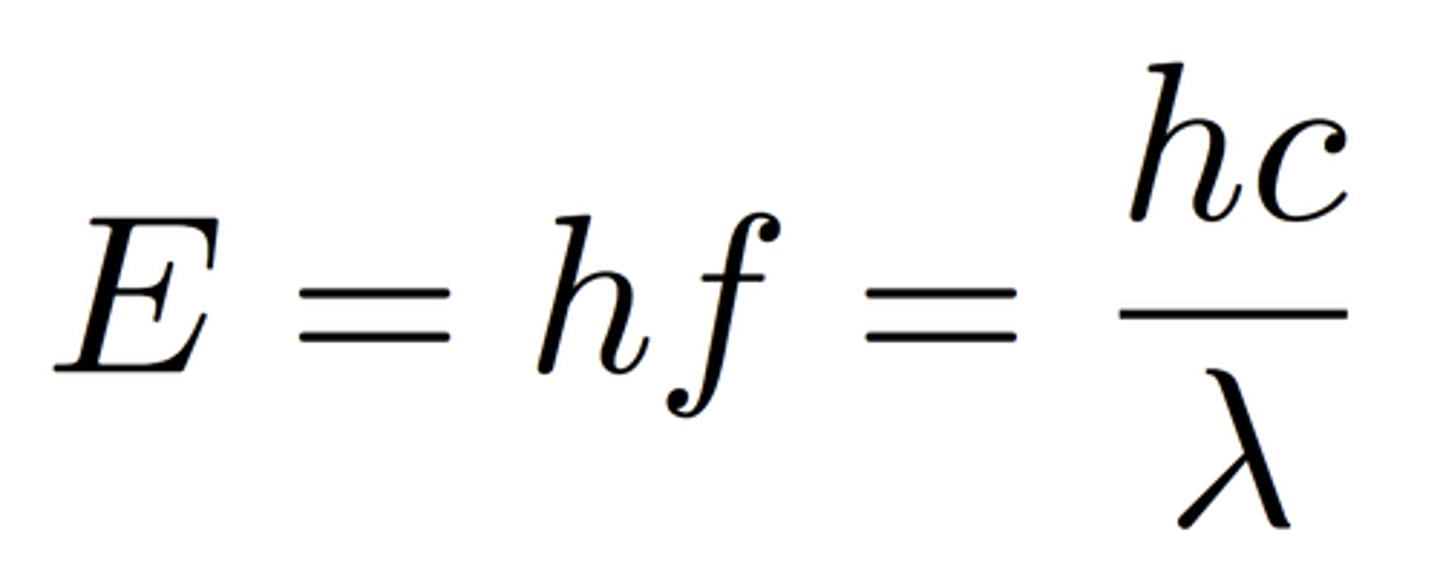Quantum Physics 9702
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Photoelectric equation
E= hf = φ + KE_max (0.5mv^2 max)
- E = hf, is energy of a photon which is transferred to an electron to release it from a material.
- f= frequency of incident photons
- φ = work function J
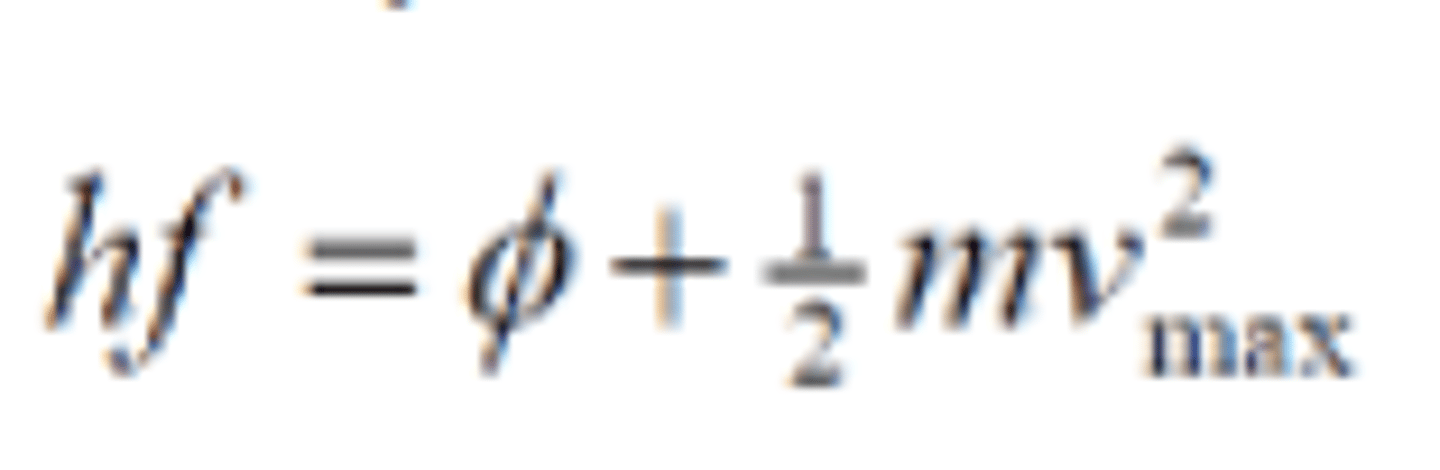
Energy at maximum k.e. = hf - φ (graph)
- Energy at maximum k.e. = hf - φ
- work function = h * threshold frequency
- The work function Φ is the y-intercept.
- The threshold frequency f0 is the x-intercept.
- The gradient is equal to Planck's constant h
- There are no electrons emitted below the threshold frequency f0

Photoelectric current relation to intensity
- Proportional
- Increasing intensity = increasing no. of photons striking metal
- Increases no. of photoelectrons emitted, as each photoelectron absorbs on photon
Wave-particle duality
Light/EM radiation can behave like particles (photons) and waves.
Evidence for particulate nature of EM radiation
Photoelectric effect.
- electron needs a minimum energy to escape, and energy is absorbed in packets related to frequency
- minimum threshold frequency required to escape
- each electron absorbs only one whole packet of energy, unrelated to the number of packets
Evidence for wave nature of EM radiation
Diffraction and interference.
- Both purely wave properties that EM radiation exhibits through electron diffraction
de Broglie wavelength
The wavelength associated with a moving particle.
de Broglie wavelength of a particle equation
λ = h/p = h/mv
- p is momentum of the particle
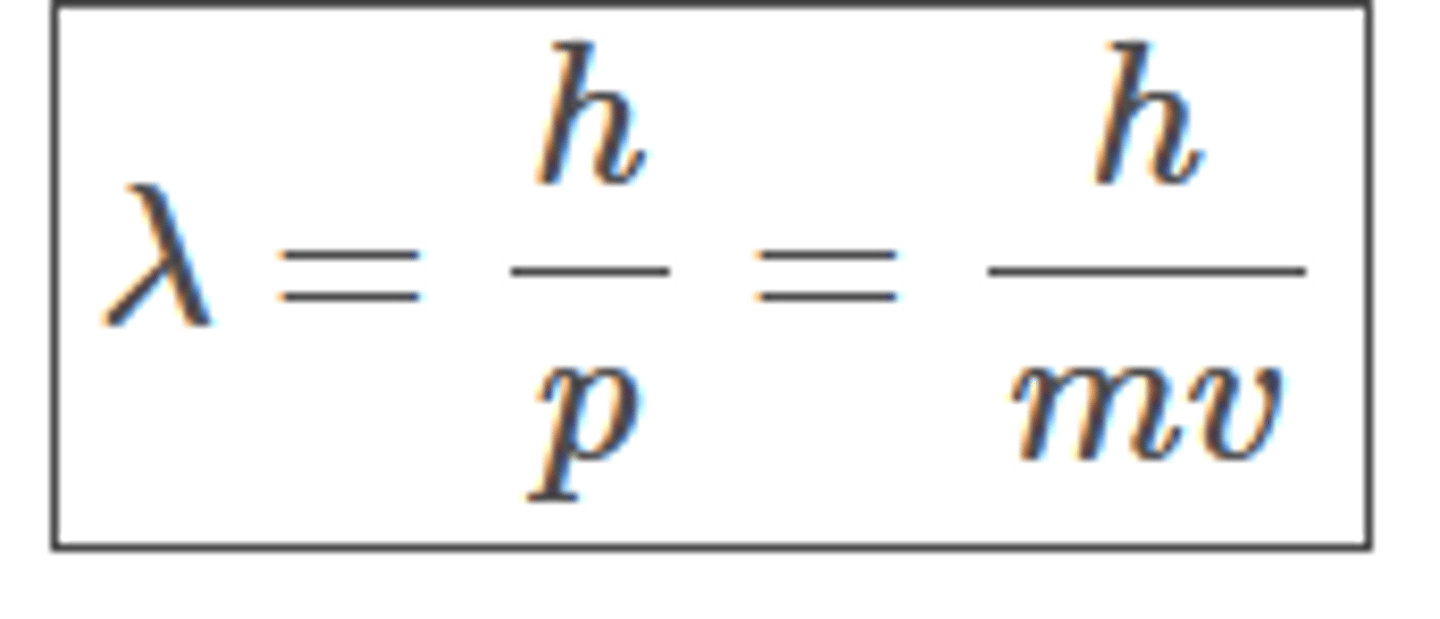
de Broglie wavelength of a particle related to kinetic energy equation
- so E= p^2/2m
- so p= (2mE)^0.5
- so λ= h/(2mE)^0.5
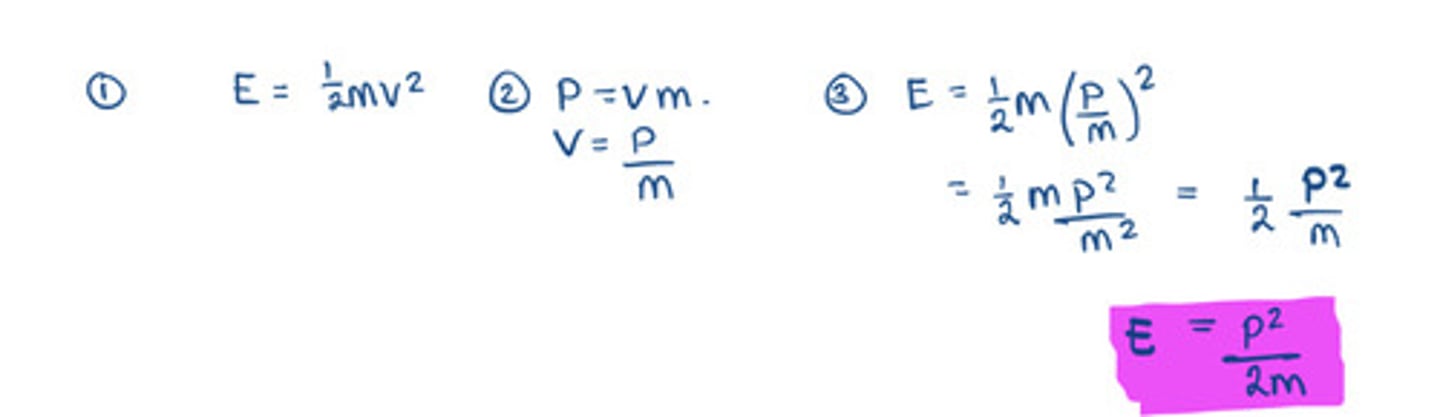
Electron energy levels
The only certain energies that an electron can have.
- Usually they occupy the lowest (ground) level
- gain energy by collisions/absorbing photons/heat
Excitation
When electrons move up an energy level.
- they're in an excited state
- enough energy to be removed from atom: ionisation
- returns to lower energy state: releases energy as a photon
Line spectra
When excited atoms emit light of certain wavelengths which correspond to certain colours.
- Each element produces a unique set of spectrum lines.
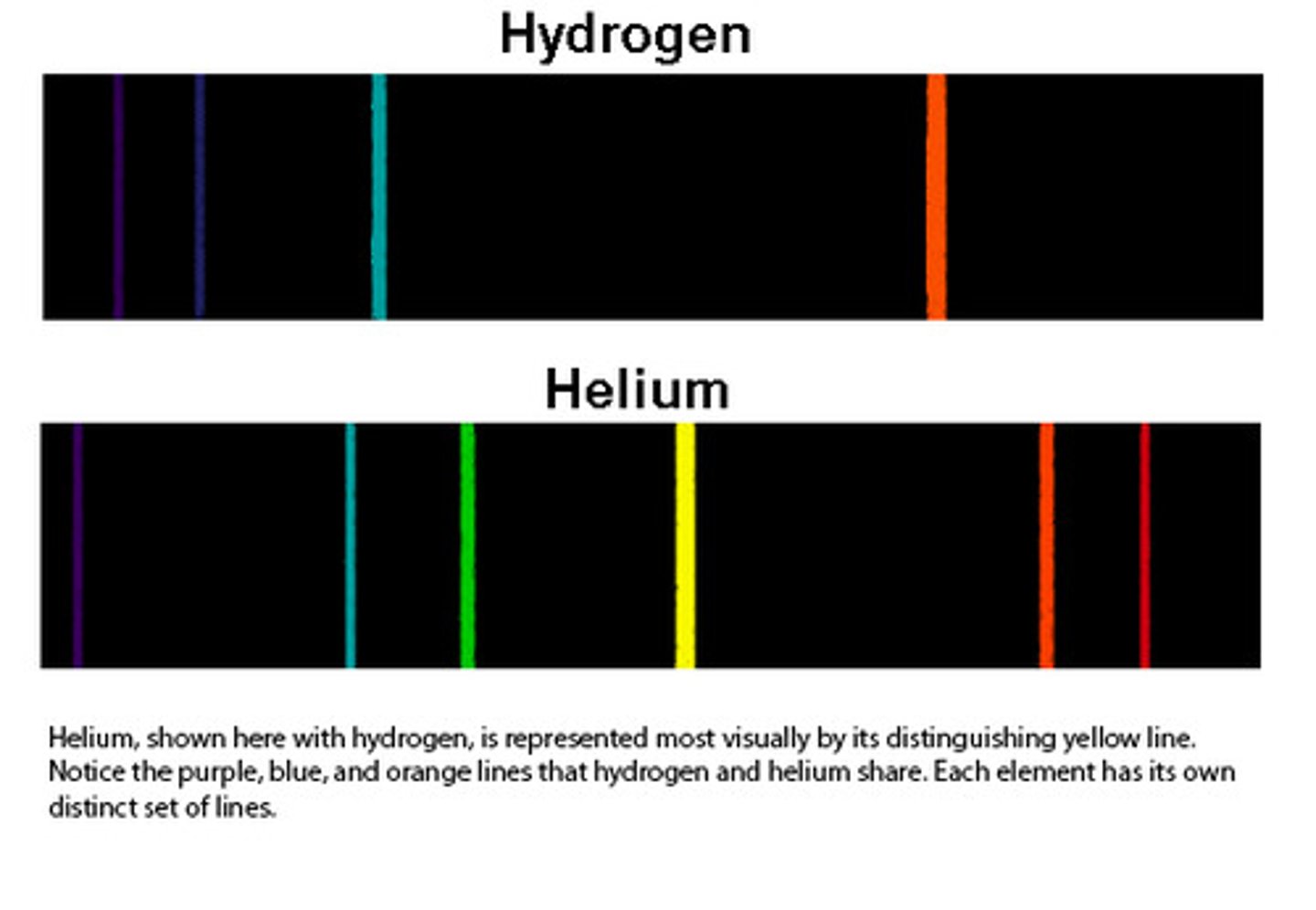
Two types of line spectra
Emission spectra and absorption spectra
Emission spectra
- emission spectrum consists of discrete wavelengths (which are different colours)
- Electron transfers higher to lower energy level: emission of photon, which has a wavelength that corresponds to the discrete energy change
- each transition is a different wavelength of light = 1 line in the spectrum
ΔE = hf or hc/λ
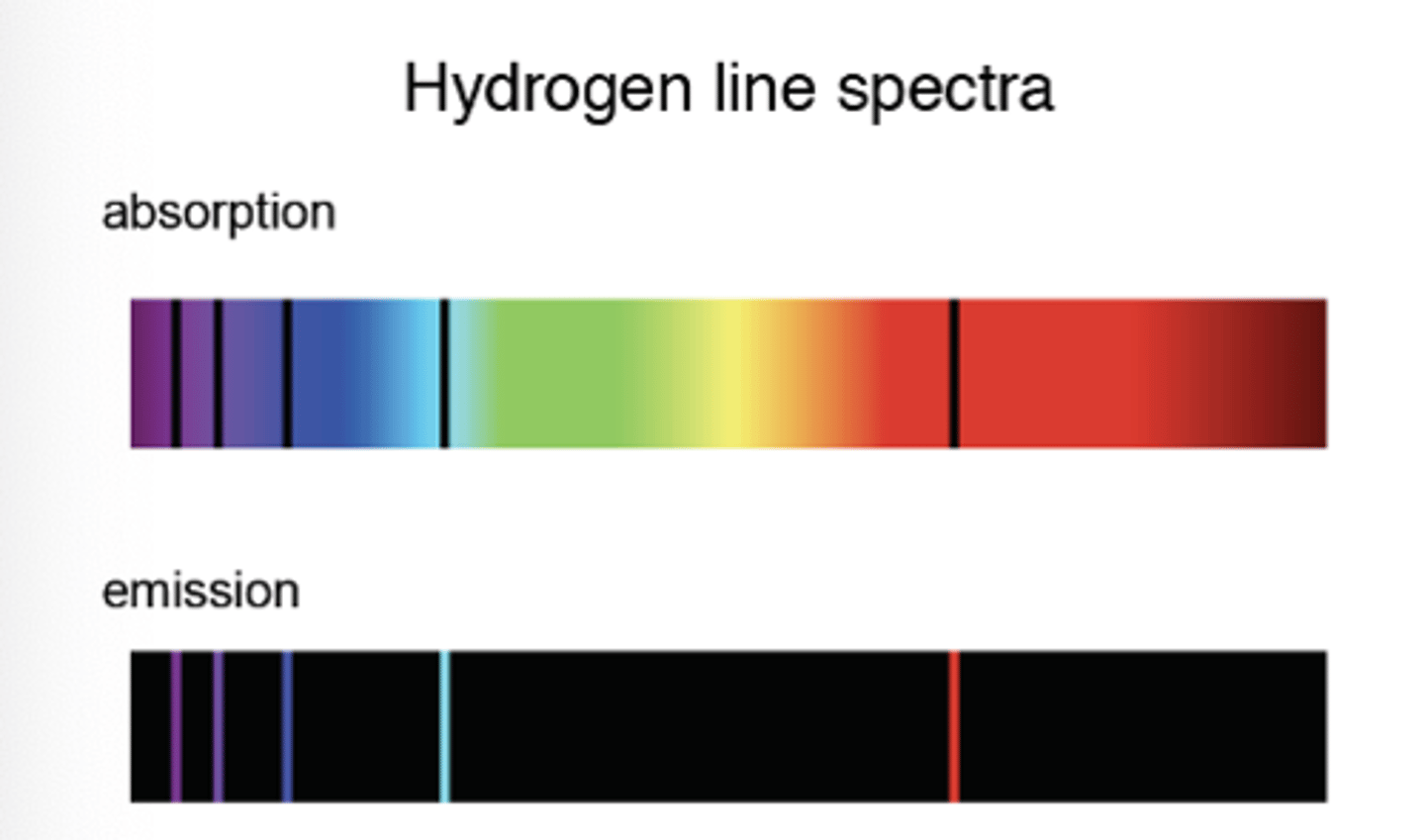
Emission spectra is evidence that
Electrons in atoms can only transition between discrete energy levels.
Absorption spectra
- An atom is raised to excited state through absorption of a photon
- consists of a continuous spectrum with dark lines at certain wavelengths which represent different energy levels of the atom
- when electrons return to lower levels, photons are emitted in all directions, so some wavelengths appear to be missing
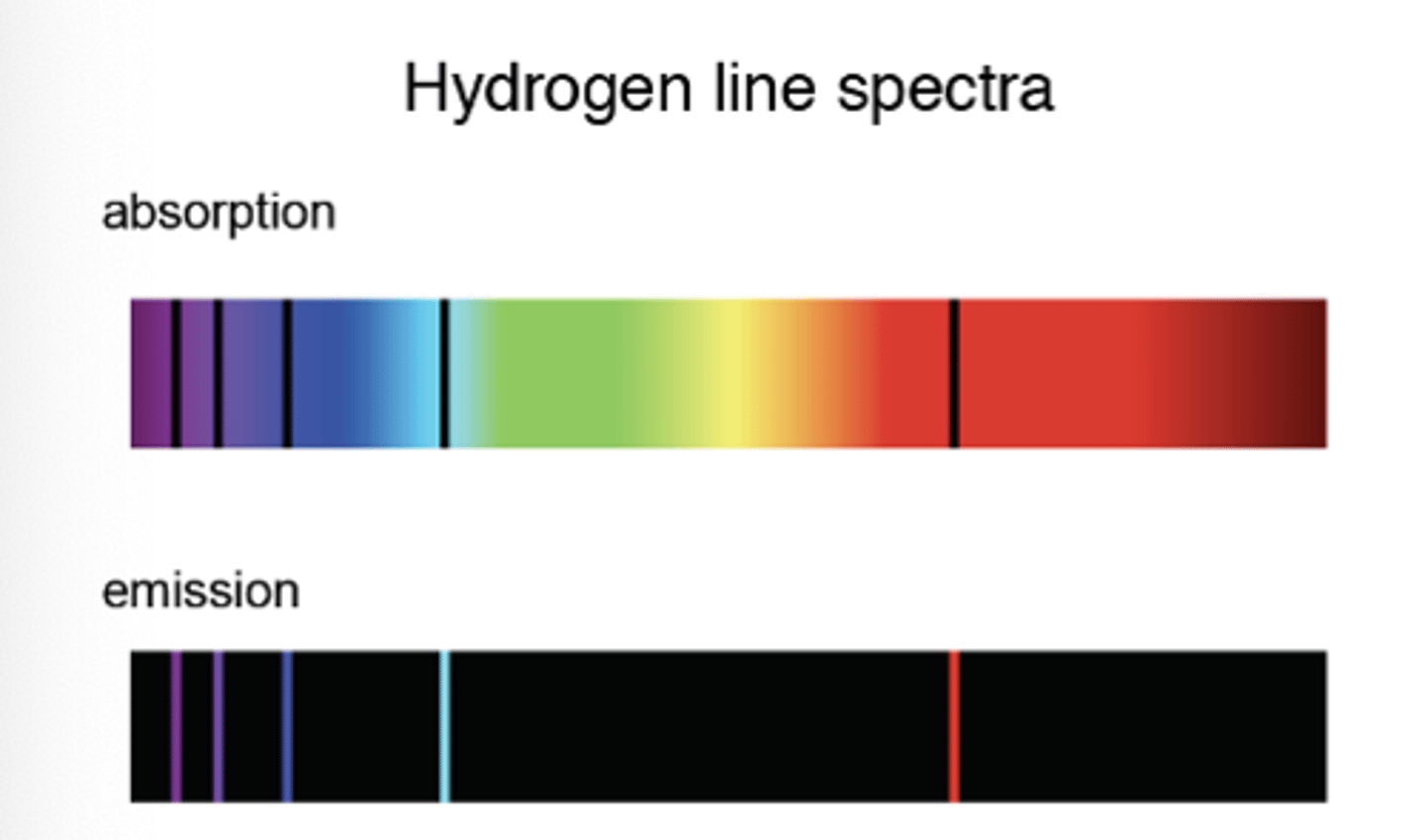
Emission and absorption spectra
The wavelengths missing from an absorption spectrum are the same as their corresponding emission spectra of the same element.
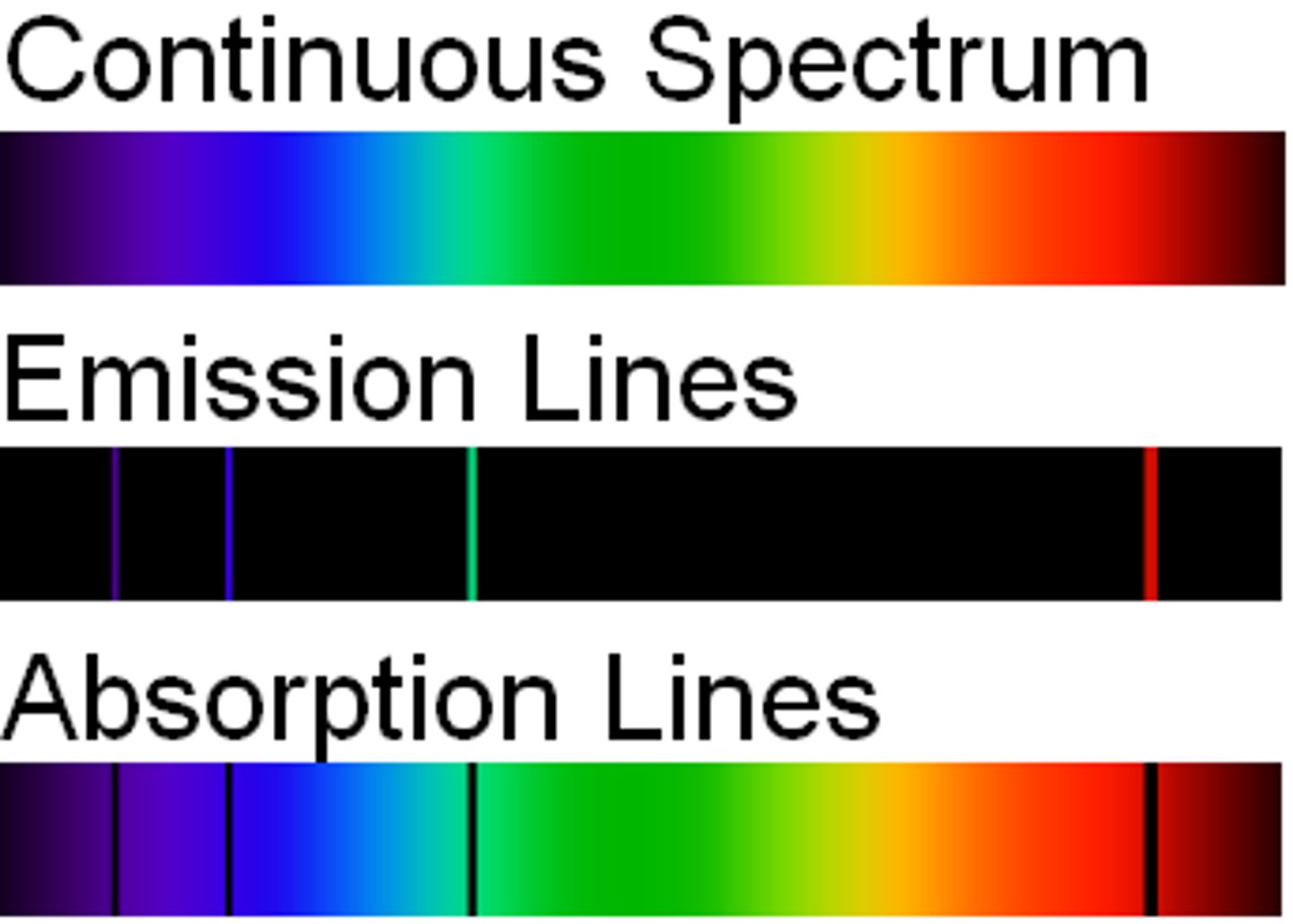
Calculating discrete energies
- The difference between two energy levels= energy of a specific photon
ΔE = hf = E2 - E1
- E1 = energy of lower level and E2= energy of the higher level
and
ΔE = hc/λ = E2 - E1
so λ= hc/(E2-E1)
so λ and energy change are inversely proportional
Photon
A quantum of energy of electromagnetic radiation.
(Fundamental particles that make up electromagnetic radiation. Light is quantised, or carried in discrete packets)
Therefore energy of photon is proportional to
- Directly proportional to frequency of electromagnetic radiation
- Inversely proportional to wavelength of electromagnetic radiation
Photon momentum
p = E/c
- p = momentum of photon
- E = energy of photon
- c = speed of light
(has no mass but has momentum)
Electronvolt (eV)
= energy gained by an electron travelling, from rest, through a potential difference of one volt
- Unit of energy.
- Derived from E = Q/V (which is from P = IV)
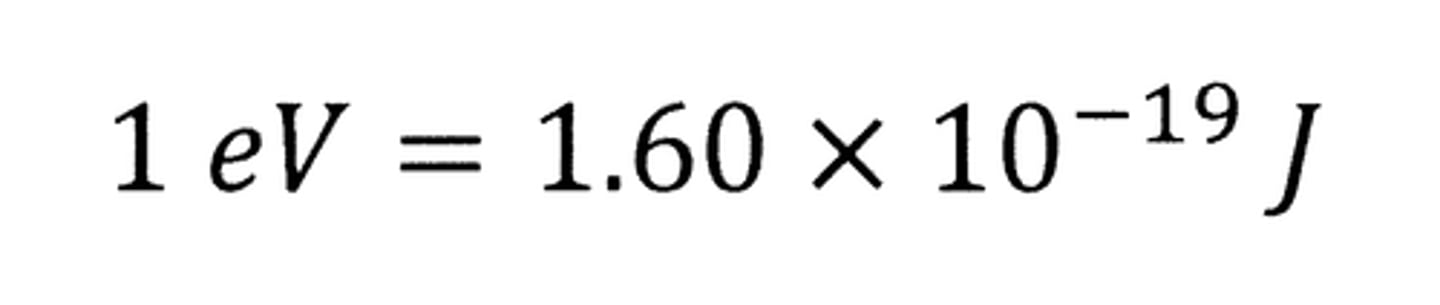
Relation to kinetic energy
- If an electron accelerates from rest, an electronvolt is equal to the kinetic energy gained:
eV = ½ mv2
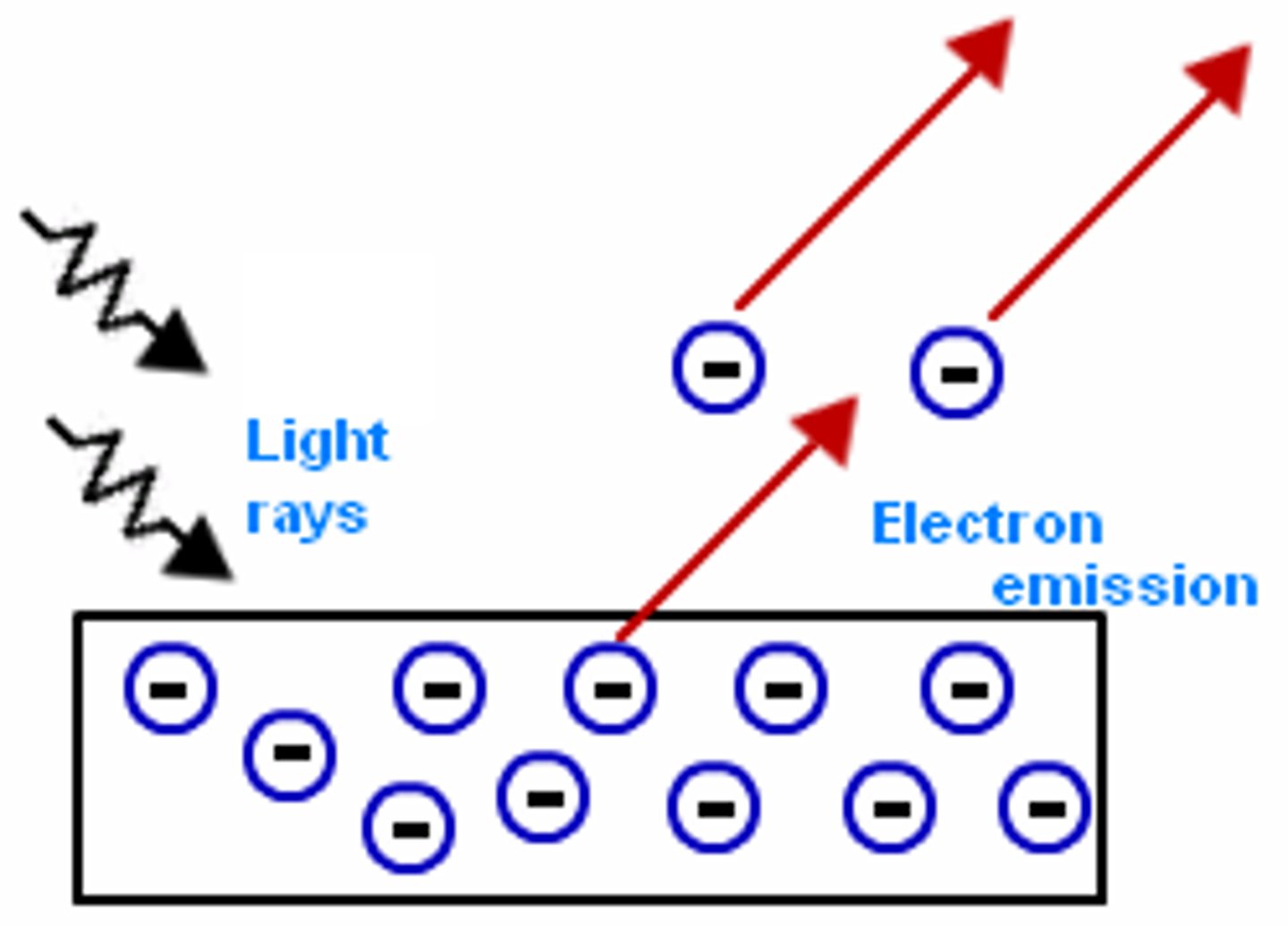
Photoelectric effect
- Electrons are emitted from the surface of a metal upon the absorption of electromagnetic radiation.
- Removed electrons are photoelectrons
- Each electron absorbs one photon.
Threshold frequency (property of a metal)
- The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal.
(Only the frequencies of light above a threshold frequency will emit a photoelectron)
Threshold wavelength (property of a metal)
The longest wavelength of incident electromagnetic radiation that would remove a photoelectron from the surface of a metal
φ Work function/threshold energy (Property of a metal)
The minimum energy required to remove a photoelectron from the surface of a metal.
- One electron absorbs one photon, so to escape the surface of a metal, an electron must absorb energy ≥ φ.
Photoelectric equation demonstrates...
- If the frequency of incident photons are too low, and energy isn't enough to overcome the work function (φ), electrons won't emit
Intensity of EM radiation
Proportional to number of photons striking/incident on the surface of the metal.
Maximum kinetic energy & incident intensity of EM radiation
- Max k.e. is independent of incident intensity
- Each electron can only absorb one photon,
- k.e. is dependent on the frequency of radiation
Photoelectric current
Number of photoelectrons emitted per second.
Electromagnetic radiation nature
Particulate nature. Made up of particles.
Photon energy formula
E = hf or hc/λ
- E = energy of photon / J
- h = Planck's constant (J s)
- f = frequency Hertz/Hz
- c = speed of light (m s^-1)
- λ = wavelength (m)