Architectures of signalling pathways LECTURE 2
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Major components of signalling processing pathways
Chemical signal→ pheromones, hormones, local hormones etc
Receptor→ G protein, tyrosine kinase-linked ion channel linked
Transducer→ G protein, non-receptor tyrosine kinases
Amplifier→ adenylate cyclase, phospholipase C
2nd Messengers→ cAMP,iP3, Ca2+, DAG
Effectors→ Protein kinases, Ca2+ binding proteins, troponin C
Response elements→Enzymes, sec vesicles, contractile proteins, ion channels, transcription elements
Response→ Metabolism, secretion, contraction, excitbility, gene transciption, cell growth
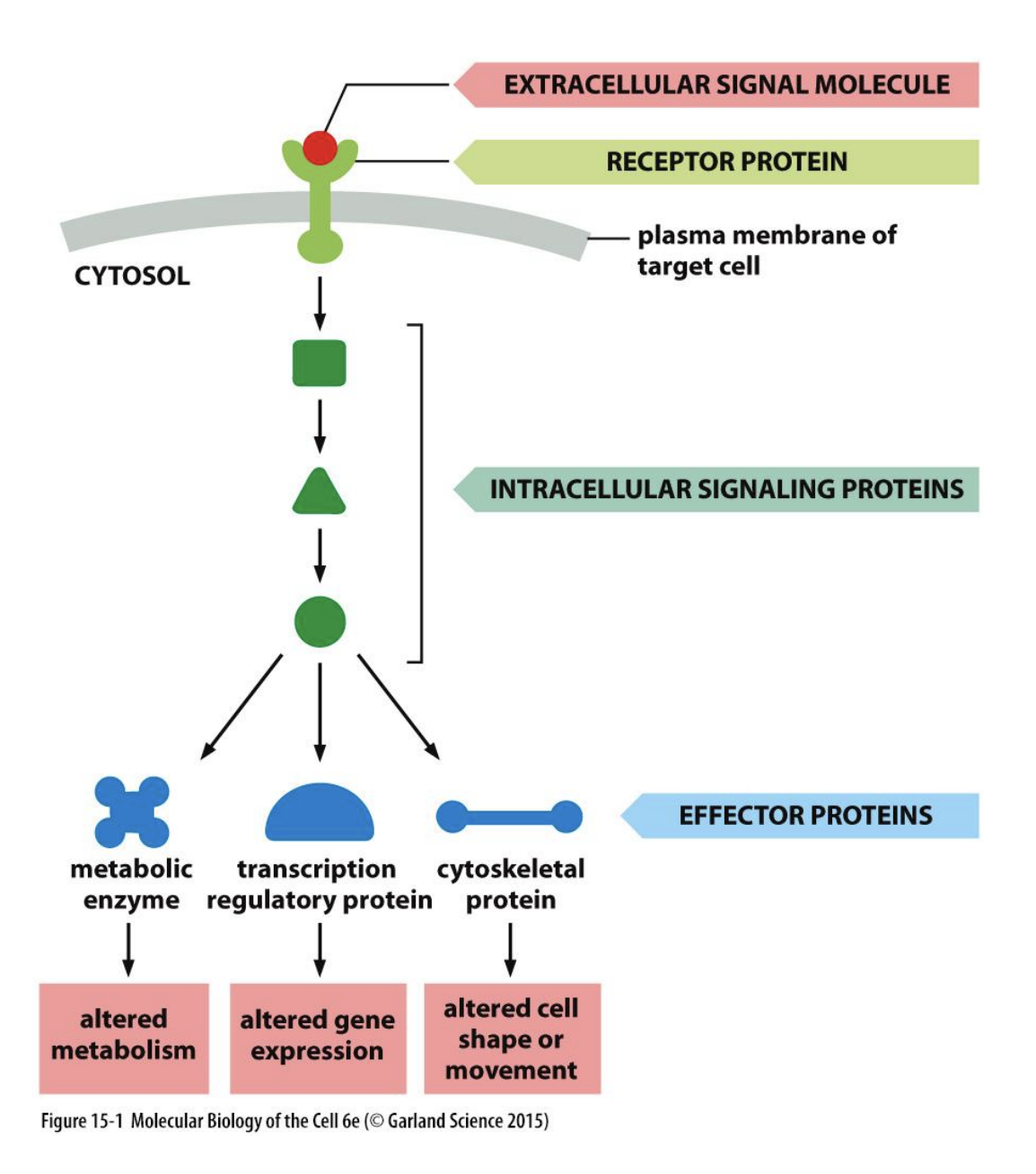
Singalling processing pathways have 5 principles
Information transfer
Amplification
Down regulation
Heterogeneity and concept of diversity
Dependence on functional properties of membranes
Information transfer
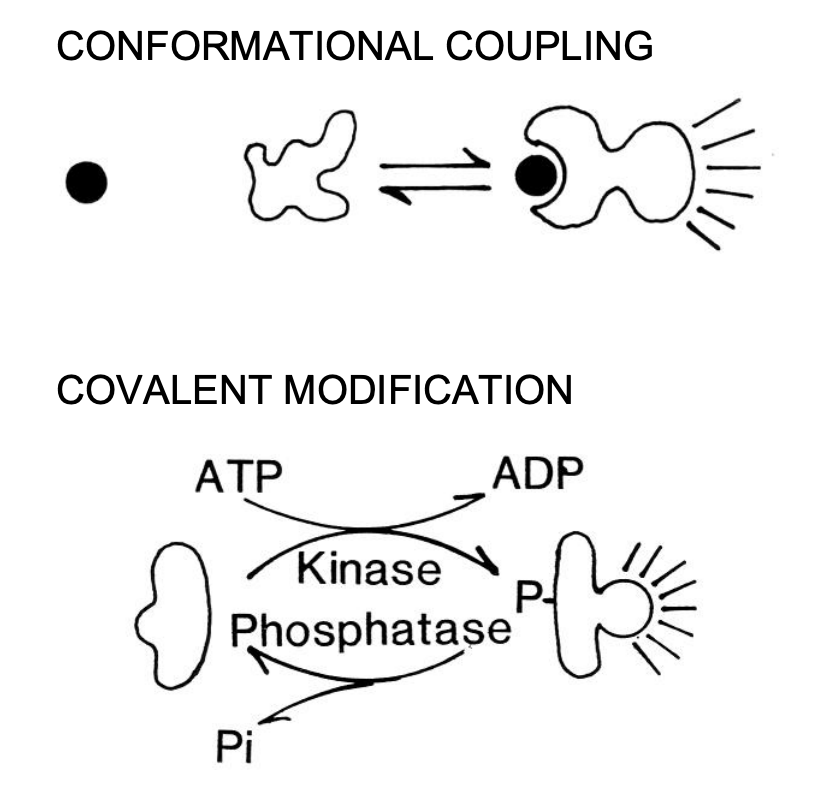
two basic mechanisms:
Conformational coupling→ highly reversible (conformational change)
Chemical signal to receptor
Receptor→ G protein (con change)
G protein→ amplifier (con change)
2nd messenger to protein kinase (conf change)
Covalent modification→highly reversible
protein kinase → proteine kinase
protein kinase→ response element
Amplification
Hormones are present at low concentrations
→have ability to amplify the initial signal
as much as 10^6
Can be at a number of steps in the signalling cascade
One signal molecule→ multiple downstream proteins
Down regulation (reset)
Once the chemical signal is removed:
need to return to unstimulated state
reversal of each step of signal transduction cascade
e.g (with covalent modification seen above:
phosphatases reverse the effect of kinase activity
target protein in dephosphoylated state when no signal
If no down reg:
→ no point in signalling
→ cannot respond to new stimulus
Heterogeneity and the concept of diversity (catering specific pathways)
many of the components come in multiple forms
different signalling pathways can interact with each other
→ OVERALL: can mix and match and interact with each other to construct precise signalling pathways that the cell needs
Dependence on functional properties of membranes
Most pathways are initially on the cell membrane
→ Membrane properties are important for this
fluidity
laterla mobility
asymmetry of membrane proteins
NOT IN STEROID HORMONES ACTION
do not initiate signal at the cell membrane
Examples of signalling pathways
Steroid hormones→receptor is nuclear protein
Ion channel-linked receptors
Pathways that involve secondary messengers:
cyclic AMP signalling pathway
Phosphoinositide signalling pathway
Tyrosine kinase-linked receptors
Steroid hormone action e.g testosterone
Hydrophobic→ diffuse across the cytoplasmic membrane of target cell
bind reversibly to specific steroid hormone receptors in the cytosol
Causes displacement of an inhibitory protein from the receptor
Exposes the DNA-binding domain
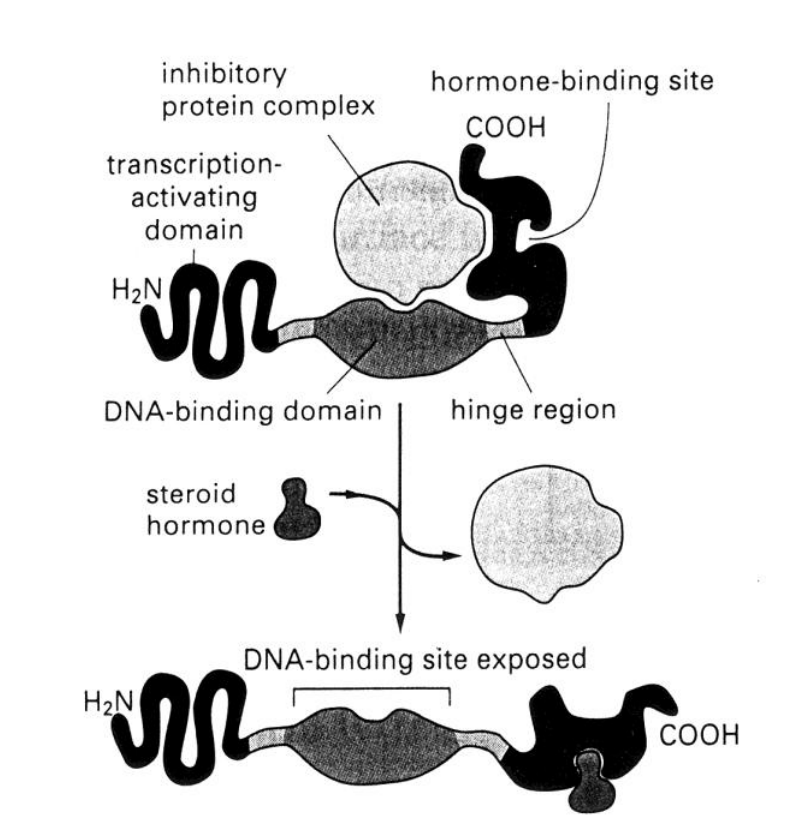
Steroid hormone receptor:
DNA binding site→ Inactive when inhib protein complex bound (and stuck in cytosol)
Steroid/ligand site→ once bound→ inhib protein complex gone (DNA binding site exposed)→ can now move to nucleus
Transciption activating domain
hinge region
Receptor example→ forms a dimer
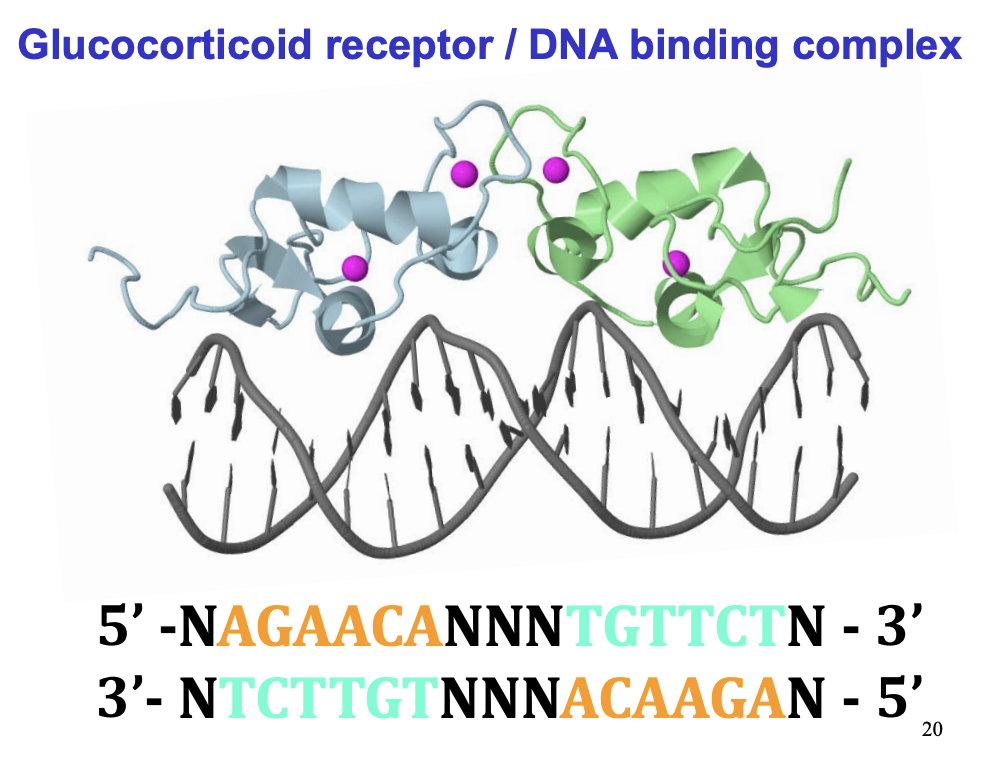
Steroid hormone action→ what happens to the hormone-receptor complex
enters the nucleus
directly regulates the transcription of specific primary response genes
encodes transciption factors
These TFs signals to activate secondary response genes
→ Amplifies the response and invokes real response
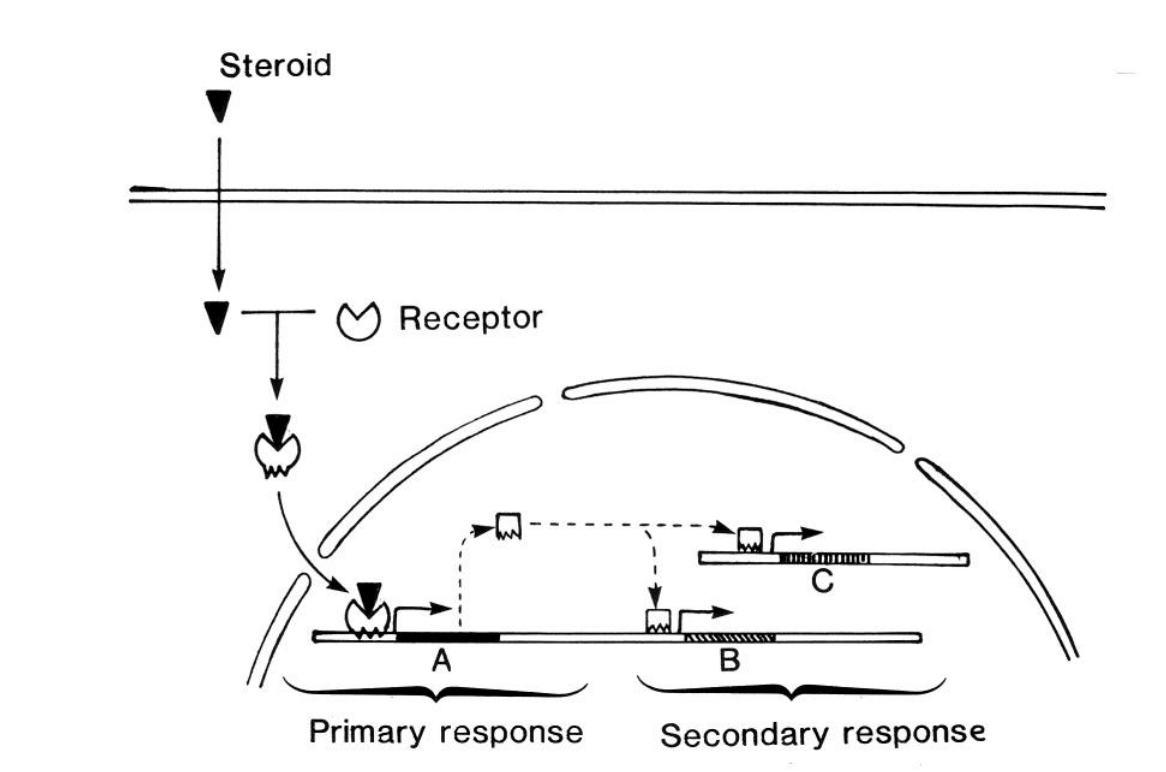
Because transcription is involved…
This is a slow process
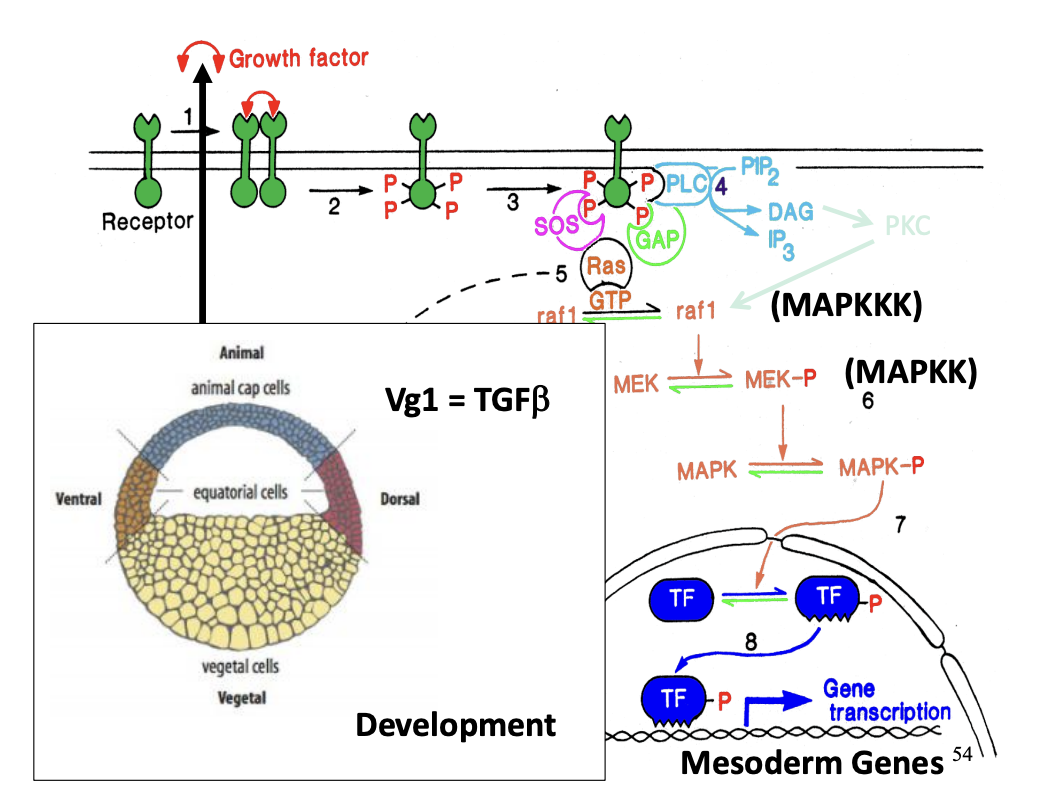
→ Used in development!\
e.g used for athlete cheating
How is it down regulated?
Lower steroids
favours dissociation from receptor
No receptor in nucleus
No more primary or secondary transciption
Ion channel-linked receptors: What used by
neurotransmitters
→ e.g acetylcholine
RAPID
Ion channel-linked receptors: E.g with ACh
ACh binds to sites on outside of the target cell
Induce conformational change
Allows Na+ entry
Membrane depolarisation
muscle contraction
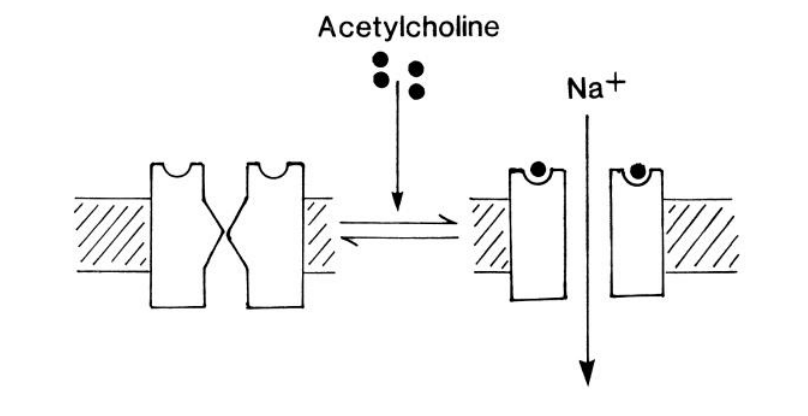
How does this depolarisation happen?
Both voltage gradient and concentration of Na+ act in the same direction
→ large net influx of Na+
up to 30 000 ions per channel per ms
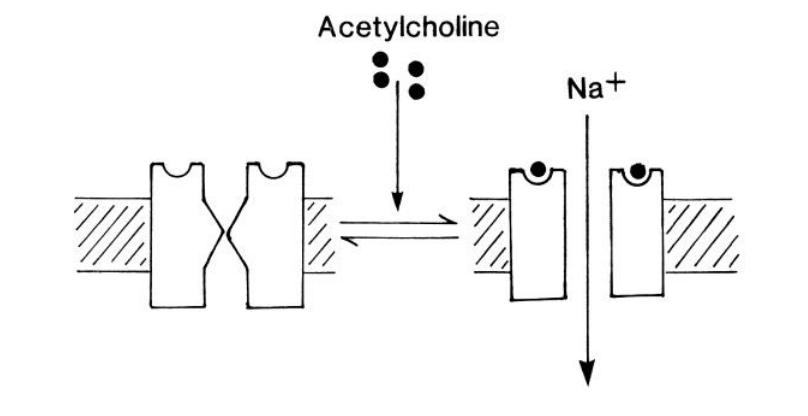
How is pathway downregulated
ACh is hydrolyses by acteylcholineesterase
found in neuromuscular junction
Na+ must be pumped out by Na+-ATPases
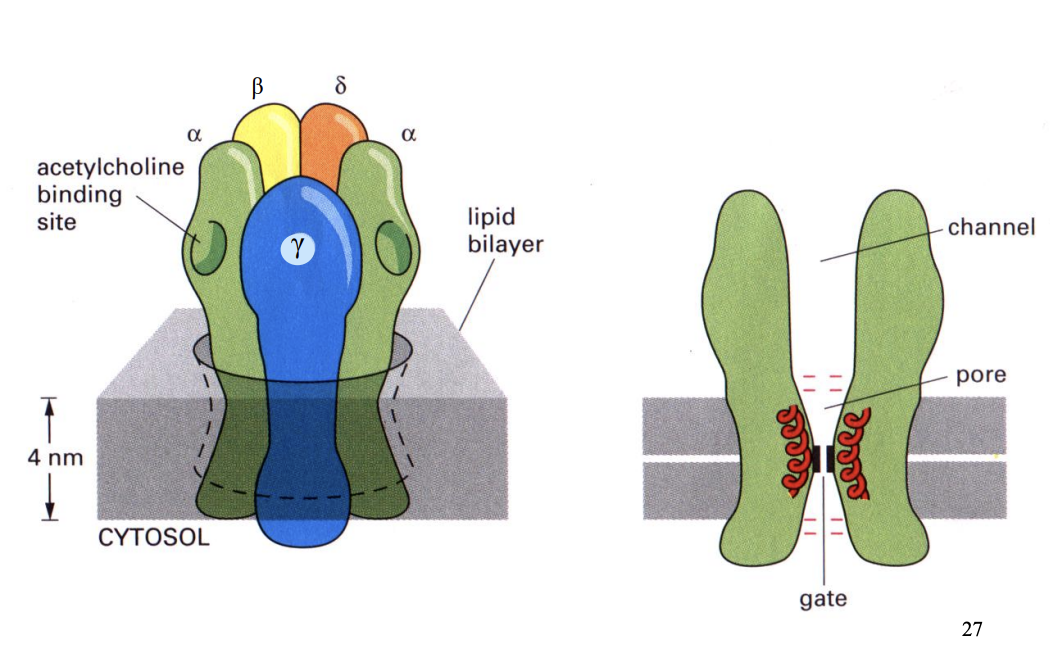
The nicotinic ACh receptor
→ When no ACh→ 10% chance open
→ When 2 ACh→ 90% chance open
What is Curare?
Antagonist of ACh
block the ACh receptor in the muscle cells
Antagonist
( blocks and no activation )
What is nicotine
Stimulates the receptor
in the absence of ACh
→ agonist
( binds and forms some sort of response )
Novichok
Acetylcholineasterase inhibitor
→ No down regulation
continuous stimulation
Why are secondary messengers needed?
The receptor not always directly involved in doing the response
(As seen above)
Help relay the signal in the cytosol
Examples of secondary messengers
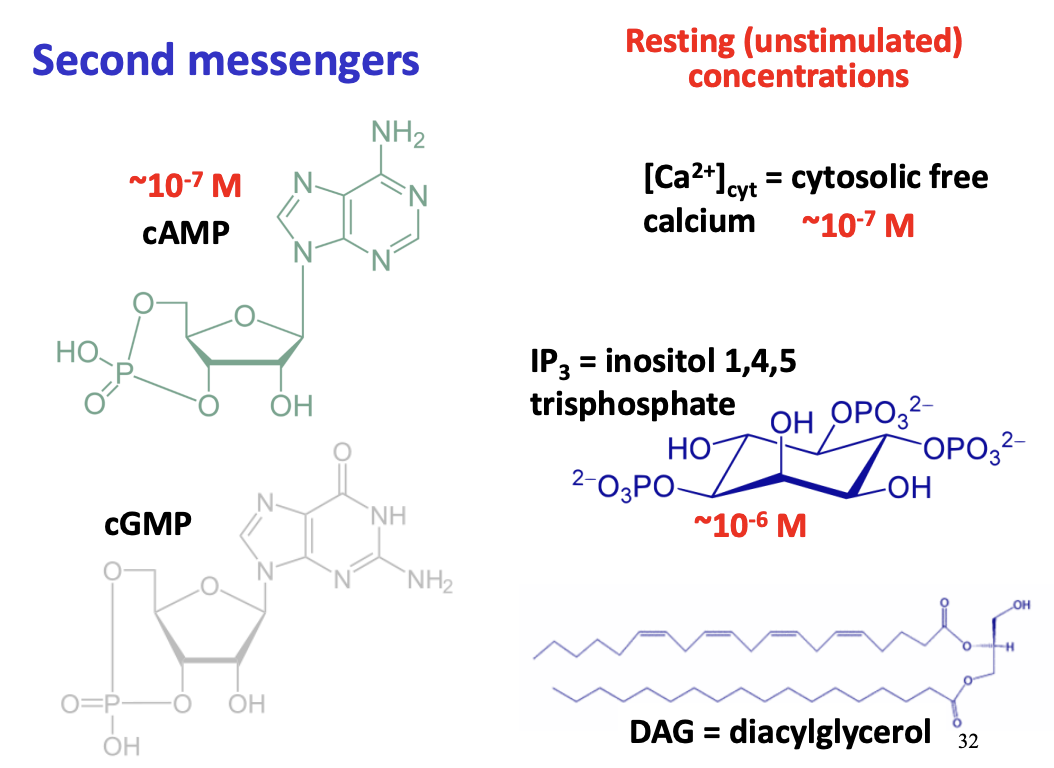
What is Cyclic AMP
synthesised from ATP by adenylate cylase
secondary messenger
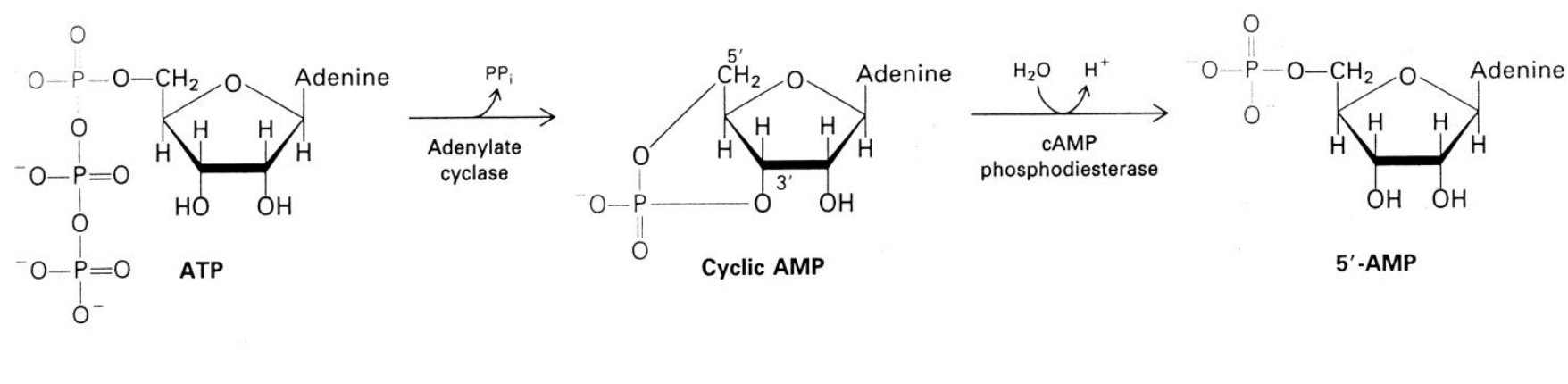
In mammals, the adenylate enzyme is embedded in the plasma membrane, what does this mean the pathway requires?
a soluble messenger is required
for it to communicate with cytosolic target enzymes
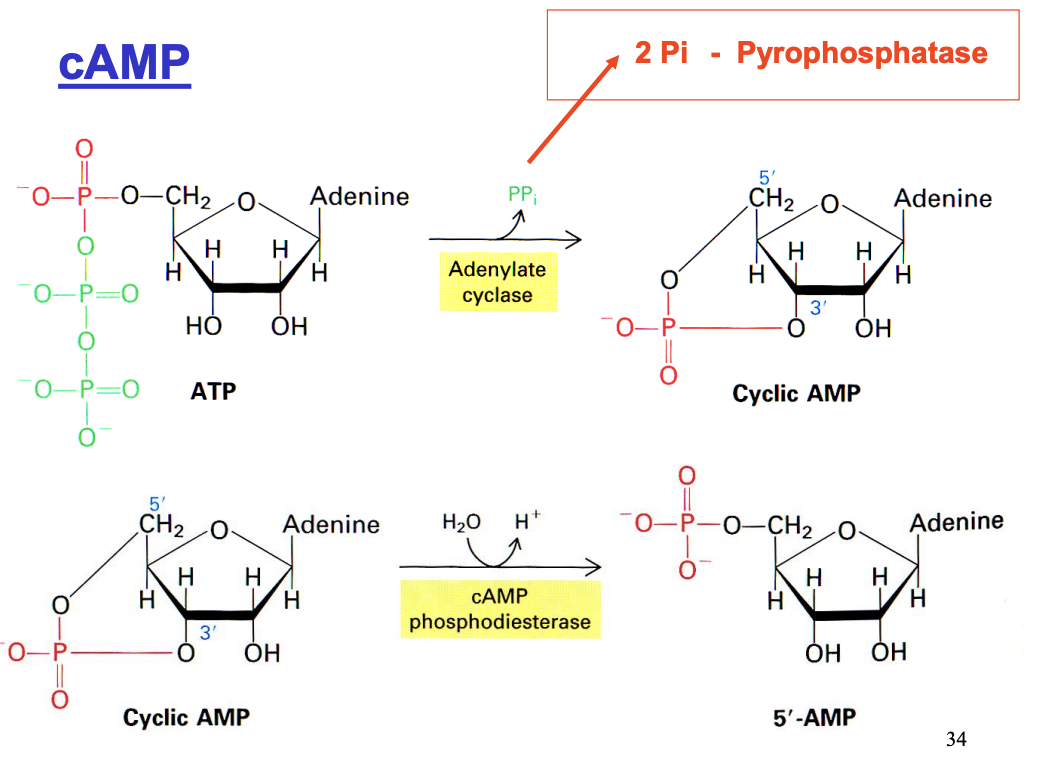
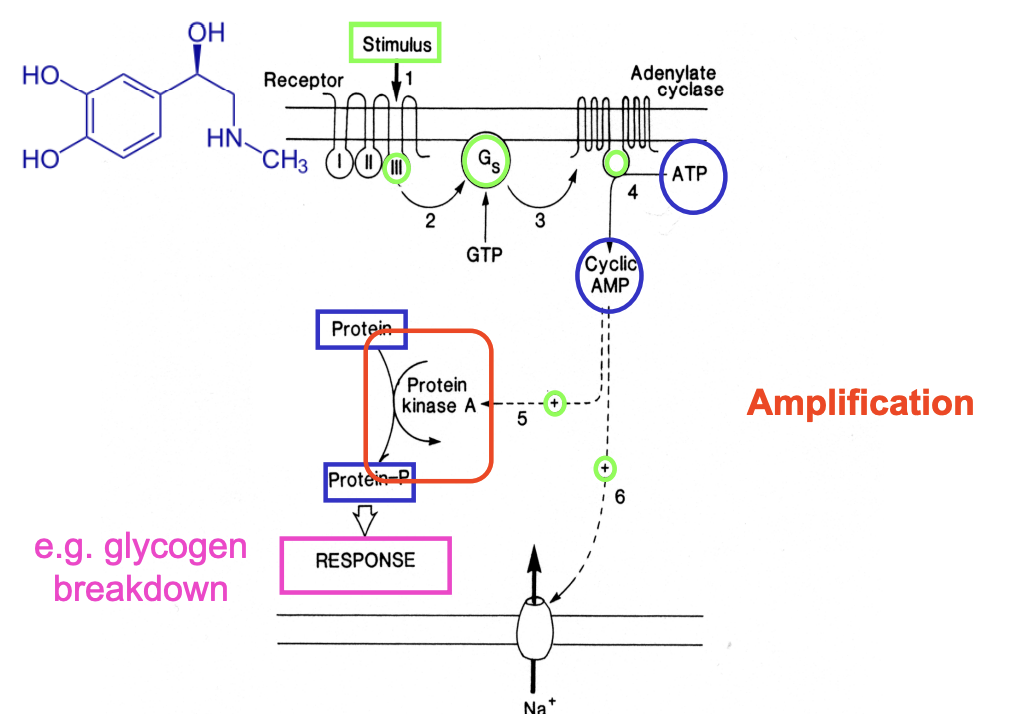
cAMP signalling pathway steps (Gs is used)→ e.g with adrenaline
chemical signal binds to membrane receptor with 7 transmembrane domains
induces a conformational change
Cytoplasmic domain III of the receptor activates→ G protein (Gs)
Adenylate cyclase (membrane bound) synthesises cyclic AMP from ATP (Amplification)
cAMP acts through protein kinase→ induce response e.g glycogen breakdown(Amplification)
ALSO
cAMP opens Na+ channels in sense organs
Membrane depolarisation and information transfer
What are these G-proteins?
Common transducer
anchored to the plasma membrane
‘G’ due to ability to bind to GTP
G protein’s interactions with GTP/GDP
When bound to GDP:
inactive
When bound to GTP
active
Types of G proteins
Heterotrimeric
Gs and Gq
Monomeric GTPases
e.g Ras (used in tyrosine kinase pathway)
Why is it important to understand G proteins
Half of all known drug molecules
→ work by targeting G-protein pathways!
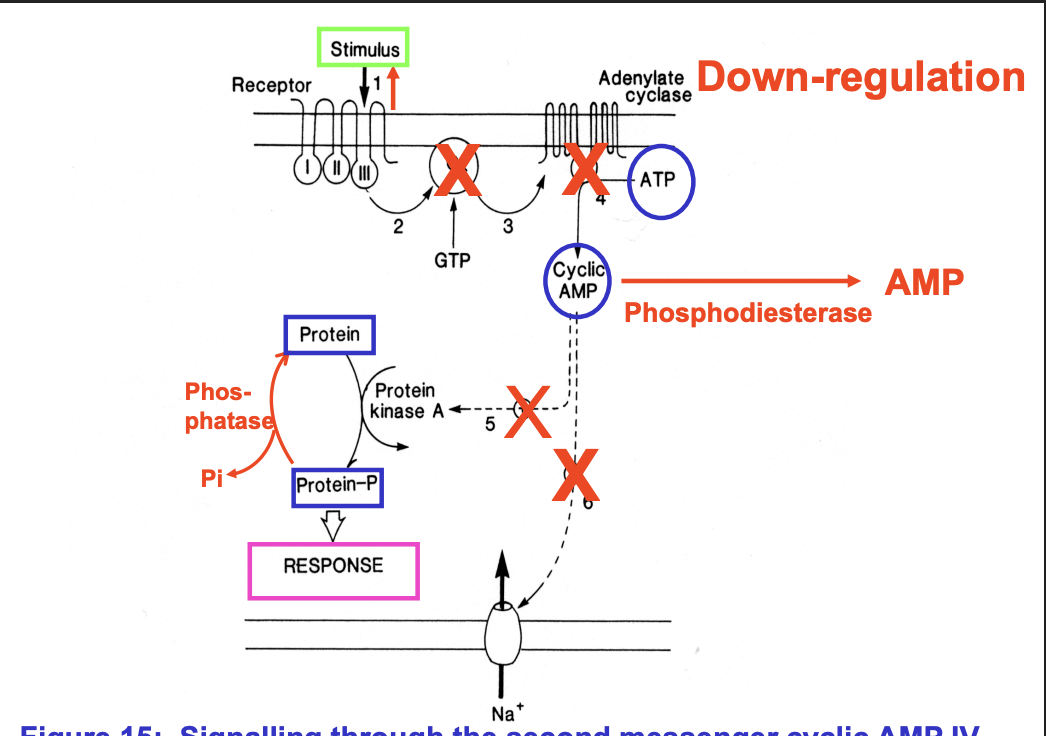
How down regulated?
No adrenaline, no conf III change, no G protein activation no cAMP made
Need to break down cAMP
phosphodiesterase→ AMP
Need to rephosphorylate the Protein-P
Use phosphatase
Na+ needs to be pumped back out
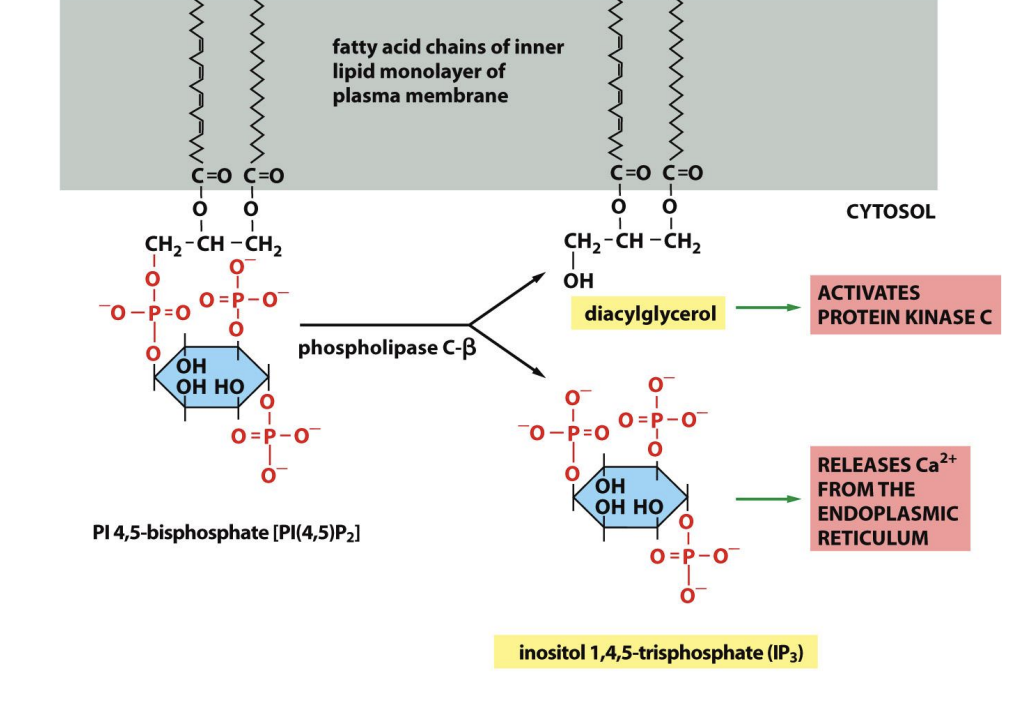
Phosphoionsitide pathway: What are the key enzymes
Phospholipase C beta:
Cleaves phospholipid in membrane from fatty acid tail
hydrolyses phosphatidyl inositl 4,5 bisphosphate (PIP2)
→ to form
inositol 1,4,5-trisphosphate (IP3) (free to move)
+ diacyl glycerol (DAG) (stays in membrane)
also
PLC:
plasma-membrane localised enzyme
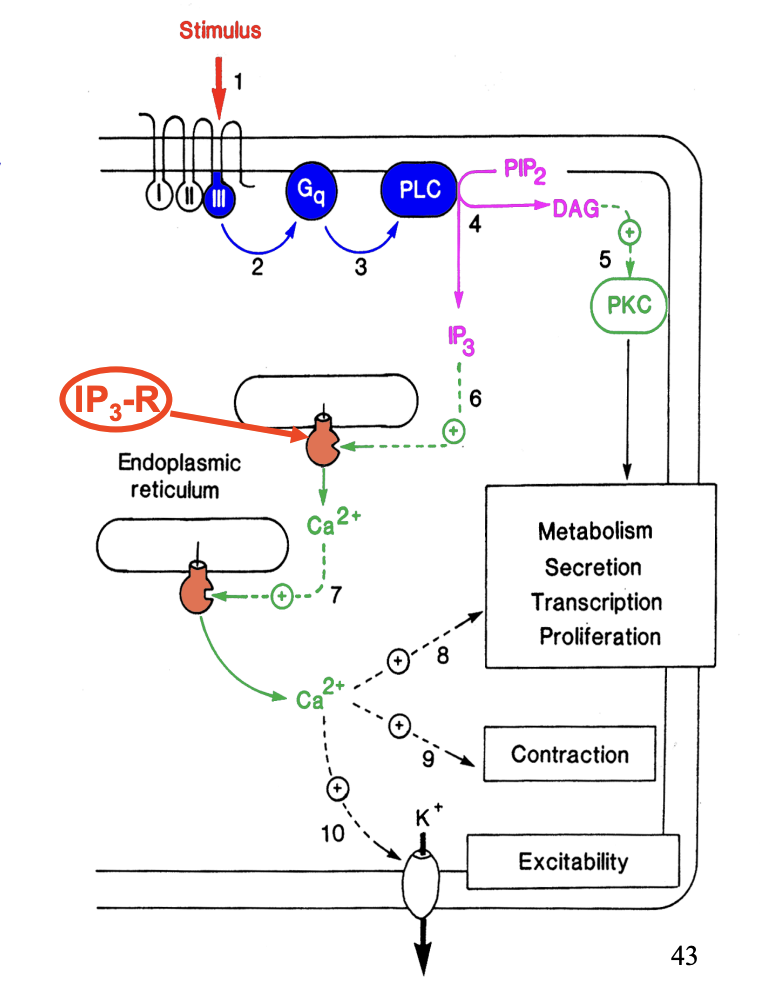
Phosphoinositide signalling pathway (Gq is used): Smooth muscles→ Uses ACh→ has multiple receptors!
Chemical signal binds to membrane receptor with 7 transmembrane domain
induces conformational change
Cytoplasmic domain III of the receptor activates a G protein (Gq)
G protein activates phospholipase C (PLC)
PLC hydrolyses PIP2 → DAG + IP3
acts as 2’ messengers:
DAG→ stimulate effector protein kinase C (PKC)
→ Response: metabolism, secretion, tasciption, proliferation
IP3→diffuses into cytosol→ acts via an IP3 receptor (IP3-R) on ion channels
Releases Ca2+ sotred in endoplasmic reticulum
Internal Ca2+ signal→ further Ca2+ release (amplification!)
Ca2+ can act through a protein kinase
or
specific binding protein (calmodulin or troponin C)
→ Induce contraction in muscle cells
or
→ act directly on ion channels→ influence excitability
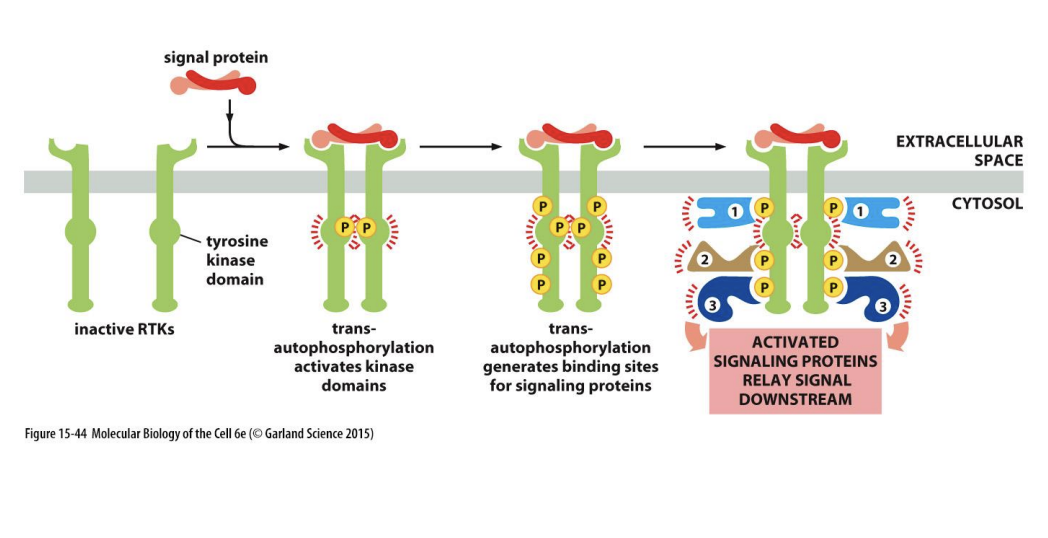
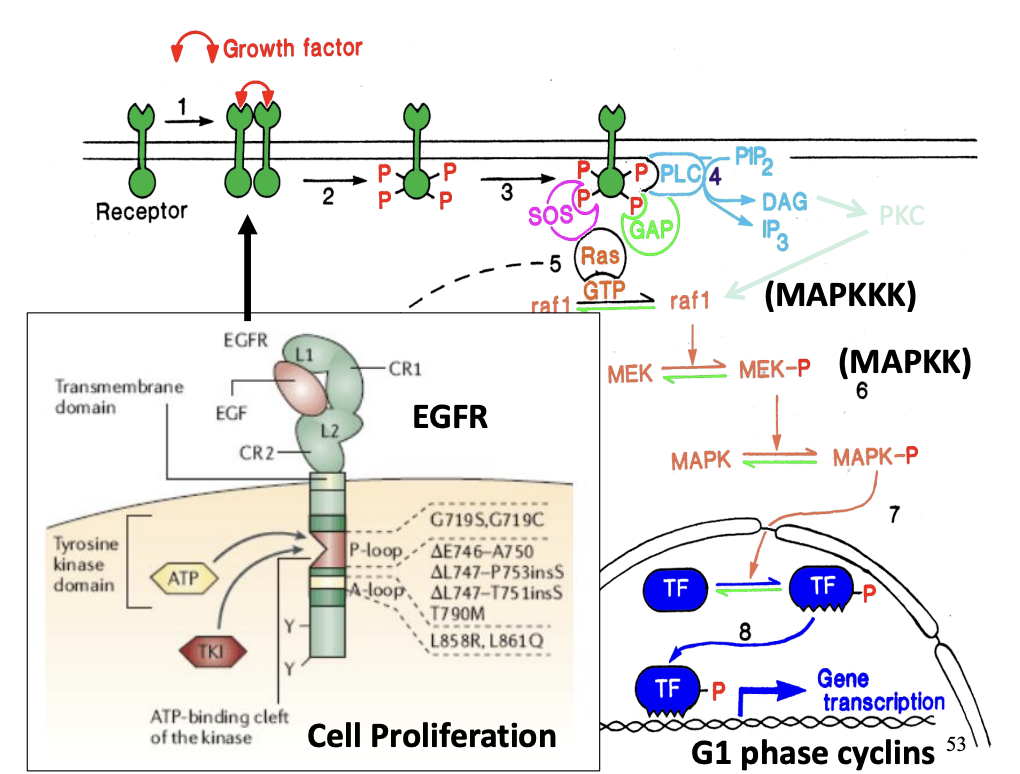
Tyrosine kinase linked receptors: role
important in cell proliferation
system transfers information from cell surface→ cell nucleus
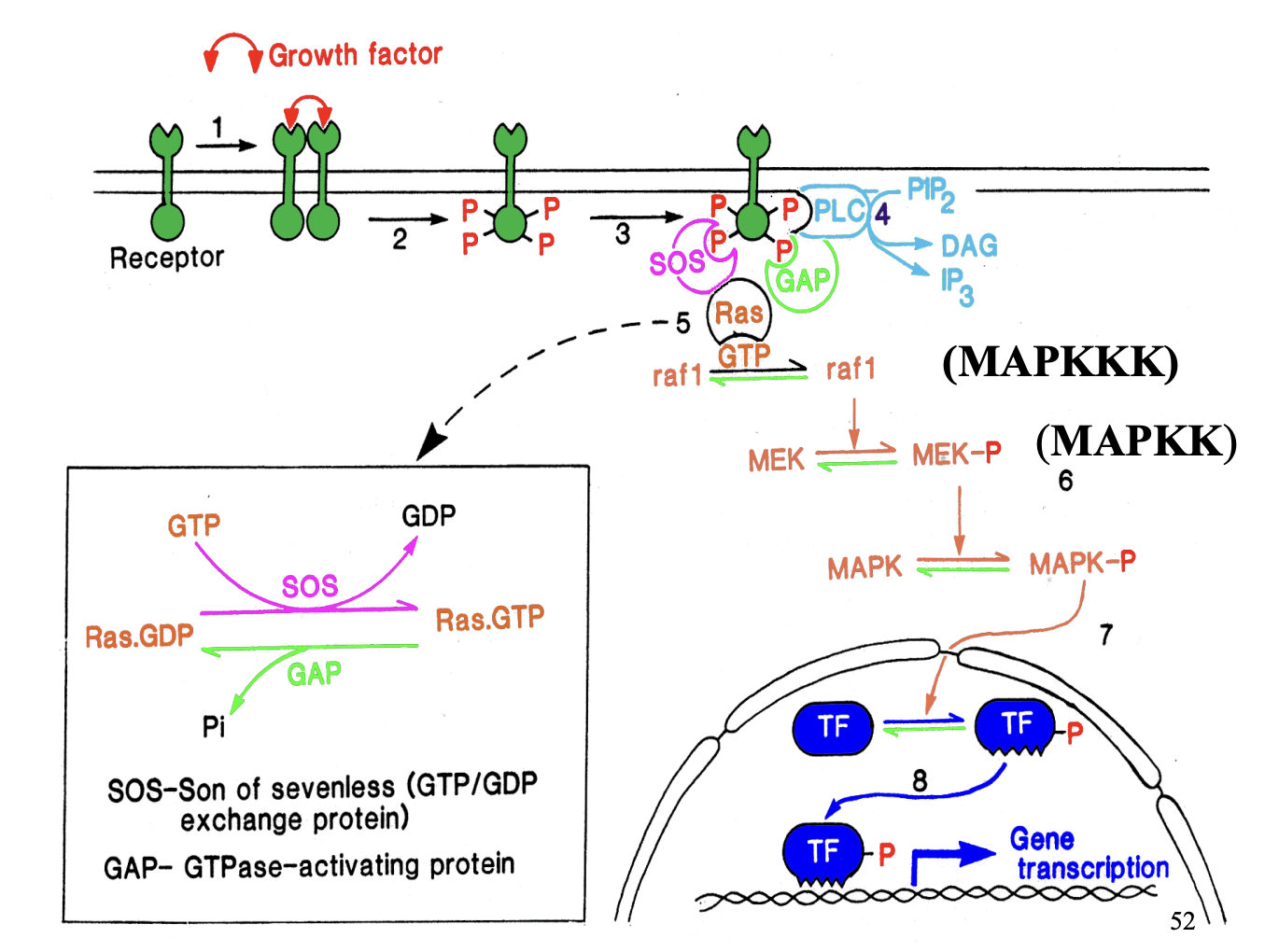
Protein phosphorylation cascade→ (no secondary messengers) →with growth factors
PDGF induces dimerisation of 2 PDGF receptors
2 tyrosine kinase domains phosphoylates each other on specific tyrosine residues
Phosphylated tyrosine residues act as docking sites
Bind different amplifiers to form multi-molecular complex → spans several signalling pathways
One amplifier: PLC→ forms DAG and IP3 (does the stuff from above…)
Phosphylated receptor also…
Attracts no. of proteins that regulate GTP-binding protein ras
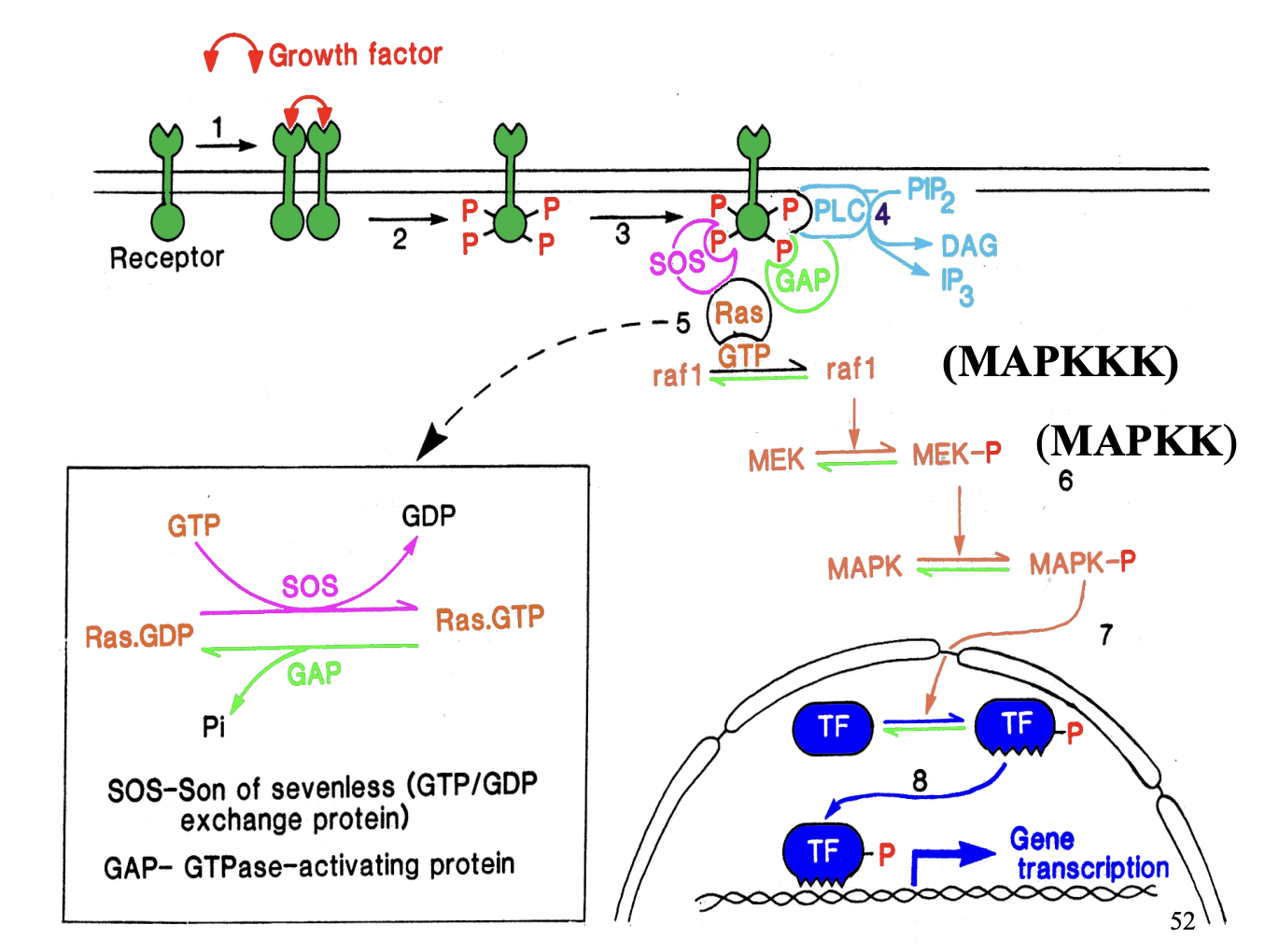
What does the trans-phosphylation of the tyrosine kinase do
Receptor has tyrosine kinase linked to it
upon signal→ transphosphoylates
→ now makes sites availabel for ptoeins to bind
Other proteins now bind:
PLC (from before)
GAP and SOS (adaptors for ras)
What is ras?
Monomeric G protein
e.g of molecular switch in a signalling pathway
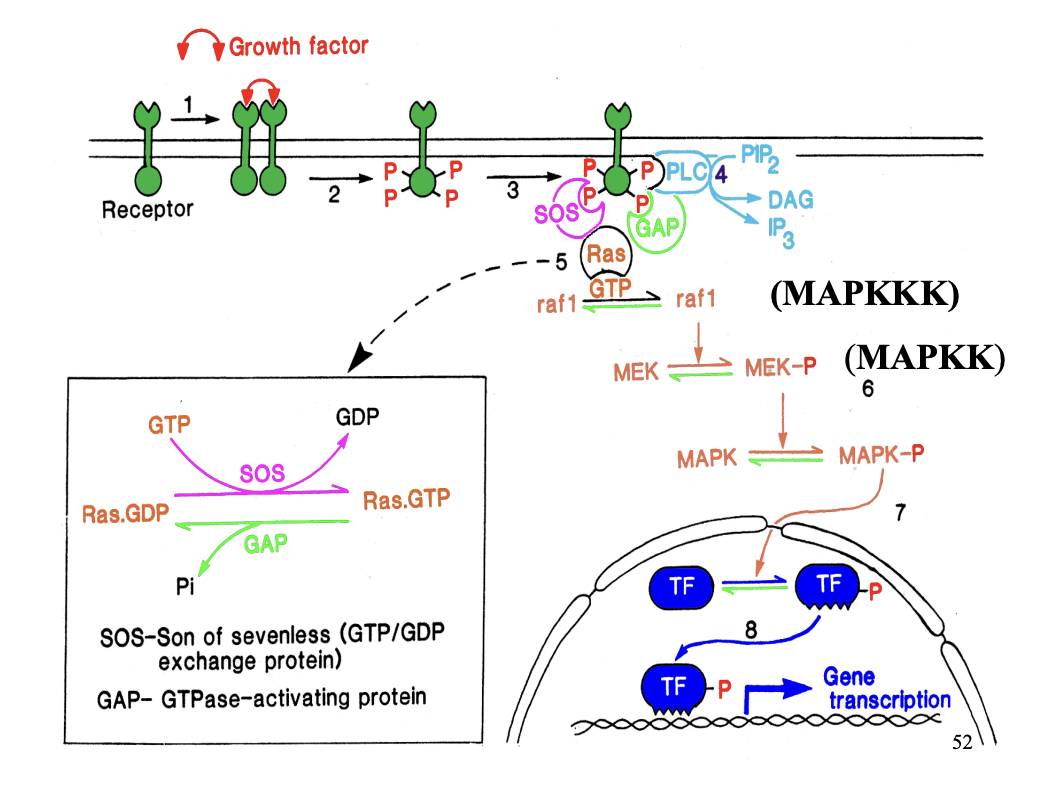
How are monomeric G proteins maintained in a default ‘off’ state?
GTPase-activating proteins (GAPs)
promote GTPase activity of monomeric G protein
How are monomeric G proteins switched on?
Guanine nucleotide exchange factors (GEFs)
promote GTP binding
→ therefore the ‘on’ state
( coz GTP is bound)
How is ras activated
GDP bound→ ras inactive
How activated: Son of sevenless (SOS) exchanges GDP for GTP
turned off with GAP
Ras GTPase activity:
has low GTPase activity
BUT
can inactivate itself with the help of GTPase activating protein GAP
Because of the GAP
Ras activation is shortlived
→ So needs to amplify the signal: phosphylation cascade
raf1 (kinases) MAPKKK
phosphylated MEK→ MEK-P (MAPKK)
phosphorylates MAPK→ MAPK-P (mitogen activator protein kinase)
→ Inolved in cell proliferation and development response
Transciptional changes!
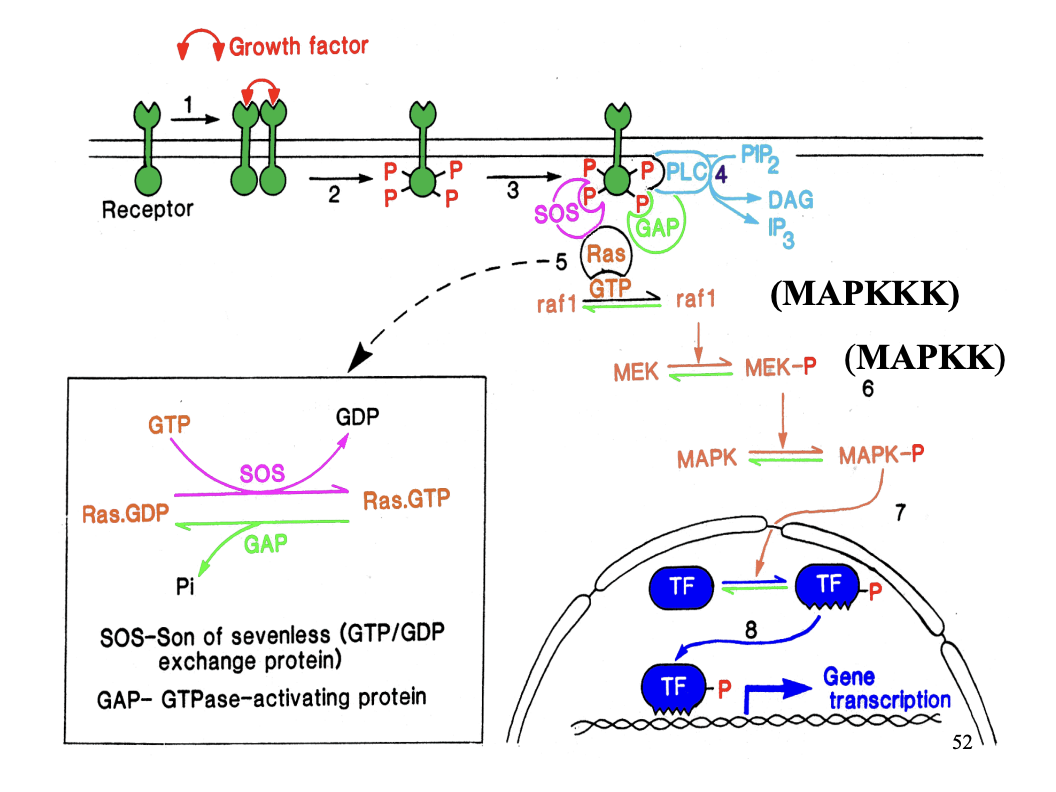
Speed of this pathway?-
Rapid activation of ras
sustain phosphylation
Causes slow (sustained) transciptional changes
Oncogenic ras?
has point mutation
causes loss of GTPase activity
→ permanently activated
(conformation change)→ if not happened→ massive downstream effects
30% of cancer due to ras mutation
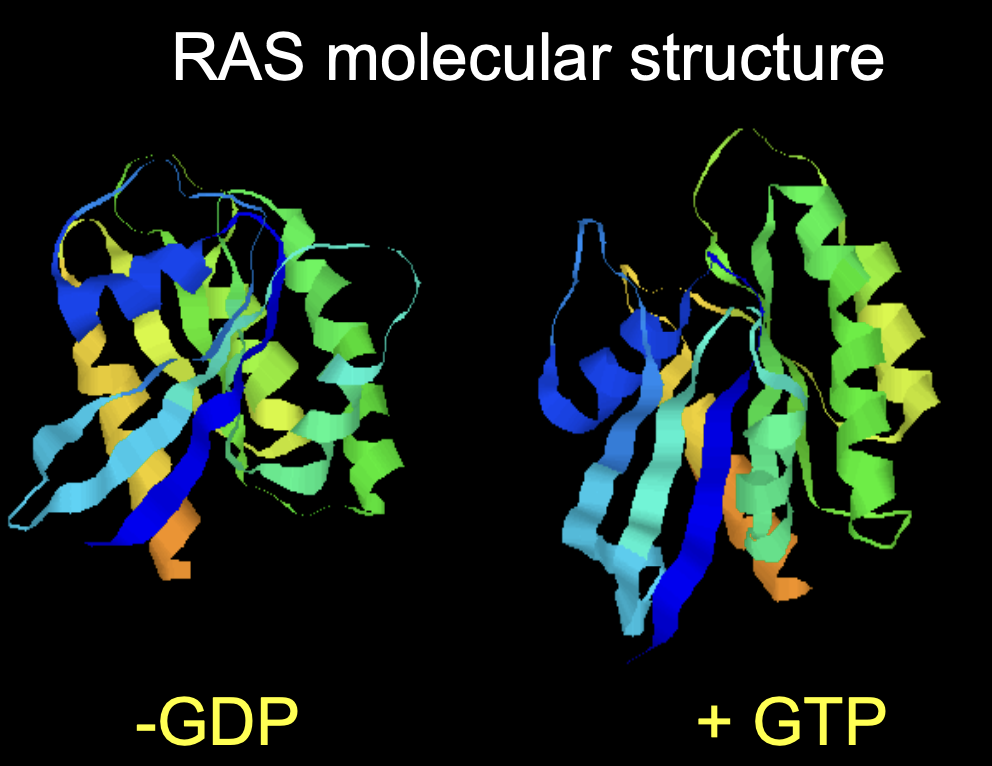
How does (now actiavated) ras contribute to this pathway?
Activated rasGTP initiates protein phosphylation cascade
→ causes phosphylation of mitogen actiavted protein kinase (MAP kinase)→ MAPK-P
MAPK-P translocates into the nucleus
phosphorylates transcription factors (TF)
Activated transcription factors (TF-P)→ initiate transcription of genes responsible for controlling cell proliferation
G phase cylins
Used for cell proliferation:
Used for development:
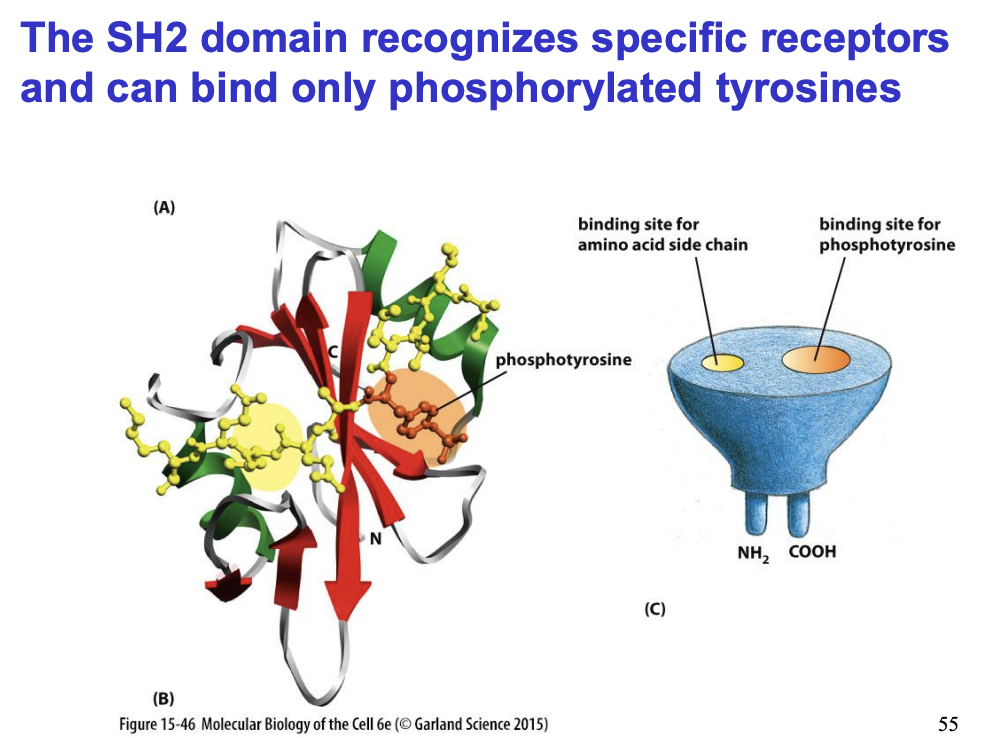
These protein adaptors have SH2 domains
Recognise specific receptors
only bind when tyrosine phosphylated
Overall different types of signalling organisation
intracellular receptors coupled with transcriptional control
plasma membane bound receptors which are ligans inotropic receptors
G-protein coupled receptors→ increase concentration of cytosolic second messengers
enzyme-linked receptors
Self assessment questions:
1. List five pathways used in intracellular signalling.
2. How are hormonal signals relayed to the nucleus?
3. How can ion channels act as receptors?
4. What is a second messenger?
Amolecular that relays a signal from a receptor to to target molecules, aplifying and propogating the signal to coordinate a response
5. Name two types of G protein.