Ribosomes
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
What determines the final destination of a protein in a cell?
Its signal sequence (amino acid "address label").
Where does protein synthesis usually start in eukaryotic cells?
On free ribosomes in the cytosol.
How are proteins transported between cytosol and nucleus?
Via nuclear pores, which allow folded proteins with a nuclear localization signal to pass.
How are proteins transported from cytosol into mitochondria or chloroplasts?
With a signal sequence recognized by membrane receptors and translocators; proteins are unfolded during import, then refolded inside.
What happens to the signal sequence after a protein enters the mitochondrion?
It is cleaved by a signal peptidase.
(Once the protein has crossed the membrane, an enzyme called signal peptidase cuts off the signal sequence.
how are proteins directed into the ER?
Proteins with an ER signal sequence bind an SRP → SRP receptor on ER → protein threaded into ER through a translocator.
What halts translation temporarily during ER targeting?
Binding of SRP to the ribosome and signal sequence.
How do soluble proteins reach the ER lumen?
Entire polypeptide is threaded through the translocator, signal sequence is cleaved, and protein folds in ER lumen.
What are polysomes (polyribosomes)?
Multiple ribosomes translating the same mRNA simultaneously.
What happens to ribosome subunits after finishing translation in the ER?
They dissociate and return to the cytosolic ribosome pool.
What modification occurs to most ER proteins?
Glycosylation – addition of oligosaccharides (first step: 14-sugar chain to asparagine).
Do cytosolic proteins undergo glycosylation?
Rarely; glycosylation mainly happens to ER proteins.
what are the three main mechanisms of protein transport in cells?
Nucleus ↔ cytosol: via nuclear pores.
Cytosol → organelles (ER, mito, chloro, peroxisome): via translocators, proteins unfolded.
ER → endomembrane system: via vesicles with coat proteins (e.g. clathrin) + SNAREs for docking/fusion
What are coat proteins and SNAREs used for in protein transport?
Coat proteins (e.g. clathrin) shape vesicles; SNAREs mediate specific vesicle fusion with target membranes.
Suppose that a protein contains an ER signal sequence at the N-terminus (the end where the protein synthesis starts), a mitochondrion signal sequence in the middle, and a chloroplast signal sequence at the C-terminus (the part that is formed last). Where will this protein be translocated?Where will this protein be located to after translation has finished?
1. Translation always starts in the cytosol
The ribosome begins protein synthesis free in the cytosol.
The very first part of the protein that emerges is the N-terminus.
2. The ER signal sequence is translated first
Since the ER signal sequence is at the N-terminus, it appears first as the ribosome synthesizes the protein.
This sequence is recognized immediately by the Signal Recognition Particle (SRP).
SRP binds to the signal sequence and ribosome, halts translation, and brings the ribosome to the ER membrane.
3. Protein enters the ER
Once docked at the ER, the polypeptide is threaded into the ER lumen through a translocator.
Translation resumes, and the entire protein is made directly into the ER lumen.
4. What about the mitochondria and chloroplast signals?
The mitochondrial (middle) and chloroplast (C-terminal) signals never get used, because the protein is already committed to the ER pathway as soon as the N-terminal ER signal sequence was recognized.
Signal recognition happens co-translationally (while the protein is being made), and the earliest signal in the sequence dominates.
5. Final destination after translation
After translation, the protein is located in the ER lumen.
From here, it can be processed, glycosylated, and eventually transported via vesicles to the Golgi, lysosome, plasma membrane, or secreted outside the cell.
if A protein has no signal sequence Where will it end up after translation?
It will remain in the cytosol, because without a signal sequence there is no targeting information.
A protein has a mitochondrial signal sequence at the N-terminus and an ER signal sequence in the middle. Where does it go?
It will go into the mitochondrion, because the N-terminal signal is the first part of the protein to emerge from the ribosome and directs it to the mitochondria before the ER sequence is even translated.
A protein has an ER signal sequence at the N-terminus and a stop-transfer sequence further down. What type of protein will it become?
It will become a single-pass transmembrane protein, because the ER sequence initiates translocation into the ER, but the stop-transfer sequence halts translocation and anchors the protein in the membrane.
A protein has an ER signal sequence at the N-terminus and two internal stop-transfer sequences. What’s the result?
It will become a multi-pass transmembrane protein, because each stop-transfer sequence locks another segment of the protein into the ER membrane, creating multiple membrane-spanning domains.
A protein has a peroxisome-targeting sequence but no ER signal sequence. Where does it go?
It will be fully translated in the cytosol and then imported into the peroxisome, because peroxisomal proteins are recognized post-translationally by receptors that direct them into the organelle.
A protein has an ER signal sequence at the N-terminus, and later a mitochondrial signal sequence. Where will it be after translation?
It will enter the ER lumen, because the N-terminal ER sequence is recognized co-translationally by SRP and commits the protein to the ER before the mitochondrial sequence is exposed.
A protein has a chloroplast signal sequence at the N-terminus but no ER signal. Where will it end up?
It will be imported into the chloroplast, because the N-terminal signal is recognized by chloroplast receptors and translocators, which pull the protein inside in an unfolded state.
A secretory protein is being translated into the ER. Translation is stopped when SRP binds. What resumes translation?
Translation resumes once the SRP–ribosome complex binds to the SRP receptor on the ER membrane, because this docking event opens the translocator and allows synthesis to continue into the ER lumen.
What are the four main functions of protein glycosylation in the ER?
Retains misfolded proteins in the ER.
Protects secreted proteins from proteases.
Acts as a transport signal for correct vesicle sorting.
Contributes to the glycocalyx, protecting the cell from mechanical damage.
Which proteins are glycosylated — cytosolic proteins or ER proteins?
ER proteins are usually glycosylated; cytosolic proteins rarely are.
What is the endomembrane system?
A group of organelles connected by vesicle trafficking: ER, Golgi, endosomes, lysosomes, and in plants, the vacuole.
How do proteins move between compartments of the endomembrane system?
Via vesicles that bud from one organelle, travel, and fuse with the target organelle’s membrane.
What role do coat proteins (like clathrin) play in vesicle trafficking?
They shape the budding vesicle from donor membranes. After budding, the coat is removed so the vesicle can fuse with the target membrane.
What is the role of the Golgi apparatus in protein processing?
The “Post Office” of the cell: receives proteins from the ER, modifies them A) By ribosomes attached to the RER.(e.g. completes glycosylation), sorts, and packages them into vesicles for delivery.
Where do proteins go immediately after leaving the ER?
They are packed into vesicles that fuse with the cis face of the Golgi apparatus.
How is the Golgi apparatus different in animal vs. plant cells?
Animal cells: usually 1 or a few Golgi stacks.
Plant cells: many scattered Golgi stacks.
At which stage is glycosylation finished?
In the Golgi apparatus, after initial glycosylation in the ER.
Trans Golgi Network (TGN)
Function: Sorting & shipping center.
Directs proteins to their final destinations: lysosomes, plasma membrane, or secretory vesicles.
Think of it as the “distribution hub.”
Medial cisterna
Function: Middle compartments of the Golgi where proteins undergo further modification (e.g., trimming & adding sugars during glycosylation).
Acts as the “processing zone.”
Cis cisternae
Function: The entry face of the Golgi (nearest the ER).
Receives vesicles from the ER.
First stage of modification (e.g., removal of certain mannose sugars).
Trans cisterna
Function: Toward the exit face, just before the TGN.
Final processing steps of proteins and lipids (e.g., addition of complex sugars, sulfation).
Transport route of excreted protiens
Protein synthesis at the ER membrane => RER => vesicle => cis en trans
cisternae => trans Golgi network => vesicle => fusion of vesicle with the cell
membrane => secretion
A certain protein in a cell is coupled to various oligosaccharides. To what location in the cell is this protein most likely translocated?
The Golgi apparatus, Endosomes, Chloroplasts, The nucleus
Glycosylation takes place in the endomembrane system. Endosomes, ER and Golgi apparatus are part of the endomembrane system in which glycosylated proteins may thus end up. Proteins that are synthesised in the cytosol (including proteins that are imported from the cytosol like mitochondrial and chloroplast proteins) are rarely glycosylated and certainly not with multiple oligosaccharides.
Exocytosis
Fusion of vesicles from the Golgi apparatus with the plasma membrane, releasing proteins outside the cell and adding vesicle membrane to the plasma membrane.
What is constitutive exocytosis?
Unregulated secretion: vesicles fuse with the plasma membrane continuously without a signal → delivers proteins and increases membrane surface.
What is regulated exocytosis?
Signal-dependent secretion: vesicles wait at the plasma membrane and fuse only when a signal arrives (e.g., neurotransmitter release in neurons).
What happens to transmembrane proteins during exocytosis?
They travel in the vesicle membrane, and upon fusion, they become part of the plasma membrane.
What is endocytosis?
Uptake of material from the extracellular space by plasma membrane invagination → vesicle formation → internalization.
Why does endocytosis balance exocytosis?
It recycles plasma membrane and surface proteins, keeping cell surface area constant despite exocytosis.
What is phagocytosis?
“Cell eating”: uptake of large solid particles (e.g., bacteria by white blood cells); non-specific.
What is pinocytosis?
“Cell drinking”: uptake of liquids and solutes; non-specific.
What is receptor-mediated endocytosis?
Highly specific uptake: macromolecules bind to receptors in clathrin-coated pits, triggering vesicle budding.
What role does clathrin play in receptor-mediated endocytosis?
Clathrin forms a coat that shapes the budding vesicle. After vesicle formation, the coat is removed so the vesicle can fuse with endosomes.
What happens to cholesterol during receptor-mediated endocytosis?
Cholesterol travels in LDL particles, binds LDL receptors in coated pits, enters via clathrin-coated vesicles → delivered to endosomes → then lysosomes release free cholesterol.
What happens to LDL receptors after endocytosis?
They are recycled back to the plasma membrane from the endosome.
How do cells communicate with each other?
Via signalling molecules.
Reception: binding of an extracellular signalling molecule.
Transduction: conversion and amplification of the extracellular signal, triggering a specific response by the cell. generally consists of a number of different steps, conveyed by intracellular signalling molecules, to attain the final response.
Response(s): cell behaviour is altered by changing the activity of effector proteins, such as opening/closing ion channels, or switching on/off certain genes.
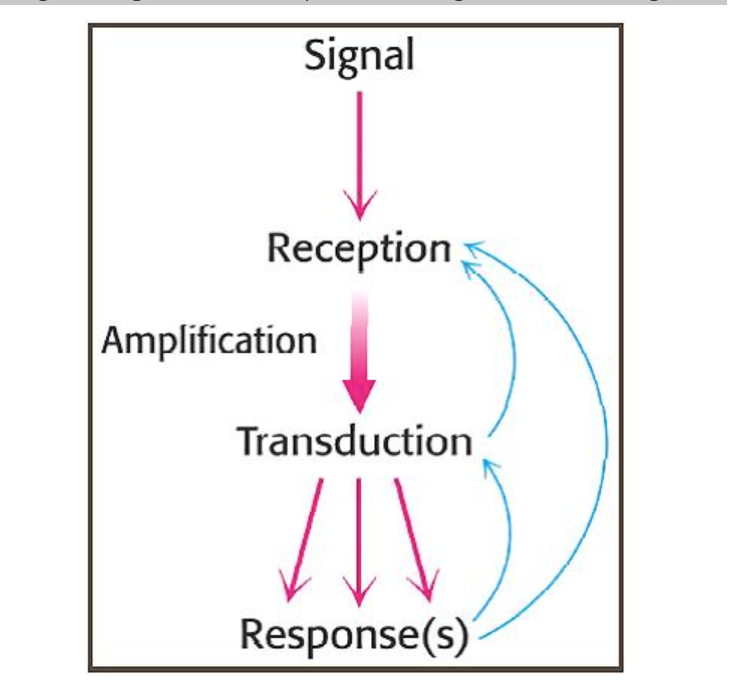
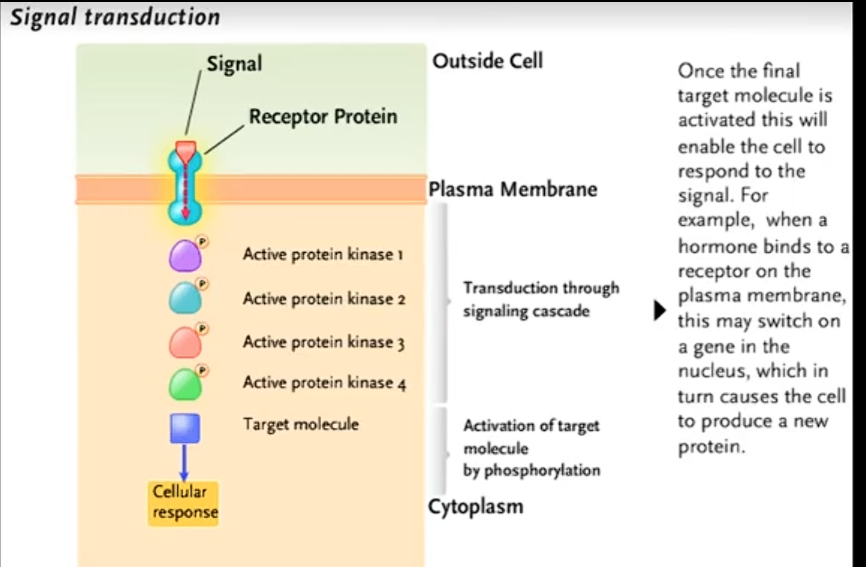
What’s signal transduction?
receptor binds with a signal molecule then transmits the signal to trigger a cellullar response.
(na protien due to a signL BINDING TO THE RECEPTOR AND CAUSING A CONFRONTATIONAL CHANGE(
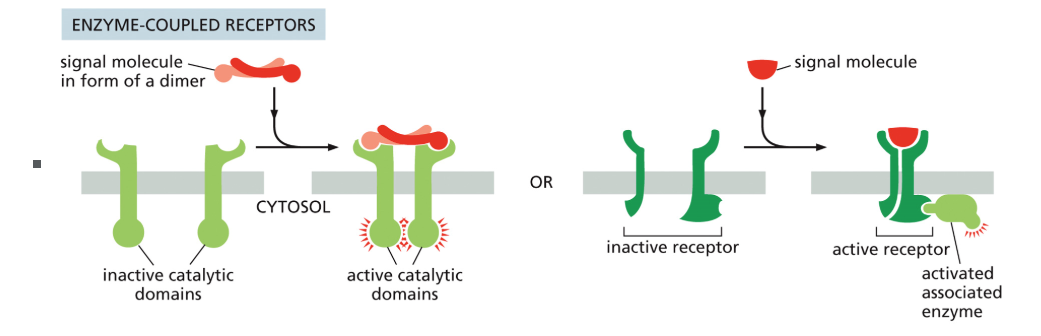
Enzyme coupled receptor
Cell-surface receptors that exist as inactive monomers; ligand binding causes dimerization and mutual activation, which then activates target proteins.
What are ligands?
Signaling molecules that bind to receptors (e.g., hormones, neurotransmitters).
What are intracellular receptors and what ligands bind them?
Receptors in the cytoplasm or nucleus; ligands must cross the plasma membrane, so they are small and/or hydrophobic (e.g., steroid hormones).
What are ion channel–coupled receptors and where are they found?
Cell-surface receptors that open or close an ion channel upon ligand binding; found mainly in neurons and muscle cells.
What are G protein–coupled receptors (GPCRs)?
Large family of membrane receptors that signal via heterotrimeric G proteins; they activate enzymes or ion channels through second messengers.
How do receptor proteins usually transmit signals inside the cell?
Via second messengers, which regulate enzymes or gene expression.
How does signal amplification work in GPCR pathways? Give an overview of the whole process
One receptor → activates many G proteins → each G protein activates many adenylyl cyclases → each makes many cAMP molecules → each cAMP activates PKA, leading to amplification.
What is direct cell–cell signaling?
Cells communicate through gap junctions (animals) or plasmodesmata (plants), or via membrane-bound ligands binding to receptors on an adjacent cell.
What is synaptic (neuronal) signaling?
A neuron transmits an electrical impulse along its axon, which triggers release of neurotransmitters at the synapse to signal the next cell.
What is paracrine signaling?
Local signaling: signaling molecules diffuse through the extracellular fluid to reach nearby target cells.
What is endocrine signaling?
Long-distance signaling: hormones are released into the bloodstream and travel via the circulatory system to distant target cells.
What receptor does epinephrine bind to in the fight-or-flight response?
A G protein–coupled receptor (GPCR), also called a 7-transmembrane helix receptor (7TM).
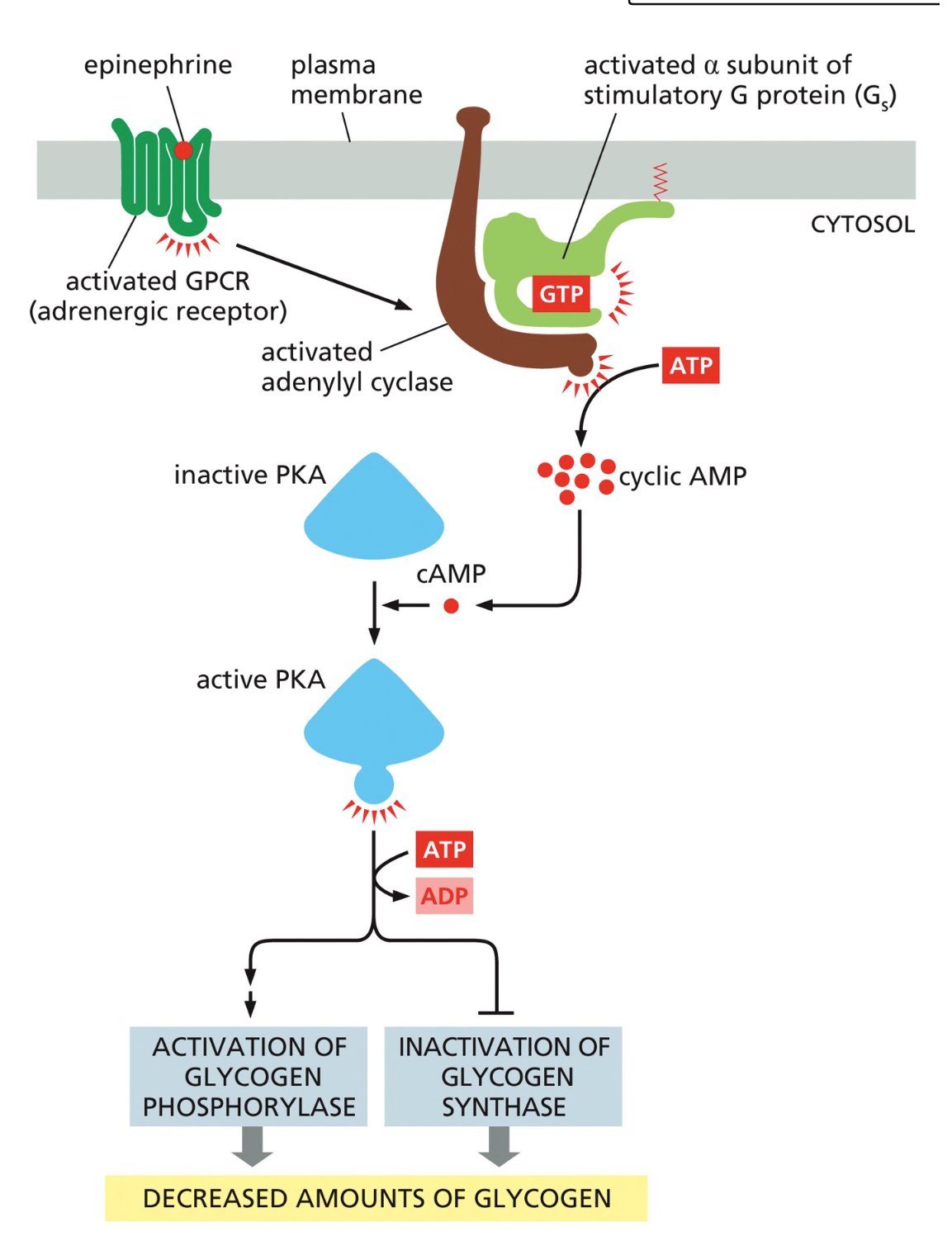
What happens to the G protein when the GPCR is activated?
The G protein binds the activated receptor.
GDP on the α-subunit is replaced with GTP, activating the G protein.
The α-subunit dissociates from βγ and continues the signal.
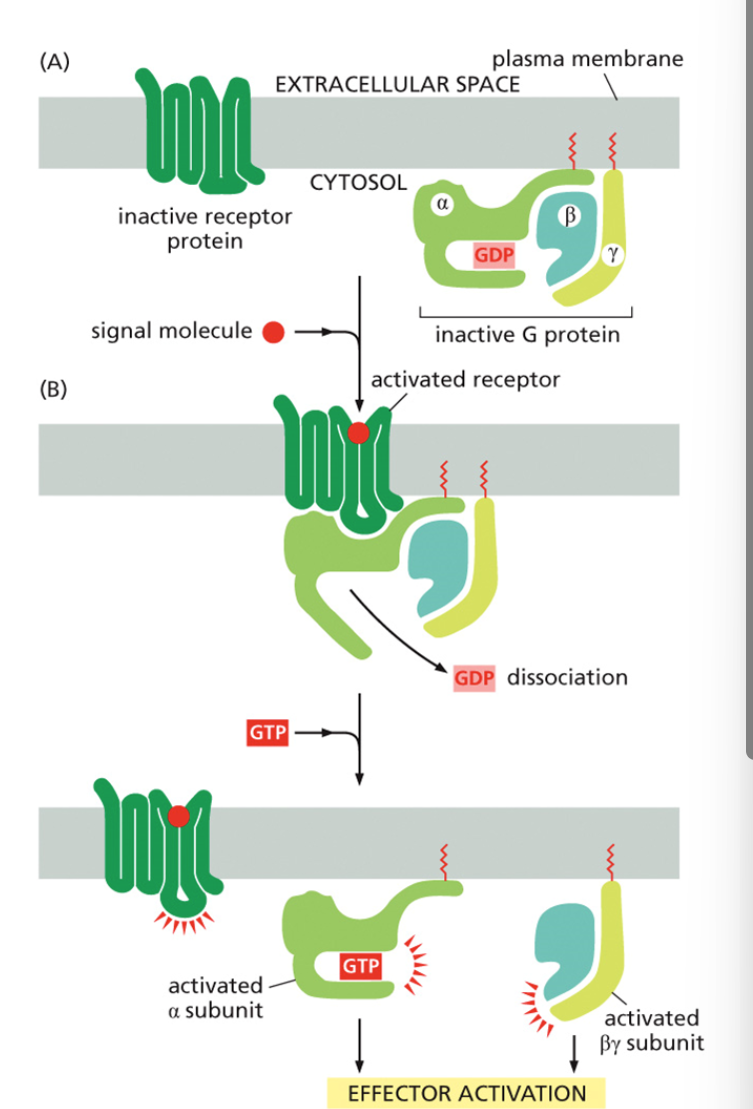
What enzyme does the activated α-subunit stimulate in the epinephrine pathway?
Adenylyl cyclase, which converts ATP into cyclic AMP (cAMP).
Why is cAMP called a “second messenger”?
Because it relays the signal inside the cell from the first messenger (epinephrine) and amplifies the response.
What does cAMP activate?
Protein kinase A (PKA) → splits into active subunits that phosphorylate target proteins.
What enzyme does PKA activate i?
Phosphorylase kinase, which phosphorylates and activates glycogen phosphorylase.
What does glycogen phosphorylase do?
Breaks down glycogen into glucose-1-phosphate → converted to free glucose → released into the bloodstream.
How is the G protein inactivated?
The α-subunit hydrolyzes GTP to GDP, reassociates with βγ, and stops signaling.
Overview of order of amplification in flight or fightsituation
Amplification: One epinephrine → many G proteins → many adenylyl cyclases → thousands of cAMP → lots of PKA activity → massive glucose release.
Temporal control: G proteins self-inactivate by hydrolyzing GTP.
Epinephrine → GPCR → G protein → Adenylyl cyclase → cAMP → PKA → Glycogen breakdown
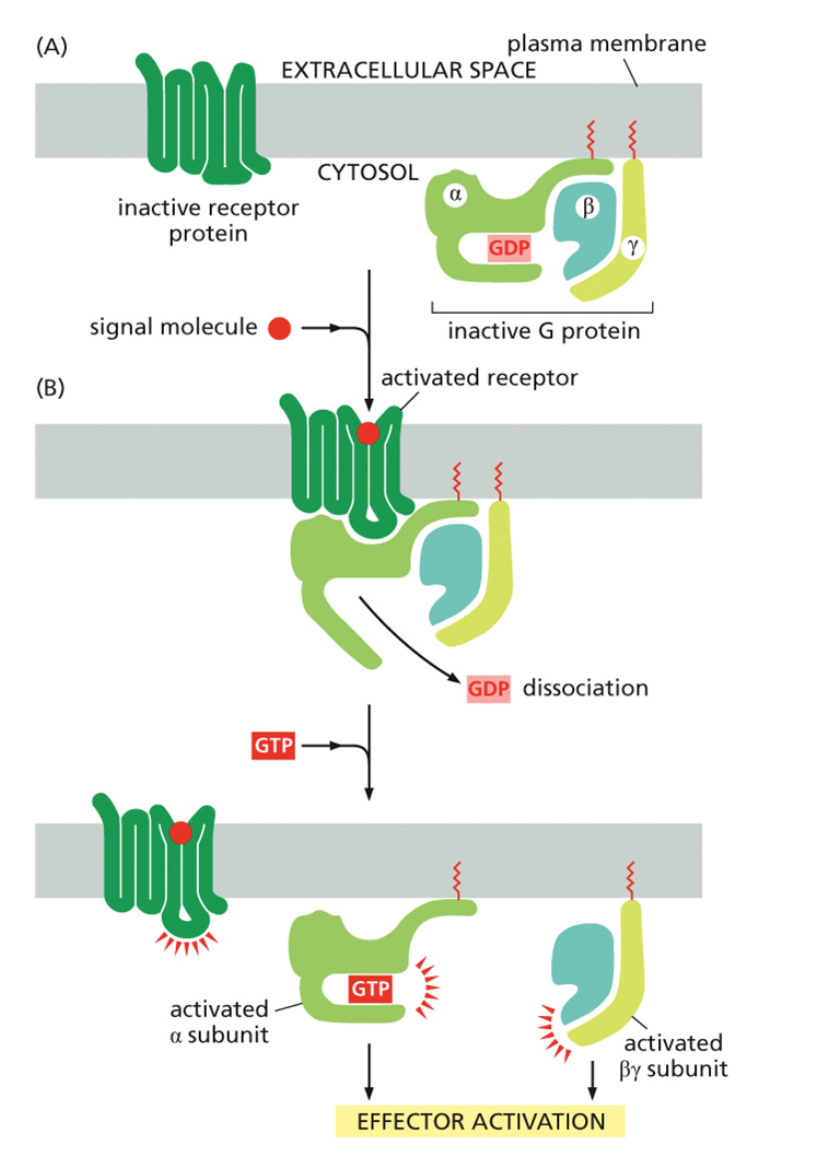
describe the activation and inaction of a G protien
A signaling molecule binds a G protein-coupled receptor.
the GDP-molecule bound to the α-subunit is released and replaced by GTP.
The a subunit is released by the ß- and y-subunits and moves along the plasma membrane.
The activated α-subunit binds to the target.
GTP is hydrolyzed to GDP.
The inactive α-subunit is released by the target and rebinds the β/γ-complex producing an inactive G-protein.
What is the difference between a first and a second messenger?
First messenger = the extracellular signaling molecule (ligand) that binds the receptor.
Second messenger = small, intracellular molecules/ions that spread the signal inside the cell (e.g., cAMP, Ca²⁺, IP₃, DAG, cGMP).
Why are second messengers effective?
They are small and water-soluble, so they diffuse quickly through the cytoplasm and amplify the signal.
What is the role of kinases in signal transduction?
Kinases phosphorylate proteins (add phosphate groups), usually activating them.
What is the role of phosphatases?
Phosphatases remove phosphate groups (dephosphorylation), usually deactivating proteins.
What happens if kinase is inhibited?
Normal pathway:
Receptor → 2nd messenger (e.g., cAMP) → Kinase 1 → Kinase 2 → Kinase 3 → Response (e.g., glycogen breakdown).
If a kinase is inhibited:
Example: Kinase 2 is blocked.
Effect: Signal stops at that point → downstream kinases (3, response) are not activated.
Result: No cellular response (no glycogen breakdown).
What happens if phosphatase is inhibited?
Normal pathway:
Receptor → 2nd messenger (e.g., cAMP) → Kinase 1 → Kinase 2 → Kinase 3 → Response (e.g., glycogen breakdown).
Example: Phosphatase that turns off Kinase 2 is blocked.
Effect: Kinase 2 stays active longer.
Result: Over-activation of downstream signaling (excess glycogen breakdown)
What is I-cell disease caused by?
A defect in the enzyme that adds mannose-6-phosphate (M6P) to lysosomal proteins in the Golgi.
Why is mannose-6-phosphate important?
It is the sorting signal that directs enzymes from the Golgi to lysosomes.
What happens to lysosomal proteins in I-cell disease?
They are not tagged with M6P → mis-sorted → secreted outside the cell instead of delivered to lysosomes.
What is a diagnostic marker for I-cell disease?
High levels of lysosomal enzymes in blood/urine, because they were secreted instead of sent to lysosomes.
What are the three pathways that deliver material to lysosomes?
Endocytosis (uptake from outside the cell).
Phagocytosis (large particles like bacteria).
Autophagy (self-eating: damaged organelles/cytoplasm delivered to lysosomes).
What type of receptor is used to detect odorants and flavors?
G protein–coupled receptors (GPCRs).
How do odorant GPCRs work?
Binding of odorant → GPCR activates a G protein → G protein activates adenylyl cyclase → ↑ cAMP → opens ion channels → neuron depolarizes → signal sent to brain.
How is the system able to detect so many smells and flavors?
Different odorants activate different combinations of GPCRs, producing unique patterns the brain interprets.