Topic 4 - Chemical Changes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What colour is an acid or an alkaline in certain indicators?
Litmus: Acid - Red Alkaline - Blue
Methyl Orange: Acid - Red Alkaline - Yellow
Phenolphthalein: Acid - Colourless Alkaline - Pink
What are some examples of strong acids and weak acids?
Strong Acids:
Sulfuric
Hydrochloric
Nitric
Weak Acids:
Ethanoic
Citric
Carbonic
What is pH?
The measure of the concentration of Hydrogen Ions.
For every decrease of 1 on the pH scale, the concentration of hydrogen ions increase by a factor of 10.
So a pH 4 has 10 times the concentration of H+ ions than pH5
Reactions of acids:
Metal + Acid → Salt + Hydrogen
Metal Oxide + Acid → Salt + Water
Metal Hydroxide + Acid → Salt + Water
Metal Carbonate + Acid → Salt + Carbon Dioxide + Water
Metal + Water → Metal Hydroxide + Hydrogen
Ammonia + Acid → Ammonium Salt
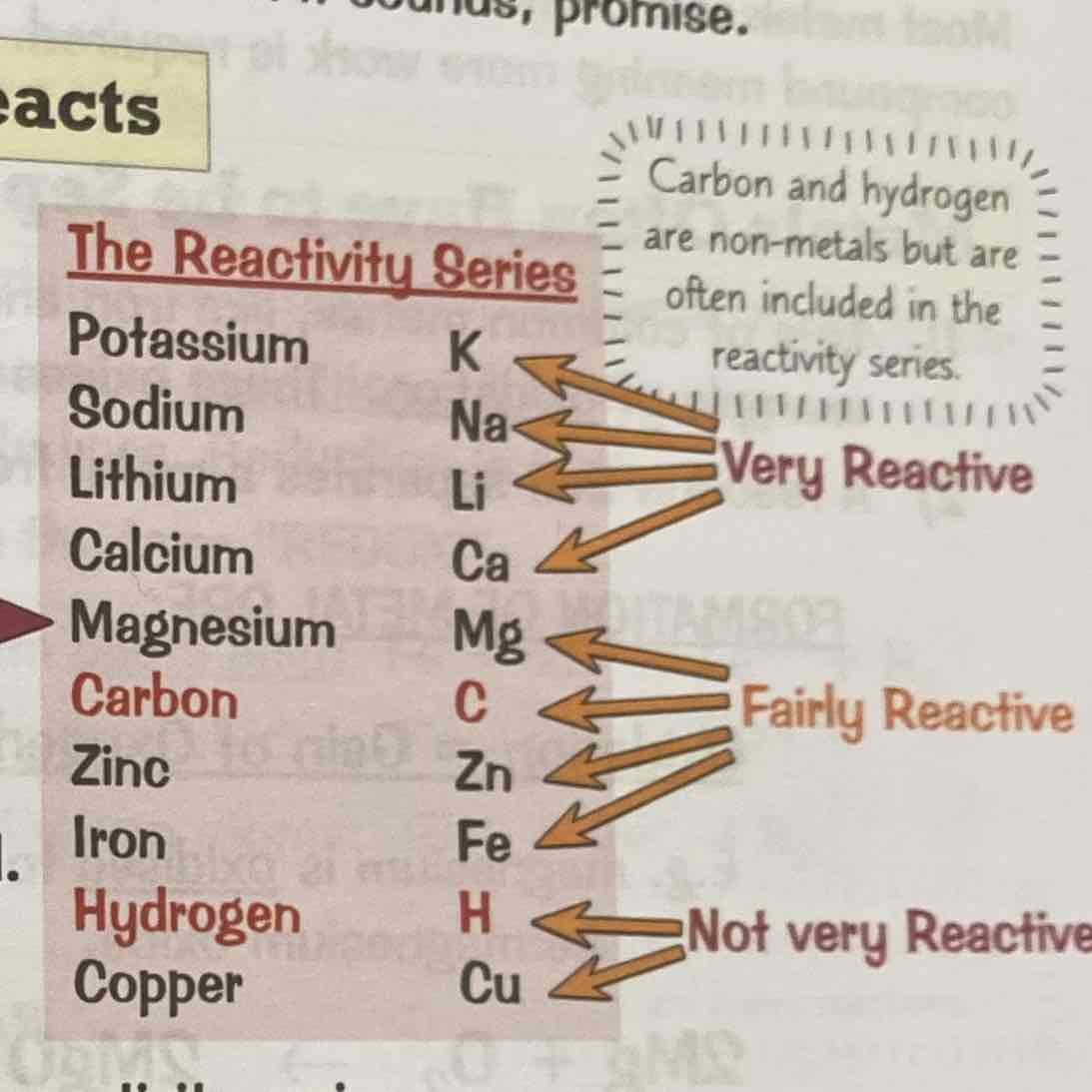
What is the reactivity Series
The list of metals in order of reactivity towards other substances.
What are Oxidation, Reduction and Redox reactions?
Oxidation - Loss of electrons Or Gain of oxygen
Reduction - Gain of electrons Or Loss of oxygen
Redox - When electrons are transferred - O and R happen at the same time.
What is Electrolyosis?
The splitting of a dissolved or molten compound with electricity. There is the cathode (-) and anode (+) electrodes which attract the opposite charge ions and they react. This creates a flow of charge throughout the structure.
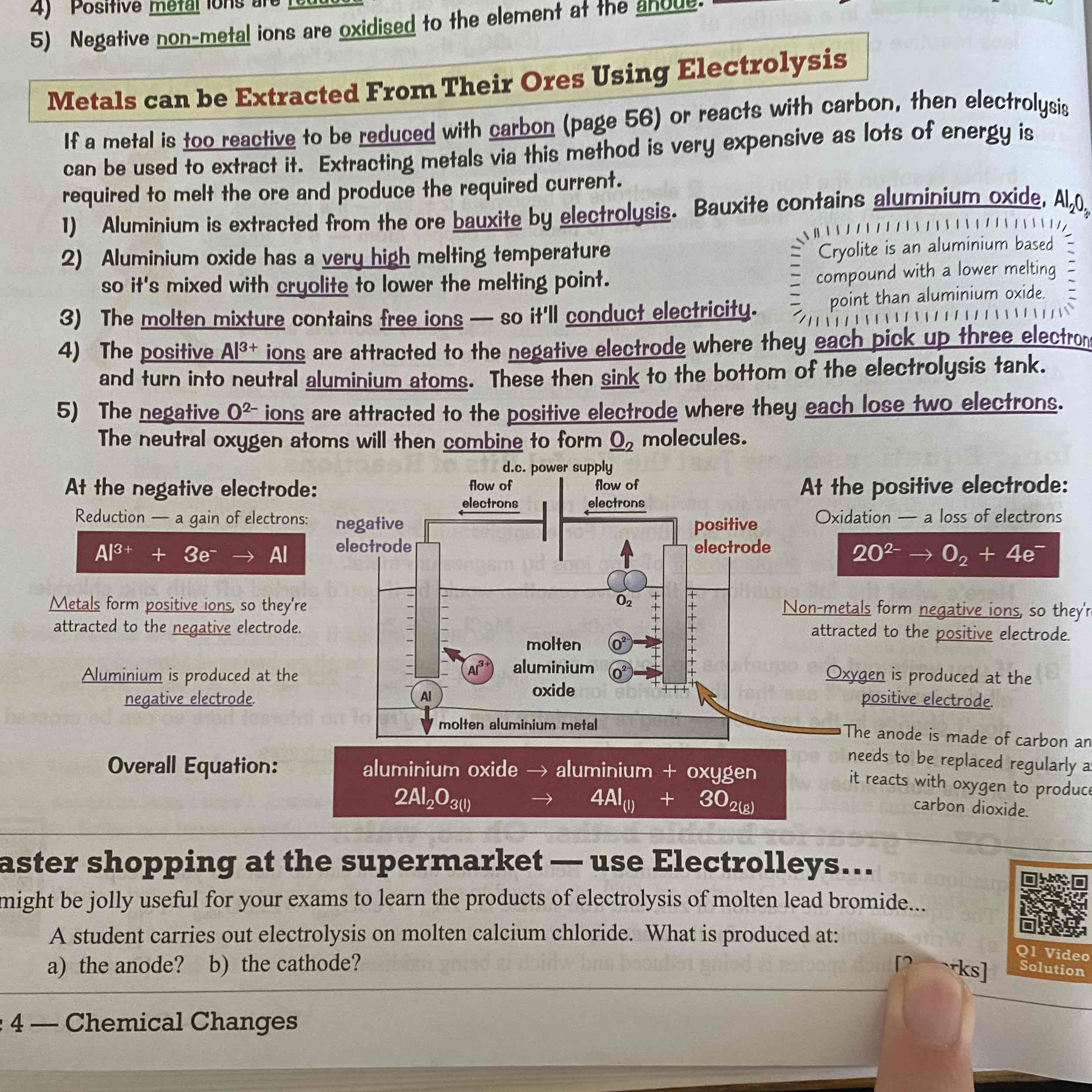
Describe an Aluminium Electrolyosis?
See image: