Adaptive Immunity 2: T cells
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
What is the main role of dendritic cells in the immune system?
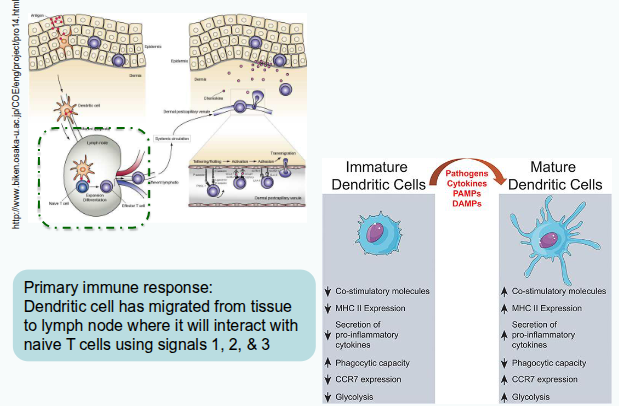
Dendritic cells integrate signals from their local environment to regulate immune responses.
From which sources do dendritic cells integrate signals?
They integrate signals from PAMPs and DAMPs, cytokines and chemokines, hormones, nutrients, and vitamins.
How do dendritic cells recognise PAMPs and DAMPs?
Through pattern recognition receptors (PRRs).
What types of hormones can influence dendritic cell function?
Stress hormones and sex hormones.
Which nutrients affect dendritic cell activity?
Lipids, carbohydrates, and amino acids.
Give an example of a vitamin that can influence dendritic cell behaviour.
Vitamin D
How does signal integration change dendritic cells?
It alters their phenotype by modifying receptor expression, cytokine production, and nutrient use.
What is meant by ‘up- and down-regulation’ in dendritic cells?
It refers to the increase or decrease in surface receptor expression and secreted product levels.
Why is nutrient availability important for dendritic cells?
Nutrients provide the energy required for dendritic cell activation and function.
What overall effect do environmental signals have on dendritic cells?
They shape dendritic cell phenotype and function, influencing the immune response.
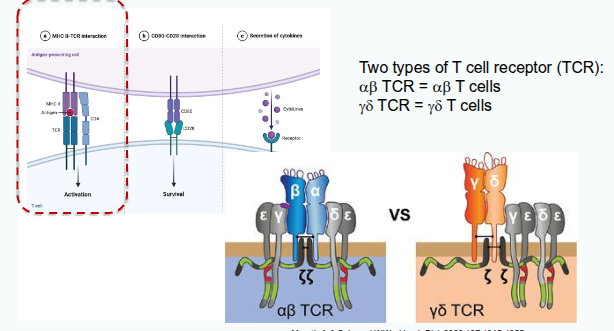
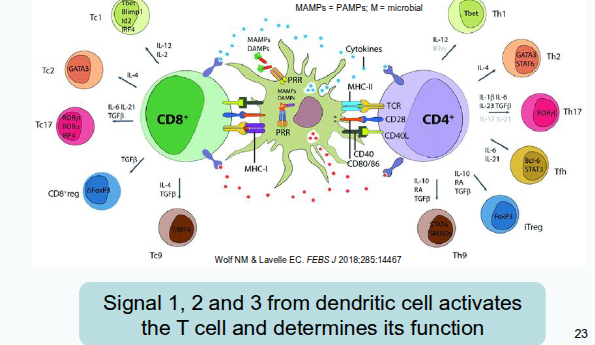
Signal 1: MHC/peptide interacts with TCR
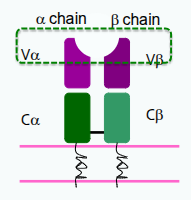
T cell receptor (TCR)

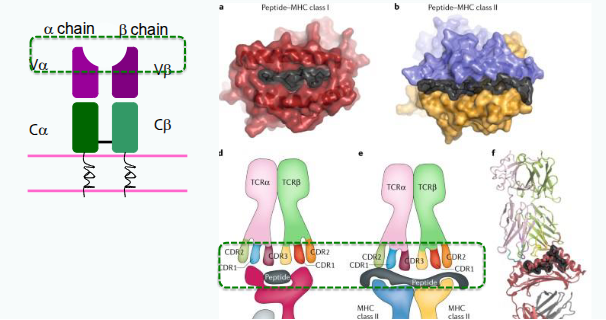
What are the structural and genetic features of the T-cell receptor (TCR) that contribute to immunological diversity?
Each TCR chain has one variable (V) and one constant (C) domain. The variable domains contain three hypervariable regions, also known as complementarity determining regions (CDRs), which provide antigen specificity. Most circulating T cells express TCRs encoded by the αβ genes, while a smaller subset expresses TCRs encoded by the γδ genes.
How is diversity generated in T-cell receptors (TCRs) and antibodies?
Diversity is generated by gene rearrangement of inherited gene segments — a process unique to antibodies and TCRs.
Which gene families are inherited and involved in generating TCR diversity?
Variable (V), diversity (D), and joining (J) gene families are inherited from one’s biological parents.
How do V, D, and J genes contribute to TCR diversity?
They rearrange randomly to select individual gene segments, creating a unique combination for each receptor.
Where does hypervariability occur in the TCR, and what does it affect?
Hypervariability occurs in the MHC/peptide binding region of the TCR, affecting antigen specificity.
How likely is it that the same αβ TCR pairing is generated in an individual?
It is extremely unlikely due to the high degree of random gene rearrangement and variability.
Immunological diversity: TCR
Thymus
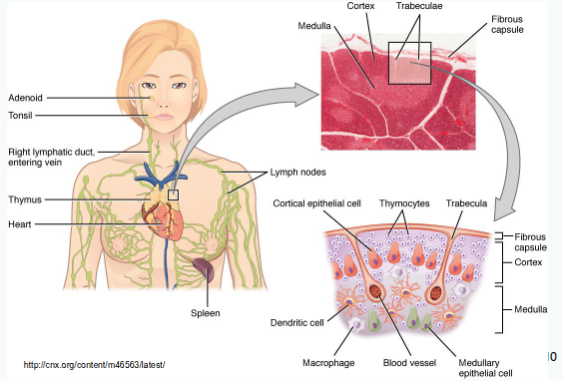
Where do T-cell precursors originate and where do they migrate to mature?
T-cell precursors originate in the bone marrow and migrate to the thymus for maturation.
What happens to T-cell precursors after arriving in the thymus?
They undergo rapid expansion under the influence of cytokines such as interleukin-7 (IL-7).
What is the role of interleukin-7 (IL-7) in T-cell development?
IL-7 promotes proliferation and induces transcription factors that commit cells to the T-cell lineage.
What major event occurs once T-cell precursors commit to the T-cell lineage?
They assemble the T-cell receptor (TCR).
What happens when T cells successfully assemble a functional TCR?
They begin to express both CD4 and CD8 surface proteins, becoming double-positive (DP) cells in the thymic cortex.
What is the lifespan of double-positive (CD4⁺CD8⁺) thymocytes?
Approximately 3–4 days.
T cell development

What happens to double-positive (DP) thymocytes if they do not receive the right signals?
They die unless they are ‘rescued’ by receiving appropriate survival signals.
How are double-positive T cells ‘rescued’ during development?
By engaging their T-cell receptor (TCR) with an MHC molecule, allowing them to mature into single-positive (SP) CD4⁺ or CD8⁺ T cells
What does positive selection ensure in T-cell development?
That the TCR of a DP cell can recognise self MHC:self peptide complexes, ensuring self-MHC restriction.
What is the purpose of negative selection in T-cell development?
To remove T cells that react too strongly with self MHC:self peptide complexes, preventing autoimmunity.
What is the outcome of positive and negative selection in the thymus?
Only T cells with moderate affinity for self MHC survive and mature into functional, self-tolerant CD4⁺ or CD8⁺ T cells.
Which cells mediate positive selection of T cells?
Cortical thymic epithelial cells (cTEC).
What role do cortical thymic epithelial cells (cTEC) play in T-cell development?
They express MHC molecules, interact with double-positive (DP) T cells, and have the machinery for MHC I and II antigen processing.
What determines whether a T cell survives positive selection?
The TCR must recognise self MHC:self peptide complexes with sufficient, but not excessive, affinity
Which cells mainly mediate negative selection of T cells?
Bone-marrow-derived antigen-presenting cells and medullary thymic epithelial cells (mTEC).
What happens to T cells that bind too strongly to self MHC:self peptide during negative selection?
They undergo apoptosis, removing potentially self-reactive T cells from the repertoire.
What is the overall purpose of positive and negative selection?
Positive selection ensures T cells can recognise self MHC, while negative selection eliminates self-reactive T cells to prevent autoimmunity.
Are all self-reactive lymphocytes deleted in the thymus?
No, not all self-reactive lymphocytes are deleted.
Why aren’t all self-reactive cells eliminated?
There is a balance between purging self-reactive cells and maintaining the ability to respond to pathogens
How does the thymus expose developing T cells to tissue-restricted antigens?
Through promiscuous expression of antigens from all major organs, controlled by the genes Aire and Fezf2.
What can happen to self-reactive T cells that escape the thymus?
They may enter the periphery and potentially contribute to autoimmune disease.
Can self-reactive lymphocytes cause disease?
Yes, some self-reactive lymphocytes can be activated and lead to autoimmune disease.
Are there mechanisms outside the thymus to control self-reactive T cells?
Yes, additional peripheral mechanisms exist to regulate autoreactive T cells that escape thymic deletion.
What are regulatory T cells and what is their role in immunity?
Regulatory T cells are a specialised population that can develop in the thymus and periphery. They suppress other immune cells, including conventional T cells, helping to prevent autoimmunity but potentially reducing anti-cancer immunity.
Where do mature naïve T cells migrate after leaving the thymus?
They migrate to the T-cell zone of the lymph node.
What do naïve T cells wait for in the lymph node?
The arrival of a dendritic cell presenting an MHC/peptide complex that matches their TCR
Signal 1, Signal 2 and Signal 3
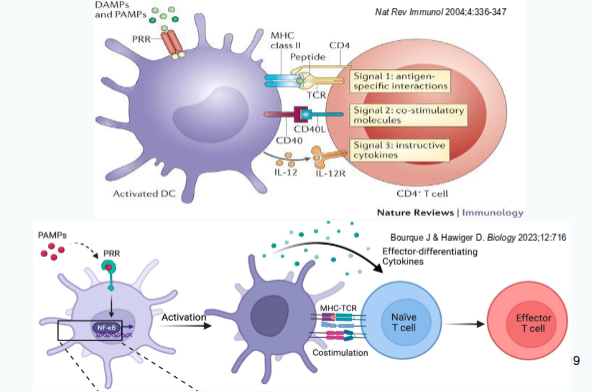
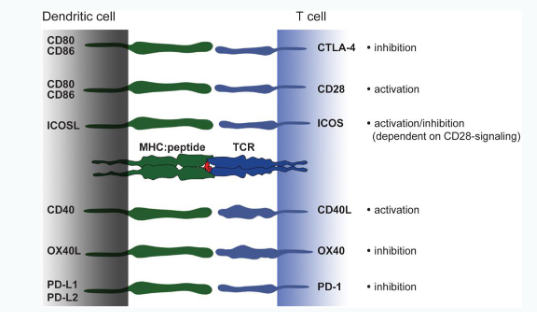
Signal 2: Co-stimulation/co-inhibition
CD = cluster of differentiation
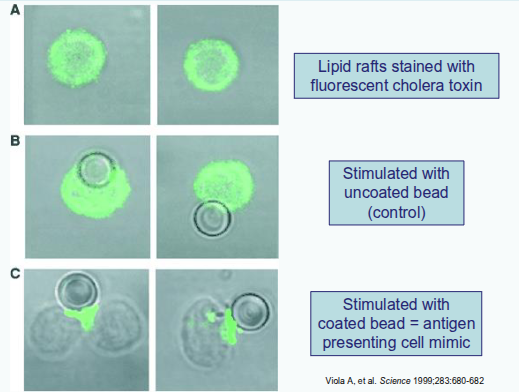
Immunological synapse

Immunological synapse
CD4 & CD8 T cell subsets
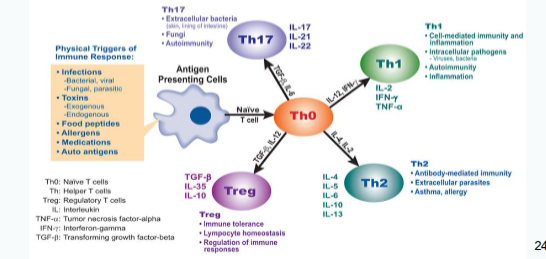
What determines the function of CD4 T helper cell subsets?
Their function is shaped to be appropriate for the trigger that activated the dendritic cells and initiated the primary immune response.
How many naïve T cells typically respond to a viral peptide-MHC (pMHC) complex?
Approximately 250–25,000 naïve T cells can be activated by 5–50 target pMHCs.
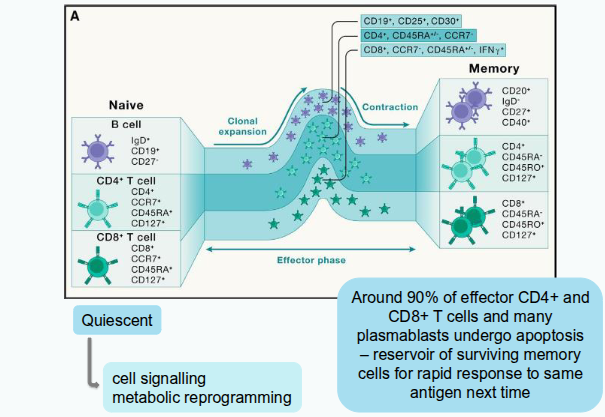
What happens when naïve T cells leave their quiescent state?
They undergo cell signalling, metabolic reprogramming, and extensive transcriptional, epigenetic, and translational changes that alter surface protein expression and cytokine secretion.
How do T cells increase in number after activation?
Through clonal expansion, producing daughter cells that carry the same TCR as the original activated T cell.
What is the division time for CD4⁺ and CD8⁺ T cells during clonal expansion?
About 10 hours for CD4⁺ T cells and 6–8 hours for CD8⁺ T cells.