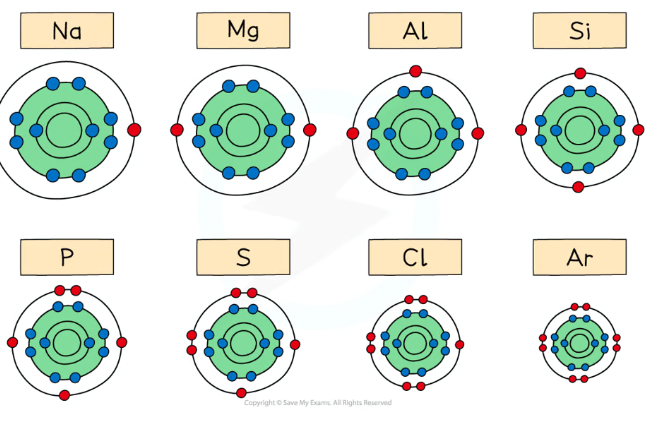
What is the trend in the first ionisation energies across period 3?
Across the period, the nuclear charge increases, so the atomic radius decreases
There are stronger attractive forces between the nucleus and the outer electrons, so it is harder to remove electrons
So the first ionisation energy increases
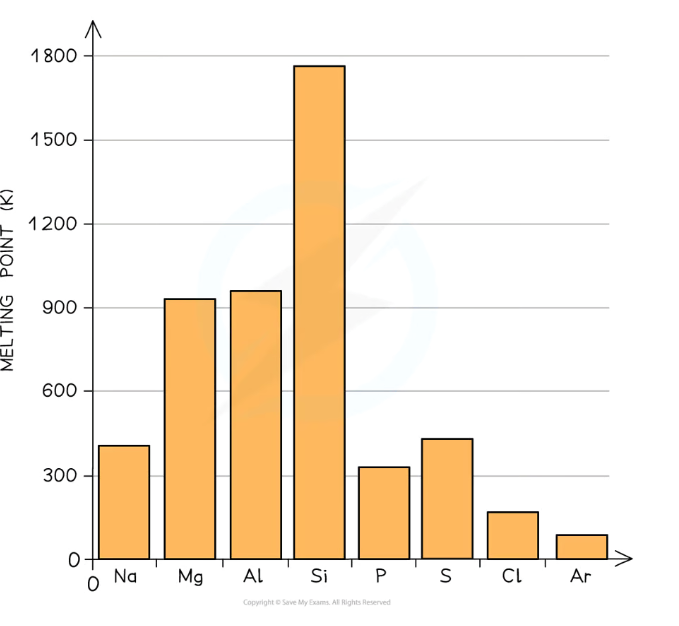
How do melting points change across period 3?
For sodium, magnesium + almuminium, the melting point increases, because they have a giant metallic structure, so:
When the ionic charge increases there are more delocalised electrons, and more attraction between cations and the sea of electrons
When the atomic radius decrease the ions are more closely packed
For silicon, there is a big peak in melting point, because it has a giant covalent structure, so:
4 covalent bonds must be broken to separate one atom, which requires a lot more energy
For phosphorus, sulphur, chlorine and argon, the melting point decreases, because they are simple covalent molecules, so:
Only weak van der Waals forces need to be broken to melt them, which don’t require much energy
(The anomaly for sulfur is because it exists as S8, which is larger than P4)
What is the reaction of sodium with water?
Sodium reacts vigorously with cold water, to produce hydrogen gas and sodium hydroxide
Melts, effervesces and moves across the surface of the water
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g)
What is the reaction of magnesium with water?
Magnesium reacts very slowly with cold water, to produce hydrogen gas and magnesium hydroxide
Mg (s) + 2H2O (l) → Mg(OH)2 (aq) + H2 (g)
However,
Magnesium reacts vigorously with steam, to produce hydrogen gas and magnesium oxide
Mg (s) + H2O (g) → MgO (s) + H2 (g)
What is the reaction of sodium with oxygen?
Burns with a yellow flame to produce a white solid (sodium oxide)
4Na + O2 → 2Na2O
What is the reaction of magnesium with oxygen?
Burns with a white flame to produce a white solid (magnesium oxide)
2Mg + O2 → 2MgO
What is the reaction of aluminium with oxygen?
Burns with a white flame to produce a white solid (aluminium oxide)
4Al + 3O2 → 2Al2O3
What is the reaction of silicon with oxygen?
Burns with a white flame to produce a white solid (silicon dioxide)
Si + O2 → SiO2
What is the reaction of phosphorus with oxygen?
Burns with a white flame to produce a white solid (phosphorus oxide)
4P +5O2 →2P2O5
4P +5O2 →P4O10
What is the reaction of sulfur with oxygen?
Burns with a blue flame to produce an acidic gas (sulfur dioxide)
S + O2 → SO2
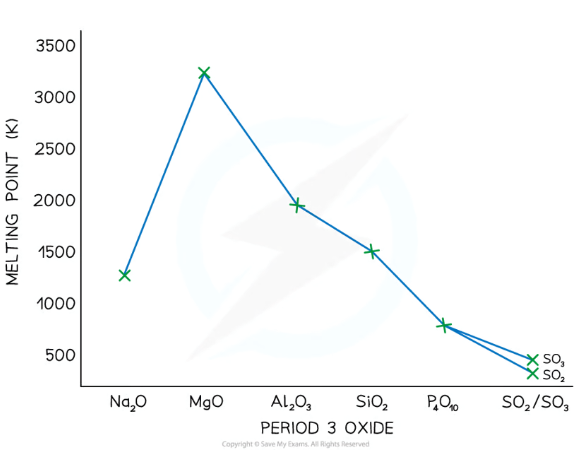
Give and explain the trend in the melting point of the period 3 oxides from Na to S?
Na-Al oxides have the highest melting points, because:
Giant ionic lattice structure- requires a lot of energy to break strong electrostatic forces between ions
MgO > Na₂O because Mg is a smaller ion and has a greater valency
Al₂O₃ < MgO because Al₂O₃ has a lower electronegativity difference and so has less ionic character
SiO₂ also has a high melting point, because:
Giant covalent structure- requires a lot of energy to break all the strong covalent bonds between atoms
P₄O₁₀ and SO₃ have low melting points, because:
Simple covalent molecules- requires little energy to overcome the weak intermolecular forces between molecules
What is the equation of the reaction of sodium oxide with water? What is the pH of the solution formed?
Na2O (s) + H2O (l) → 2NaOH (aq)
Dissociates readily to produce OH- ions, so the pH is 13-14
What is the equation of the reaction of magnesium oxide with water? What is the pH of the solution formed?
MgO (s) + H2O (l) → Mg(OH)2 (s)
Mg(OH)2 is only sparingly soluble, so few OH- ions formed, so the pH is only 10
Why don’t aluminium oxide and silicon dioxide react with water?
The ionic lattice of aluminium oxide is very strong so it is insoluble
The giant covalent structure off silicon dioxide is very strong so it is insoluble
So they have no effect on pH, as they don’t produce H+ or OH- ions
What is the equation of the reaction of tetraphosphorus decoxide with water? What is the pH of the solution formed?
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Dissociates readily to produce H+ ions, so the pH is 1-2
What are the equations of the reactions of sulfur dioxide and trioxide with water? What are the pHs of the solutions formed?
SO2 (g) + H2O (l) → H2SO3 (aq) - sulfurous (IV) acid
Dissociates weakly to produce some H+ ions, so the pH is 2-3
SO3 (g) + H2O (l) → H2SO4 (aq) - sulfuric (VI) acid
Dissociates strongly to produce H+ ions, so the pH is 0-1
What is the trend in the reactions of period 3 oxides with water?
The metal oxides (ionic) produce alkaline solutions
Aluminium oxide and silicon dioxide are too strongly bonded to dissolve and react with water
The non-metal oxides (covalent) produce acidic solutions
What is the equation of the reaction of sodium oxide and hydrochloric acid?
Na2O (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l)
What is the equation of the reaction of magnesium oxide and hydrochloric acid?
MgO (s) + 2HCl (aq) → MgCl2 (aq) + H2O (l)
What are the equations of the reactions of aluminium oxide with hydrochloric acid and sodium hydroxide? What label do we give to aluminium because of these reactions?
Al2O3 (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2O (l)
Al2O3 (s) + 2NaOH (aq) + 3H2O (l) → 2NaAl(OH)4 (aq)
Aluminium can react with both acids and bases, so we call it amphoteric
What is the equation of the reaction of silicon dioxide with sodium hydroxide?
SiO2 (s) + 2NaOH (aq) → Na2SiO3 (aq) + H2O (l)
What is the equation of the reaction of tetraphosphorus decoxide with sodium hydroxide?
P4O10 (s) + 12NaOH → 4Na3PO4 + 6H2O (l)
What are the equations of the reactions of sulfur dioxide and trioxide with sodium hydroxide?
SO2 (g) + 2NaOH (aq) → Na2SO3 (aq) + H2O (l)
SO3 (g) + 2NaOH (aq) → Na2SO4 (aq) + H2O (l)