TB2: Absorption and Partitioning
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Define Drug Absorption
The transport of a un-metabolised drug from the site of administration to the circulation system.
Describe the Mechanism that Drug Absorption follows
Passive diffusion (high → low concentration) without use of energy.
Carrier-mediated membrane transport; active and facilitated diffusion and other non-specific drug transporters.
Name the factors that influence Absorption
Lipid/water partition coefficient
Water solubility/dissolution
Molecular weight
Chemical structure
Particle size
Crystalline/polymorph/amorphous
Describe the importance of Aqueous and Non-Aqueous Solubility
Orally administered drugs depends heavily on:
Aqueous (hydrophilic) and non-aqueous (lipophilic) solubility,
Drugs must dissolve in water-based fluids before crossing lipid-rich cell membranes.
A drug should be hydrophobic enough to dissolve and lipophilic enough to penetrate membranes.
Define Partition Coefficient P
Ratio of drug in lipid vs. water (only non-ionized form).
Helps predict if a drug can pass through cell membranes.
Define Distribution Coefficient (D)
Ratio of drug in lipid vs. total drug (ionized + non-ionized) in water.
Shows how the drug behaves in real body conditions, where pH affects ionisation.
Note the relation between logP and site of absorption
Low Log P (<0) → Injectable
Medium (0-3) → Oral
High (3-4) → Transdermal
Very high (4-7) → Toxic build up in fatty tissues
High logP means they are likely to build up in the fatty tissues, causing tissue toxicity.
A logP of >2 is likely to penetrate the CNS, causing side-effects such as depression and strange dreams.
Describe the influence of LogP on Drug Formulation
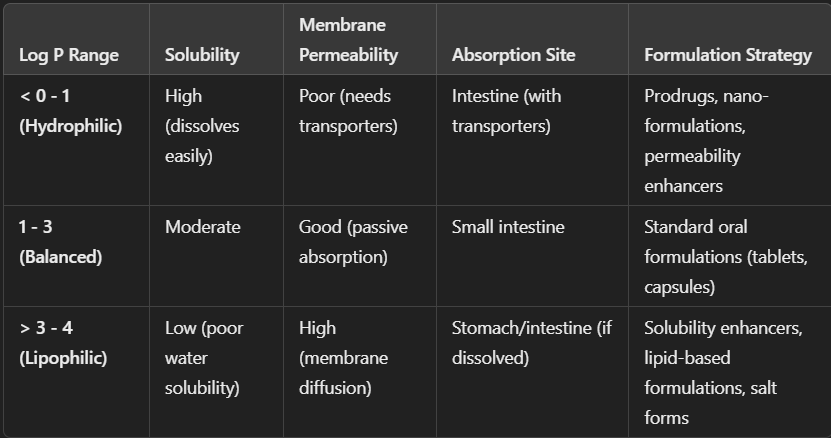
What are the defects of Salt Formulations in Lipid Solubility and Membrane Permeability?
Poor Lipid Solubility:
Salt forms of drugs are ionized, making them more water-soluble but less soluble in lipids (fats).
Since cell membranes are made of lipids, ionized drugs struggle to diffuse across them.
Reduce Passive Absorption:
Passive diffusion favors lipophilic, non-ionized drugs. Salt forms, being ionized, require transporters or specific pH conditions for absorption.
Absorption is slower or incomplete if the drug cannot efficiently cross cell membranes.
Why use Co-solvents and not Salt forms?
Salts are not soluble in lipids, therefore cannot cross biological membranes → not absorbed into circulation.
Co-solvents are added to increase solubility of poorly water-soluble drugs by modifying polarity.
What are the Essential Requirements of Co-solvents?
Miscibility with Water: Should blend well with water to ensure a homogenous solution.
Low Toxicity: Safe for human consumption or injection.
Biodegradability: Should be easily eliminated from the body without harmful accumulation.