The Lymphatic System pt.2 : Lect. 19-20
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Inflammatory Response
Resident leukocytes: mast cells, dendritic cells and macrophages sound the alarm when tissue is damaged from trauma or infection.
—> initiates the inflammatory response which makes it easier for many elements (leukocytes, proteins, platelets) in the blood to be recruited to the site
Functions:
Prevents the spread of damaging agents to nearby tissues
Disposal of cell debris and pathogens
Sets the stage for repair processes
Alerts the Adaptive Immune System
4 major events of an Inflammatory Response:
Inflammatory chemicals are released from damaged or stressed tissue cells (+ toll-like PRRs) and resident immune cells (& other immune cells that arrives)
Arterioles dilate and capillaries become more permeable in response to inflammatory chemicals causing redness, heat and swelling
Phagocyte mobilization. Phagocytes from the blood move to the site of injury/infection
Phagocytes arrive at the site of injury and consume pathogens (phagocytosis) and cell debris
Chemical Signals released (5):
Histamine (from mast cells)
Cytokines (from tissue cells and macrophages)
Bradykinin (from damagedBlood vessels)Prostaglandins (from damaged tissue cells)
Compliment proteins
Histamine
Released by resident mast cells & basophils in response to mechanical injury, presence of certain microbes & chemicals released by neutrophils
Effects:
Vasodilation of local arterioles, increased permeability of local capillaries (fluid moves into the tissues)
Also causes bronchial constriction (smooth muscle) & mucus production —> becomes evident during allergic reactions
Cytokines
Small proteins that act as chemical messengers, that calls for the innate and adaptive immune systems
Resident macrophages or dendritic cells present in skin & mucous membranes tissues are usually the first immune cells to encounter microbes when these barriers have been broken
—> Recognize PAMPs on microbes = initiates phagocytosis & release of cytokines to promote the inflam. resp. (binding of PAMP to a toll-like PRR initiates a transduction cascade —> cytokines)
Bradykinin
A plasma protein released when there is damage to the endothelial lining of blood vessels
Effects:
Vasodilation
Increased capillary permeability
Induces chemotaxis by leukocytes
Stimulates nociceptors (pain receptors) = causing individual to protect an injured area
*Blood vessel injury triggers: inflam. resp. & blood-clotting cascade
Prostaglandins
Formed and released by damaged tissue cells
Effects:
Vasodilation
Increased capillary permeability
Induces chemotaxis by leukocytes
Stimulates nociceptors (pain receptors) = causes individual to protect an injured area
Increased vascular permeability leads to edema
The surge of protein-rich fluids into the tissue space serves several functions:
Dilutes harmful substances that may be present
Brings in large amounts of oxygen and nutrients needed for repair
Allows the entry of clotting proteins which form a gel-like fibrin mesh in the tissue space. This effectively isolates the injured area and prevents the spread of bacteria and harmful agents into surrounding tissues. It also forms a scaffolding for permanent repair
Increased Vascular Permeability Promotes Immune Function
When blood flow is increased d/t vasodilation & cap. permeability, important immune proteins called compliment proteins and immune cells like neutrophils & monocytes can exit the blood and enter the affected tissue
Phagocyte Mobilization (4 steps)
Chemical signals released:
Attract neutrophils and then monocytes to the site
Activate bone marrow to produce the appropriate innate immune cells: neutro, mono, baso, eosino
Mobilization of phagocytes occurs in 4 steps:
Leukocytosis – more leukocytes enter blood from bone marrow
Margination - accumulation and adhesion of leukocytes to capillary walls
Diapedesis – the migration of leukocytes through vessel walls
Chemotaxis – leukocytes follow chemical trail to site of injury/infection
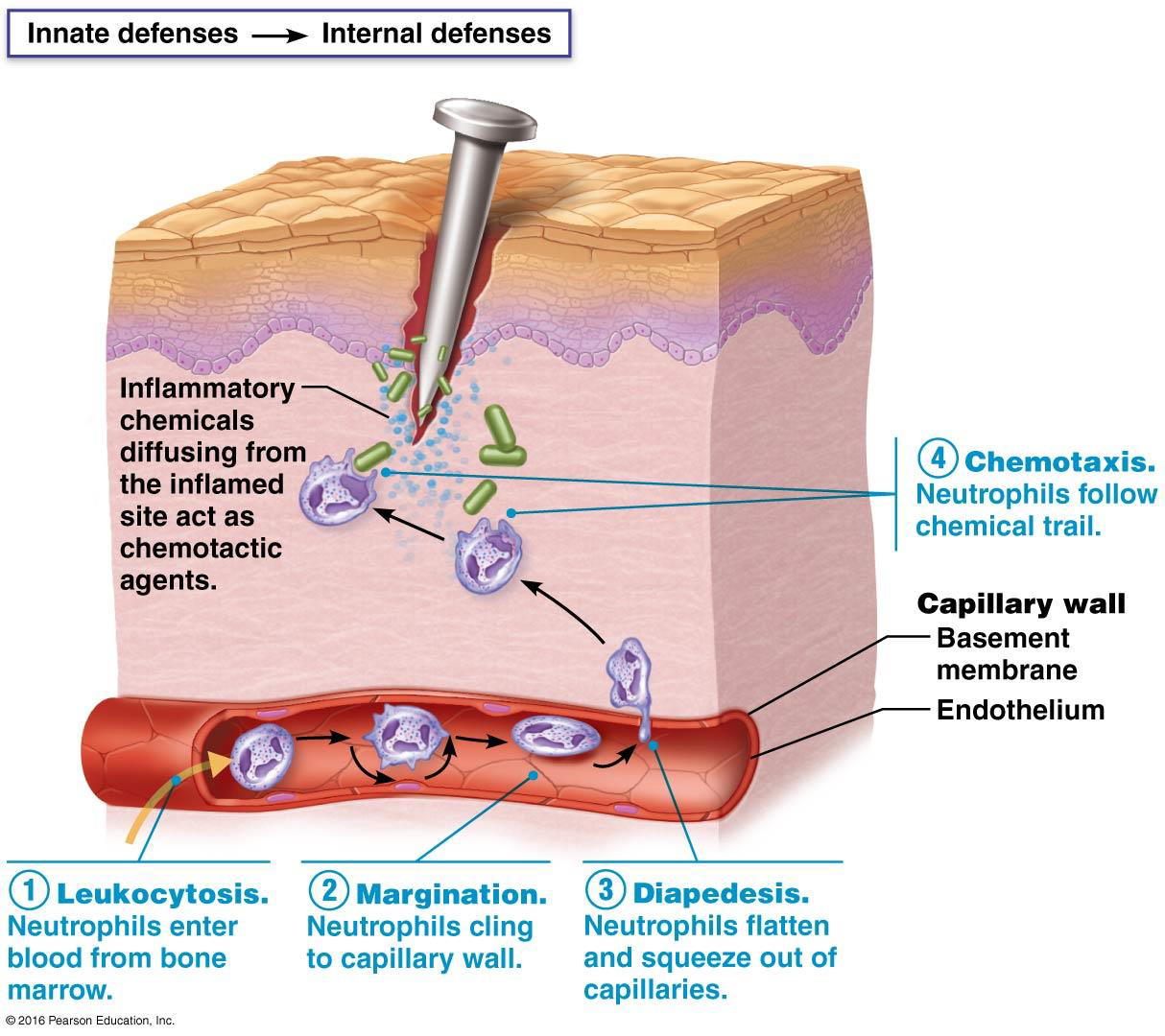
Timeline of inflamm. response
Immediate — resident mast cells, dendritic cells and/or macrophages
6-24 hours later — neutrophil numbers will increase in blood, many will enter the damaged tissue and begin phagocytosis. Neutrophils lifespan is short
Within 24-48 hours — macrophages derived from circulating monocytes predominate at the injury
Abscess
A walled-off localized collection of pus
Emigration of neutrophils, along with tissue destruction may lead to abscesses
Commonly produced by pyogenic (pus-producing) bacteria (e.g. Staphylococci)
Treatment: lancing the abscess (cutting in and draining it) & antibiotics
Cellulitis
When pus forming bacteria spread into surrounding tissues
Pus and the microorganism that produced it cause the reaction in the surrounding tissues
These spreading infections are serious life-threatening events. Must be treated by vigorous:
elimination of the infectious agent (usually with antibiotics)
incision and drainage of the lesion
Interferons
Antimicrobial proteins produced to block further infection of body cells by viruses
Viruses inject their DNA (or RNA) into a body cell where it takes over the cell’s machinery to make more copies of itself
A virus infected cell will die
However, before that happens the presence of viral DNA switches on interferon genes in the cell’s nucleus
They are transcribed and translated into interferon proteins which are released by that cell
Released interferon proteins bind to receptors on uninfected cells = stimulates them to produce antiviral proteins
If a virus tries to invade, the antiviral proteins will block it from reproducing
Therefore, interferons give resistance to our healthy cells against viral attack
*They cannot help a cell that is already infected with virus
Complement System
A group of at least 20 plasma proteins that normally circulate in the blood in an inactive state. They provide a major mechanism for destroying foreign substances in the body
Activated helps by:
Acting as inflammatory chemicals
Promoting phagocytosis by:
Acting as chemotactic factors to attract phagocytes
Acting as opsonins
Lysing and killing certain bacteria
Enhancing the effectiveness of both innate and adaptive immune responses
3 Pathways leading to Complement Activation:
Classical complement cascade begins with the formation of an antibody-antigen complex (antibody bound to an epitope on a pathogen). The 1st compliment protein binds to the antibody-antigen complex which triggers the compliment cascade. (Adaptive Immunity)
Lectin pathway: Lectin Proteins (a type of PRR, pattern recognition receptor) attach to a PAMP on surface of pathogen. 1st compliment protein will then bind and start the compliment cascade. (Innate Immunity)
The Alternative complement cascade begins with one of the complement proteins binding directly on the surface of a microbe. (Innate Immunity)
Classical Complement Pathway
Primarily activated by either an IgG or IgM antibody binding to an epitope (specific part - tag) on a pathogen
IgG and IgM are classes of antibody molecules
Antigen binding sites on antibodies have shapes that are complementary to specific epitopes - portions of proteins found on the surface of microbes
The stem region of the antibody can activate the Classical Complement Pathway by providing a site for the 1st compliment protein in the pathway to bind to
When the first of the Complement proteins is triggered by an antibody interlocked with a pathogen’s epitope it sets in motion a cascade of reactions
Each compliment component is activated in turn, acting upon the next in a precise sequence of carefully regulated steps known as the “Complement cascade”
These 2 pathways do not involve antibodies, they are both part of the innate immune system
Lectin Pathway: Involves a class of pattern recognition receptors (PRRs) called lectins which attach to carbohydrate PAMPs on the surface of microbes. This initiates the complement cascade.
Alternative Pathway: The 1st protein in the complement cascade binds to the surface of the microbe directly
All pathways lead to the splitting…
of a key protein C3
C3 splits into C3a and C3b
C3a enhances inflammation
C3b acts in 2 ways: Opsonization & MAC
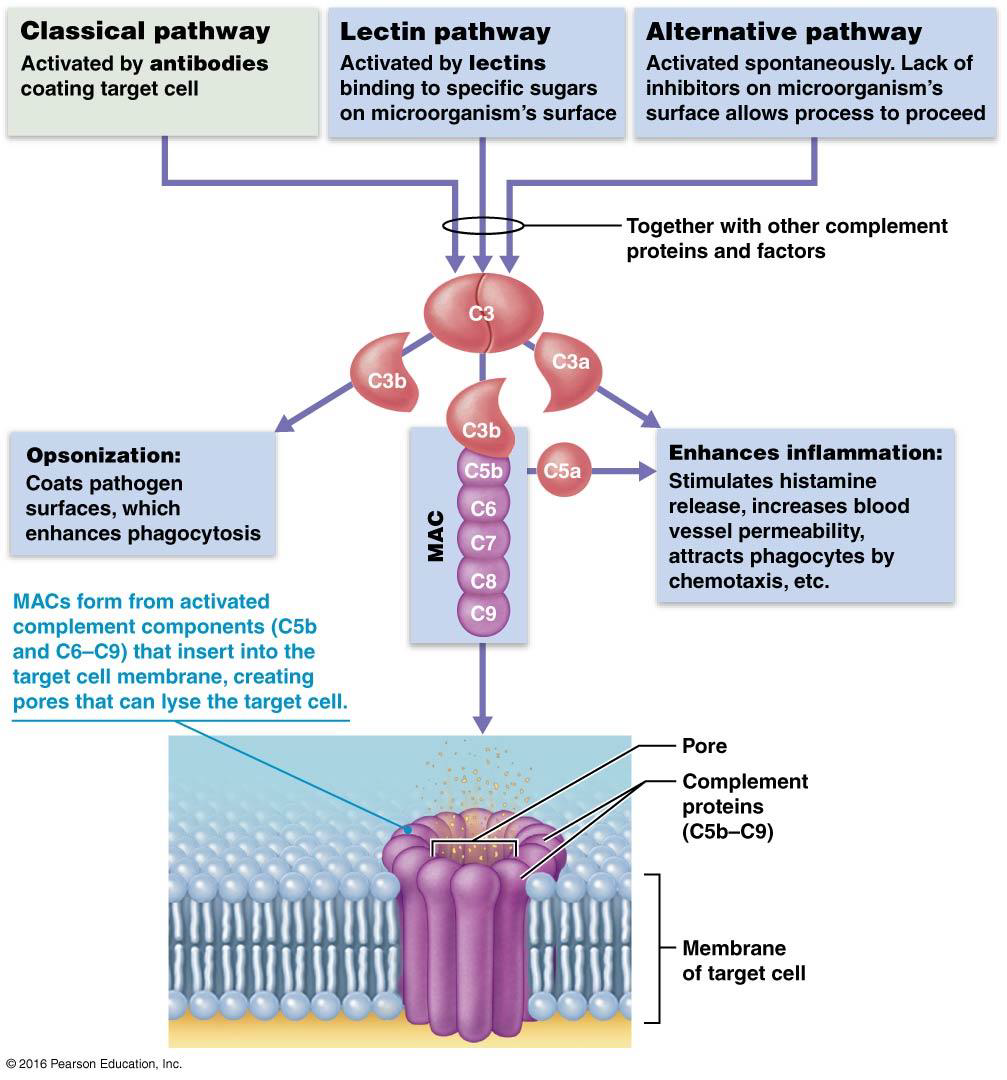
C3b Activates MAC
C3b triggers the complement cascade (made from a group of proteins that assemble together) which results in the formation of a protein complex known as the Membrane Attack Complex (MAC)
MAC will self-assemble in the plasma membrane of a target cell (e.g. a bacterium) and form a pore. These pores allow the passage of water into the cell and eventually cell lysis
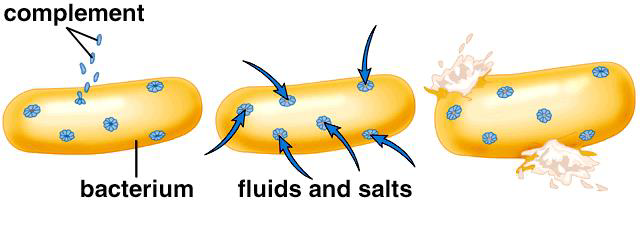
Besides lysis, what else does the complement system do?
Enhance phagocytosis (through opsonization)
Act as chemotactic factors (attract phagocytes to the site)
Stimulate mast cells and basophils to release histamine which amplifies the inflammatory response