BME 2010 Prelim 2 (Musculoskeletal system, Renal system, Endocrine system))
1/125
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
126 Terms
L13: Main components of musculoskeletal system
Joints
Cartilage
Bones
Muscle
L13: Components of skeletal system
Bone (osseous tissue)
hard, dense connective tissue that forms most of the adult skeleton
support structure of the body
Cartilage
In the areas of the skeleton where bones move (ex: ribcage and joints)
Semi-rigid form of connective tissue)
Provides flexibility and smooth surfaces for movement
Joints (a.k.a. articulations)
sites where 2 or more bones meet
L13: How bones does the human body have?
206 bones
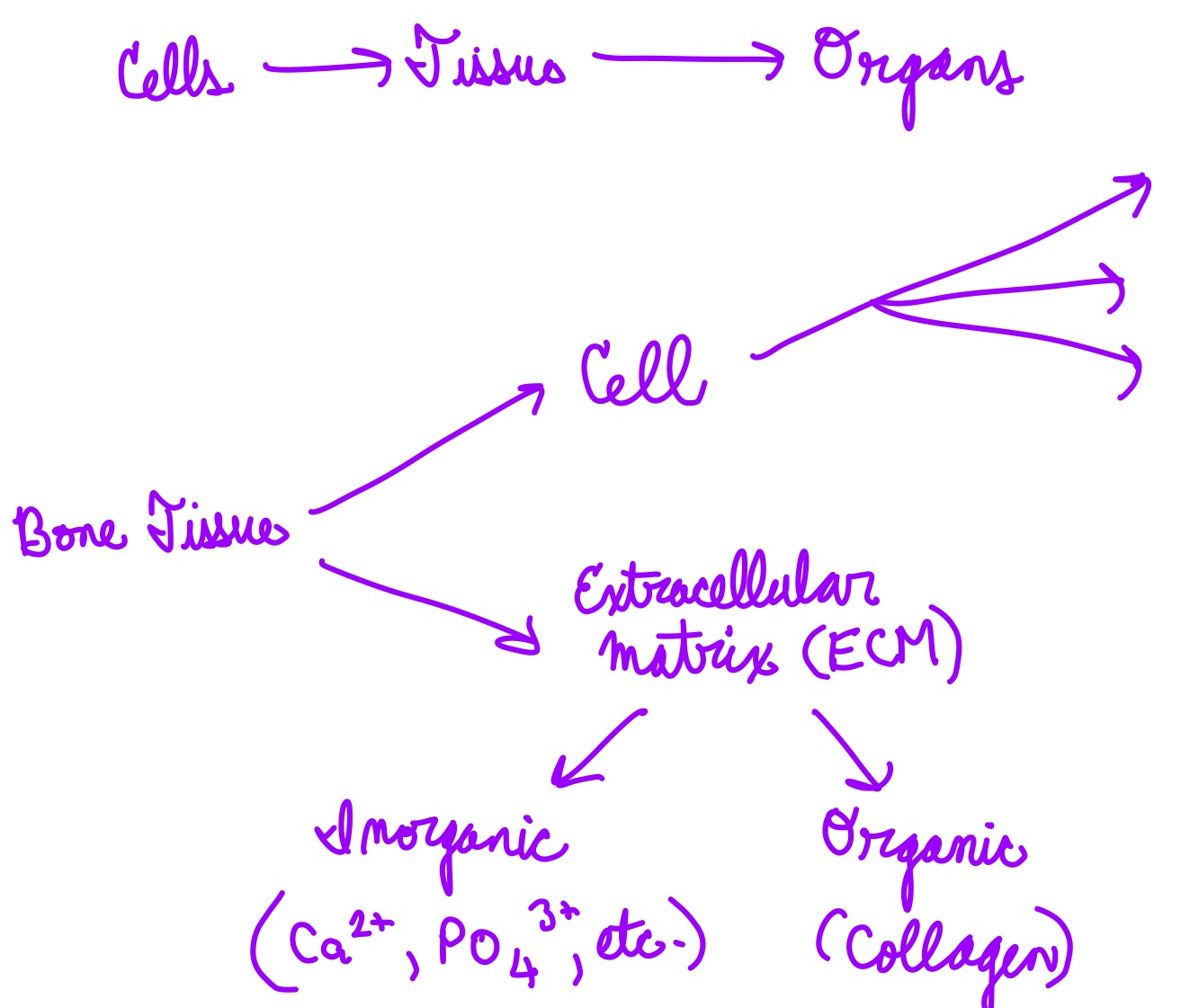
L13: Bone composition
Relatively small number of cells entrenched in a matrix of collagen fibers provide a surface for inorganic salt crystals (no carbon) to adhere
Collagen: highly abundant protein that forms fiber like structure
organic, has carbon
Salt crystals form when calcium phosphate and calcium carbonate combine to create hydroxyapatite, which incorporates other inorganic salts like magnesium hydroxide, fluoride, and sulfate as it crystallizes, or calcifies (becomes hard)
Hydroxyapatite crystals give bones their hardness and strength, while the collagen fibers given them flexibility so that they are not brittle
Calcium I think
Probably won’t degrade
L13: Bone types
Flat bone
Long bone
Sesamoid bone
Short bones
Irregular bone
*Don’t need to remember all of these though
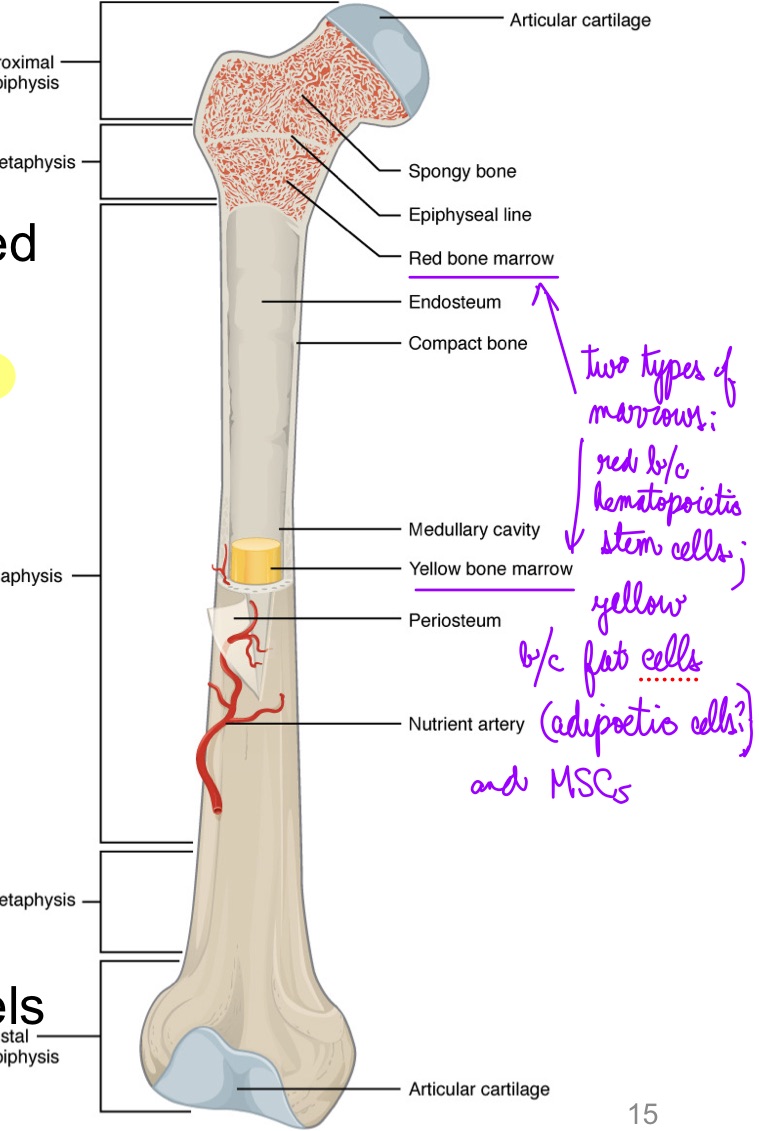
L13: Anatomy of a long bone
Main parts
Diaphysis (tubular shaft)
contains yellow bone marrow filled medullary cavity
Epiphysis (wider region at the end)
contains red bone marrow
Metaphysis
Contains the epiphyseal plate (growth plate)/line
Endosteum - lining of the medullary cavity
Periosteum - fibrous membrane covering the bone
Contains blood vessels, nerves, and lymphatic vessels that nourish the bone
L13: Type soft bone tissue: Two types
Cortical (compact)
Cancellous (trabecular, spongy)
Compact bone is dense so that it can withstand compressive forces, while spongy bone has open spaces and supports shifts in weight distribution
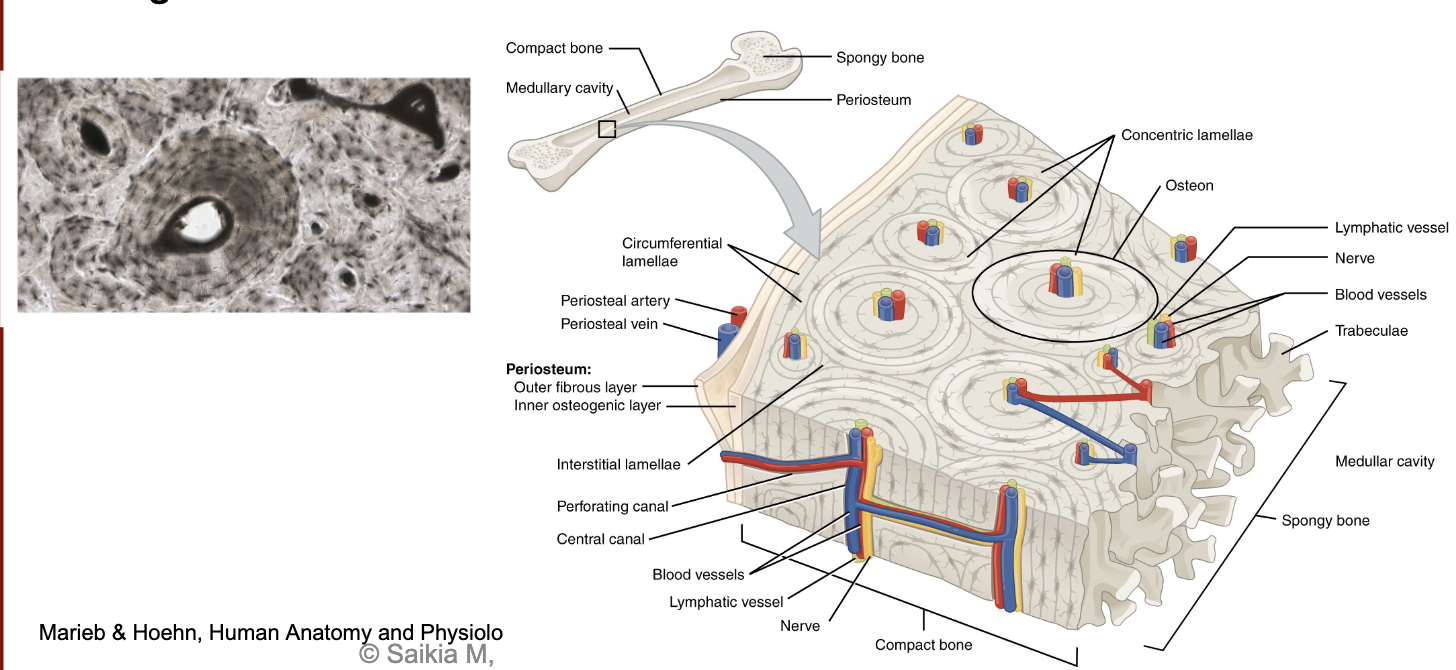
L14: Bone tissue organization (picture)
L14: Compact bone structure
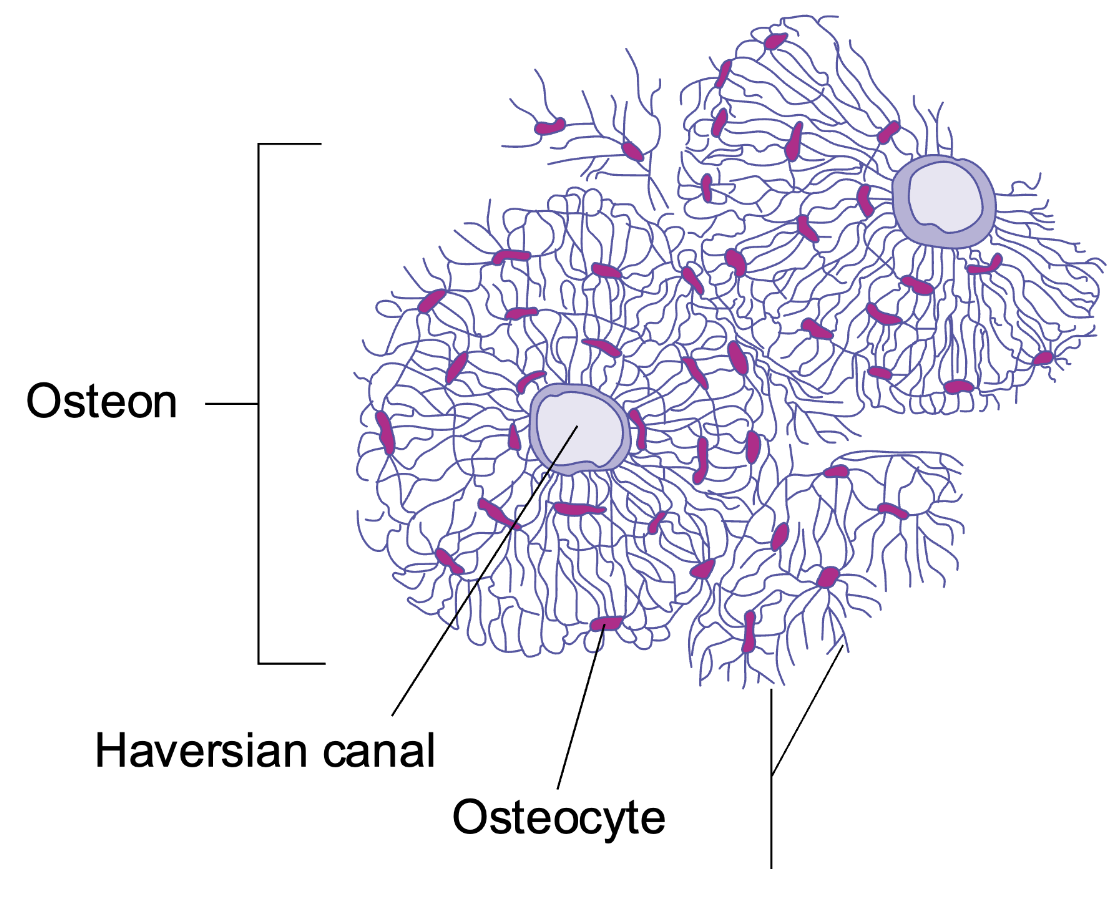
Microscopic structural unit of compact bone is called an osteon
Each osteon is composed of concentric rings of calcified matrix called lamellae
Running down the center of each osteon is the central canal
At the center of the osteons are blood vessels
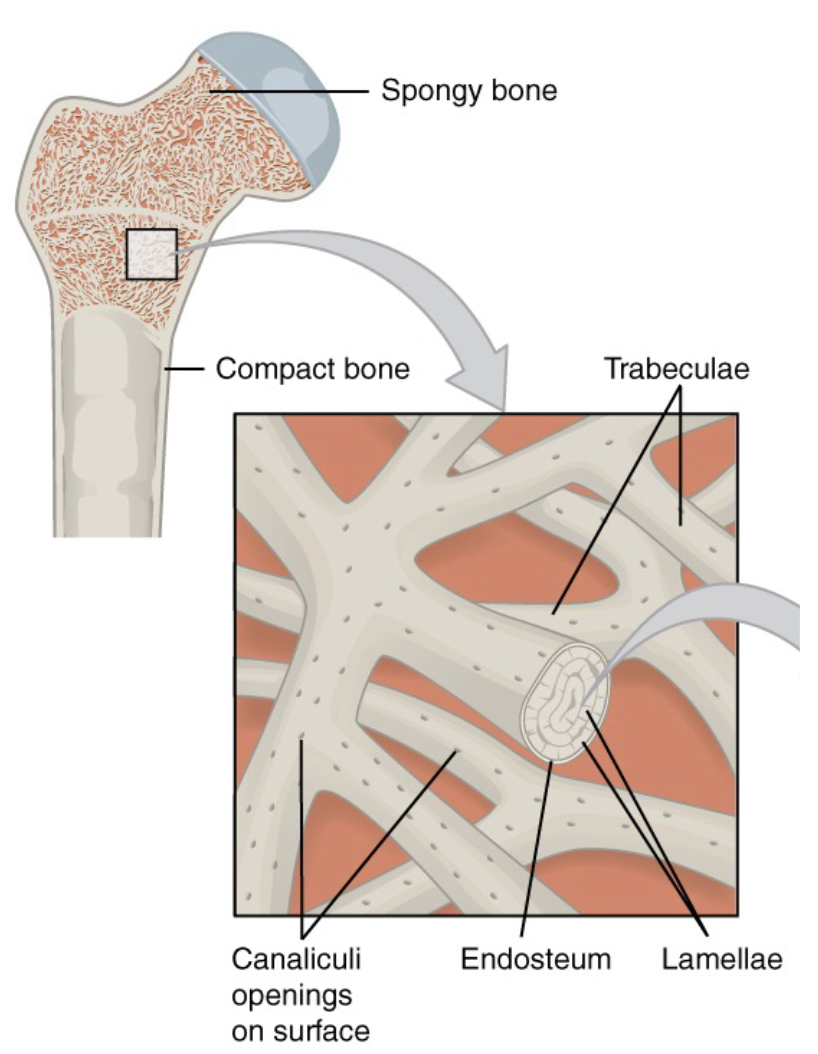
L14: Spongy bone tissue structure
Spongy bone tissue contains a network of matrix called trabeculae
Each trabecula forms along lines of stress to provide strength to the bone
the spaces of the spongy bone provides balance to the heavy compact bone by making bones lighter so that muscles can move them more easily
The spaces in some spongy bones contain red marrow
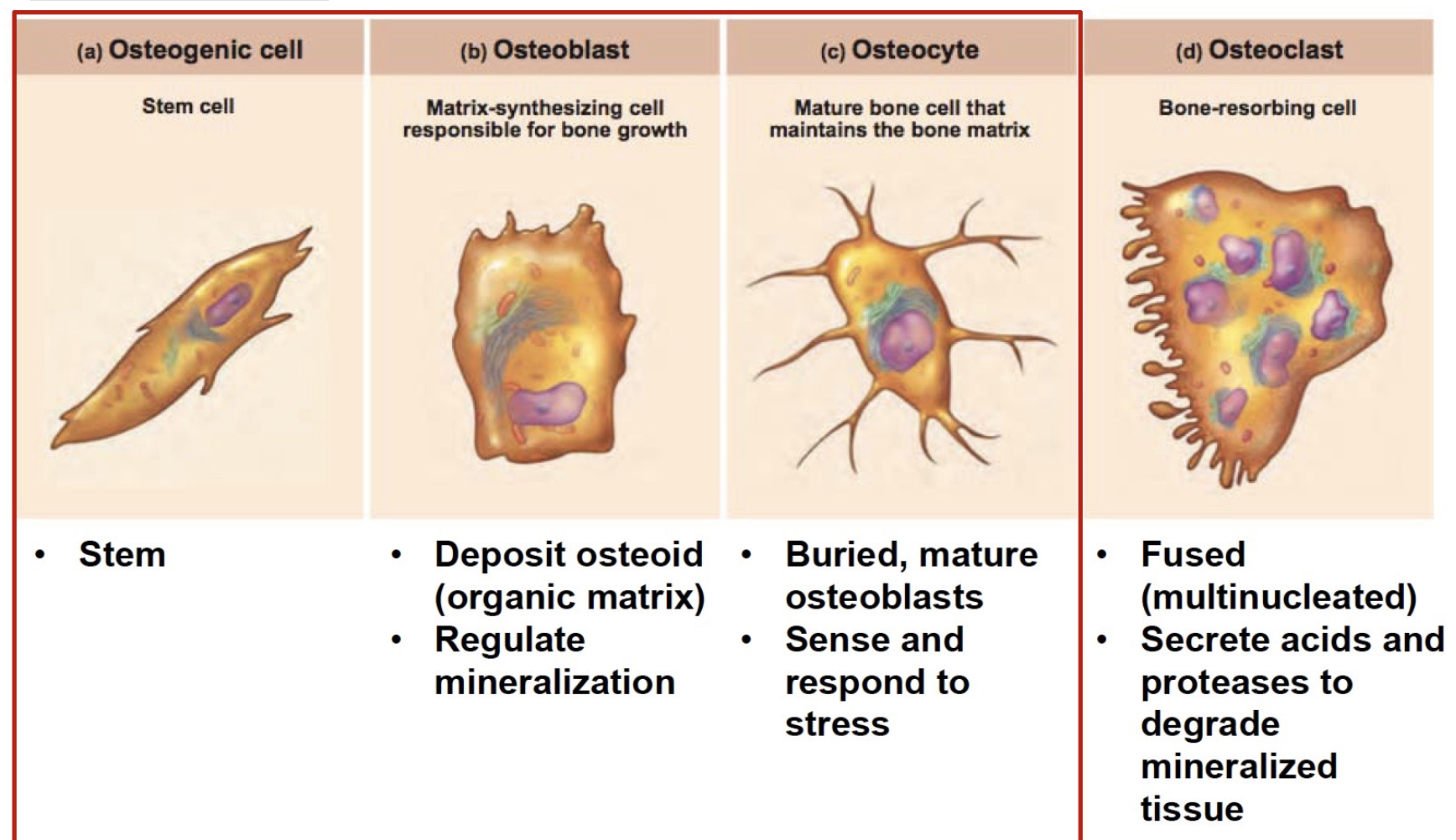
L14: Bone cells
Bone cells age
Over time, cells eventually get surrounded by ECM; they get less nutrients and eventually die
Cells with more similar functions
Osteogenic cell
Stem cell
Osteoblast
Deposit osteoid (organic matrix)!
ECM: includes collagen and minerals
regulate mineralization
Osteocyte
Mature bone cell that maintains the bone matrix
Buried, mature osteoblasts
Sense and respond to stress
*All derived from osteogenic cells
Cell with different function:
Osteoclast
Bone-resorbing cell
Fused (multinucleated)
Secrete acids and proteases to degrade mineralized tissue
*Not derived from osteogenic cells I think
L14: Osteocyte structure
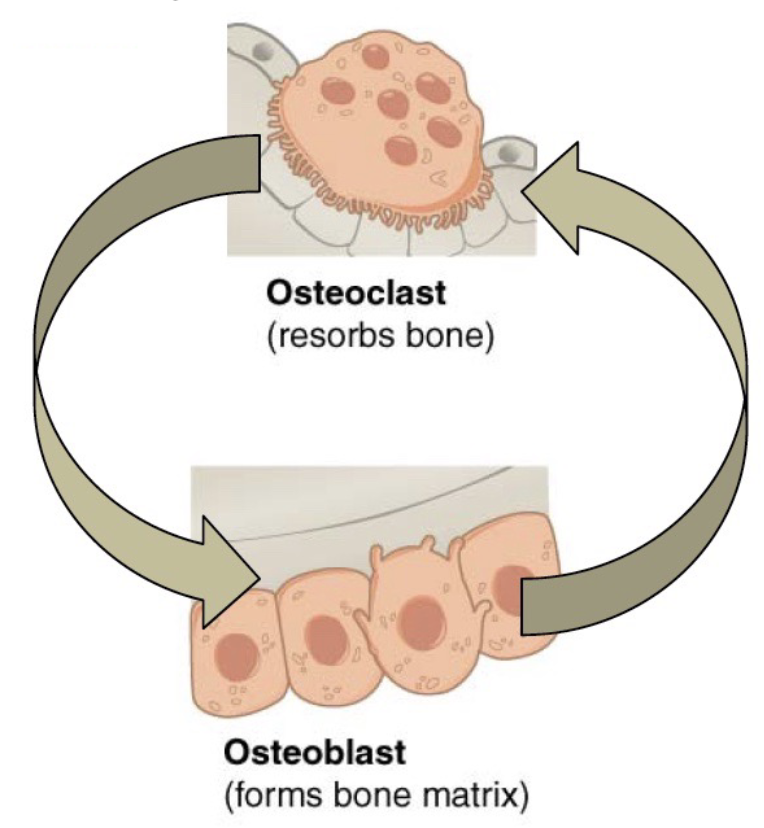
L14: Bone homeostasis and remodeling
Bone is a dynamic tissue!
10% is replaced annually
Tight controlled process
Bone resorption (degradation)
Bone formation (deposition)
Keeps bone strong
Osteoblast activity = osteoclast activity
When osteoclasts resorb damaged, or old bones, calcium is released from the bones into blood circulation; Osteoclast action is regulated by hormones
L14: Osteoporosis
A disease characterized by a decrease in bone mass that occurs when the rate of bone resorption (degradation) exceeds the rate of bone formation, a common occurrence as the body ages
Histologically, osteoporosis is characterized by a reduction in the thickness of compact bone and the number and size trabeculae in the cancellous bone
A bit of osteoporosis might happen with age
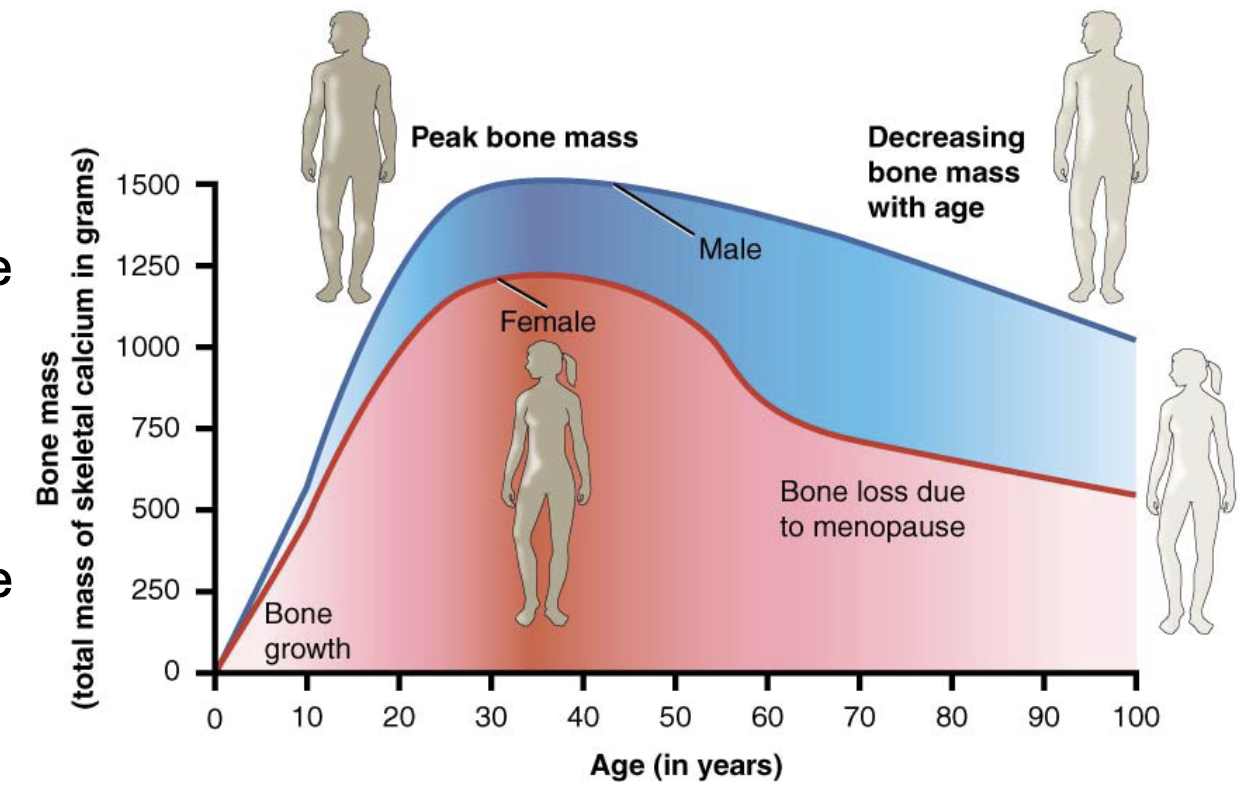
L14: Bone and gender and ageing
Women lose bone mass more quickly than men starting at about 50 years of age (around menopause)
Ovaries reduce in size and cease the production of estrogen, a hormone that promotes osteoblastic activity and production of bone matrix; Thus, osteoporosis is more common in women than in men
L14: Physical factors
Bone remodeling is affected by mechanical stress (muscle pull, gravity)
Wolff’s law: Bones will adapt based on the stress or demands placed on them
When you work your muscles, they put stress on your bones; In response, your bone tissue remodels and becomes stronger
If you don’t use the muscles surrounding a bone much, the bone tissue can weaken
L14: Joints (articulations)
The human body has 206 bones, and with the exception of one, each bone is connected to at least one other bone; Sites sites where 2 or more bones meets are the joints
Bones articulate with each other (join together)
Many joints allow for movement between between the bones, at these joints bones can move smoothly over another
Conversely some joints, have little or no mobility, are strongly united to each other; example? (see next card)
probably fibrous
At other joints, the bones are held together by cartilage, which permits limited movements between the bones
*One bone is a floating bone (only stuck to cartilage - hyoid bone)
L14: Classes of joints
Synovial (freely mobile)
Fibrous (mostly immobile)
Cartilaginous (limited mobility)
Greater mobility → lower stability
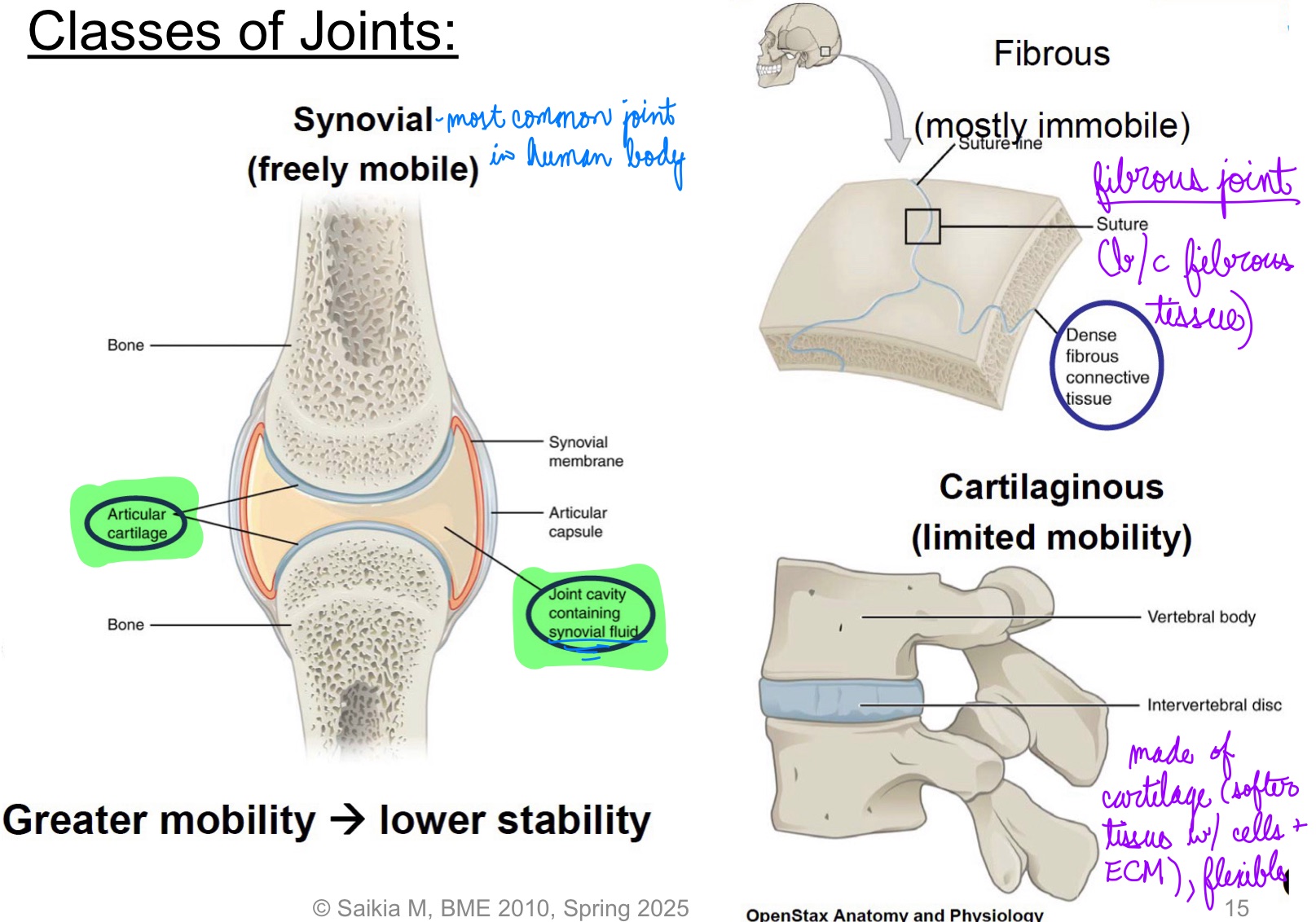
L14: Synovial joints
Most common type of joint in the body
A key structural characteristic for a synovial joint is the presence of a joint cavity
This fluid-filled space is the site at which the articulating surfaces of the bones contact each other
The articulating bone surfaces at this joint are not directly connected to each other; Allowing bones to move smoothly against each other, resulting in increased joint mobility
*Can be uniaxial, biaxial, multiaxial
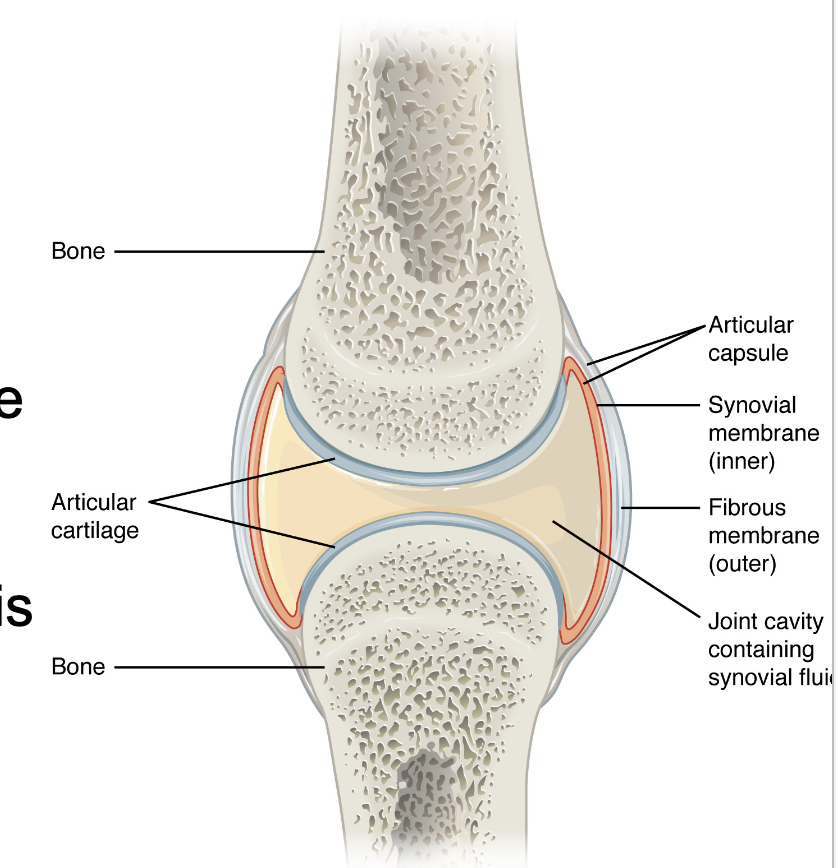
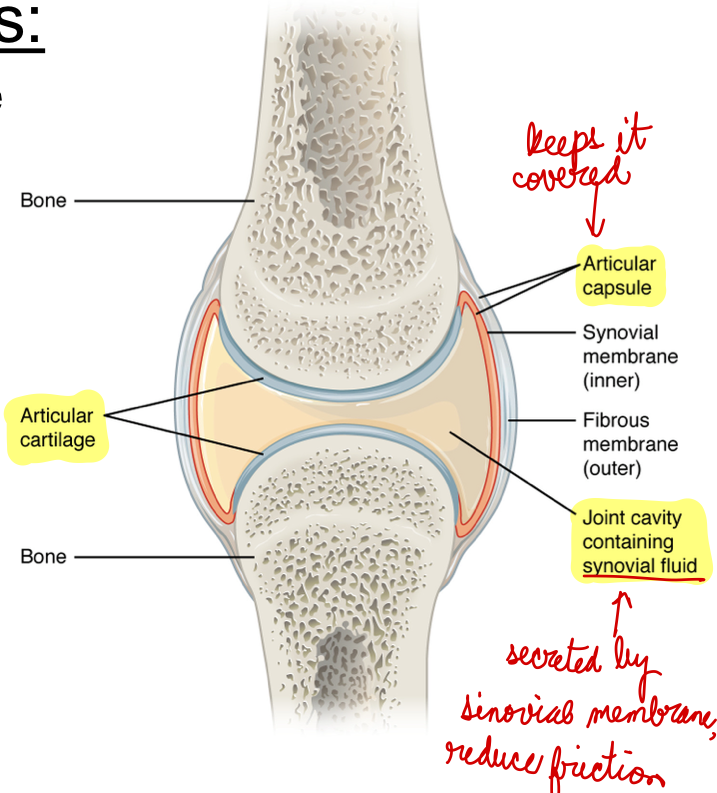
L14: Components of Synovial Joints
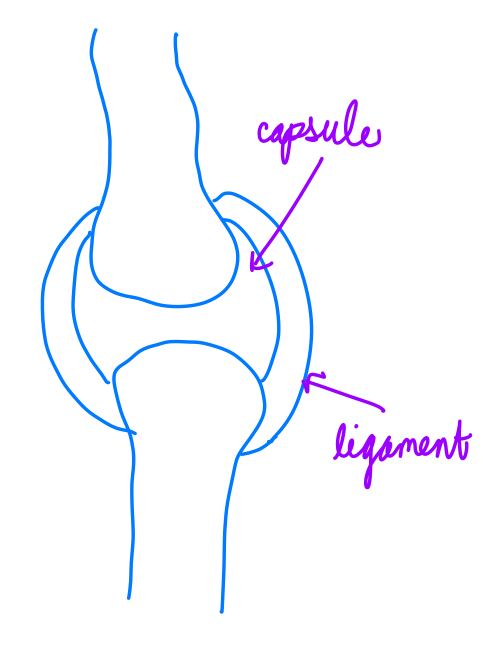
Articular capsule: The articular capsule surrounds the joint and is continuous with the periosteum of articulating bones; it consists of two layers
Fibrous layer (outer) consists of white fibrous tissue, known as the capsular ligament. It hold together the articulating bones
Synovial membrane (innter): Thin lining of the inner surface of the articular capsule; secrete synovial fluid; thick slimy fluid that lubricates the joint
Articular cartilage: a thin layer of hyaline cartilage that covers the entire articulating surface of each bone; prevents friction (like a cushion)
Note: most of the problems occur in the articular cartilage (wear and tear - osteoarthiritis)
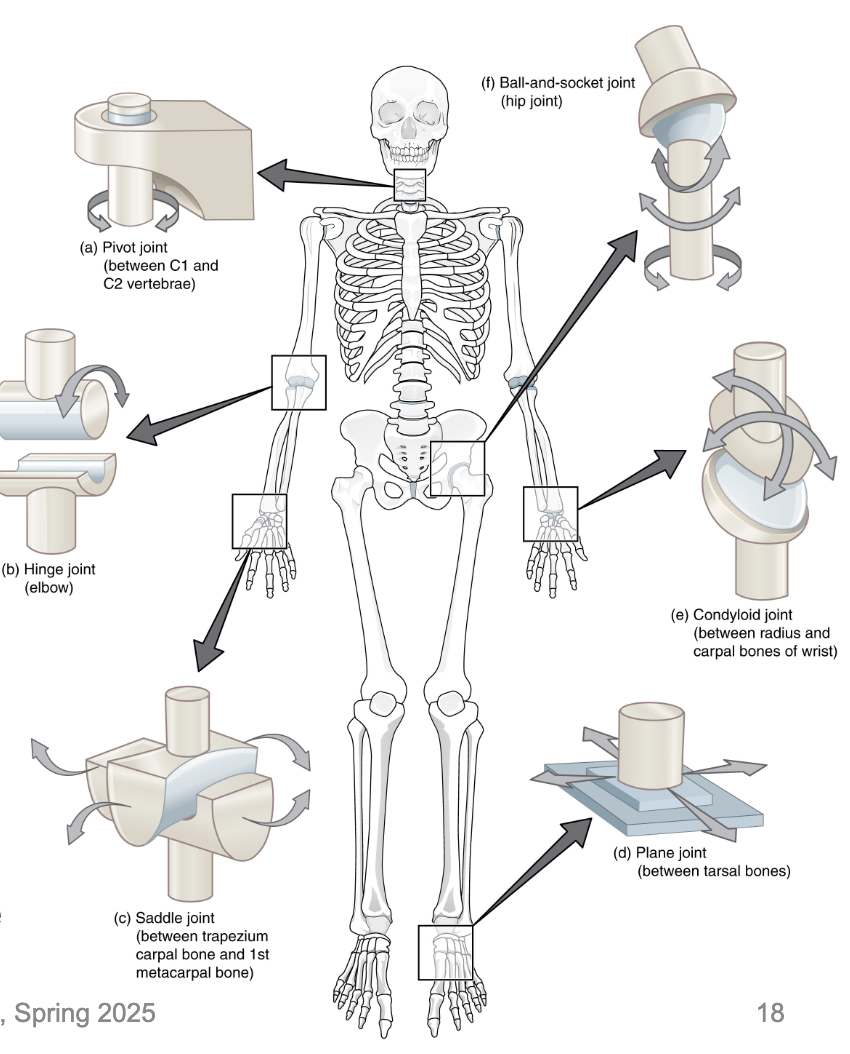
L14: Types of Synovial Joints
Six types in total
The synovial joint with the greatest range of motion is the ball-and-socket joint
At these joints, the rounded head of one bone (the ball) fits into the concave articulation (the socket) of the adjacent bone
The hip joint and the shoulder joint are the only ball-and-socket joints of the body
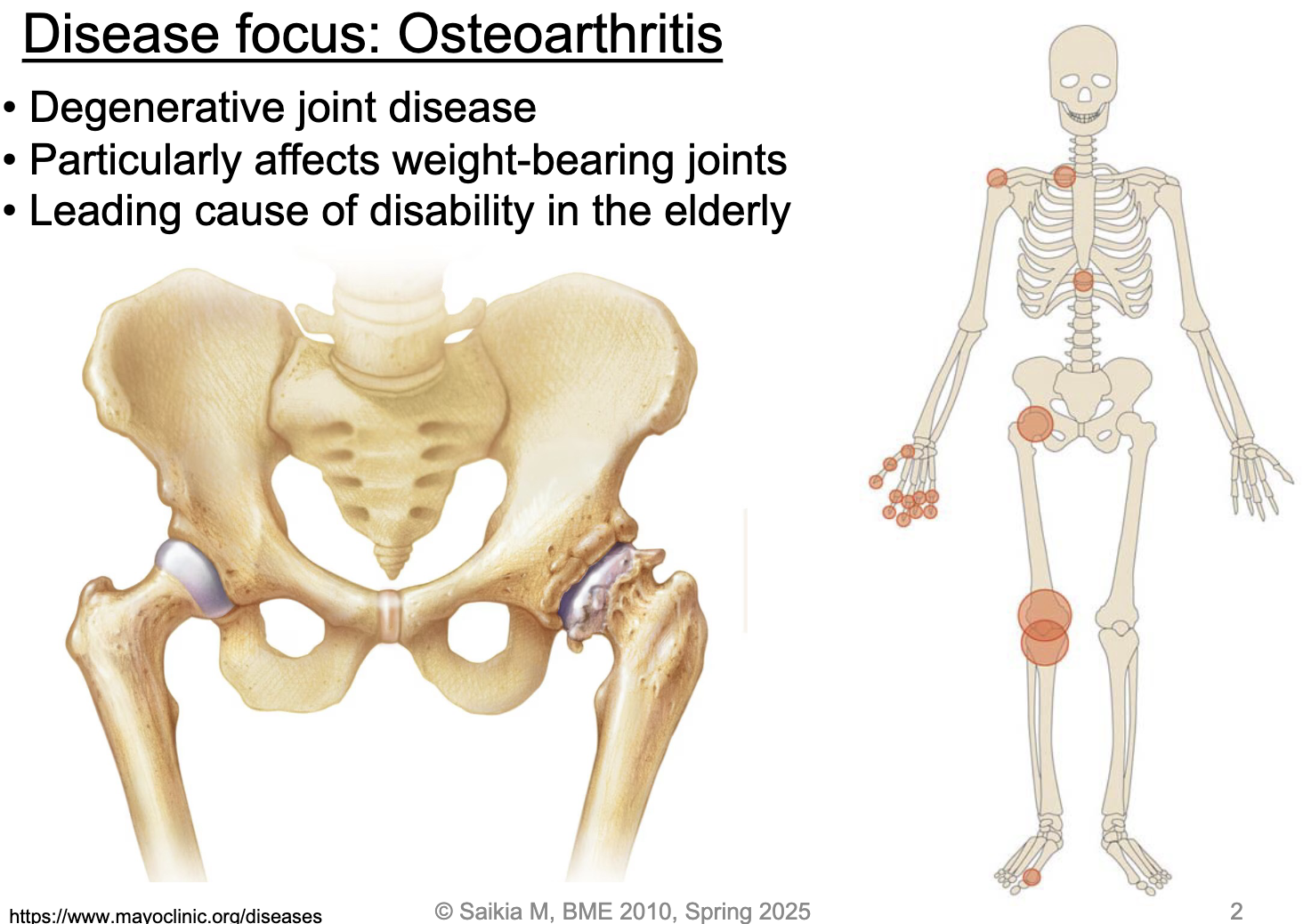
L15: Disease focus: Osteoartiritis
degenerative joint disease
particularly affects weight-bearing joints
leading cause of disability in the elderly
L15: Disease focus: Osteoarthritis (continued)
Common disorder of synovial joints: involves inflammation of the joint; Often results in significant joint pain, along with swelling, stiffness, and reduced joint mobility
Stress on the articular cartilage that covers the surfaces of bones at synovial joints, causes the cartilage to gradually become thinner; as the articular cartilage layer wears down, more pressure is placed on the bones
the joint responds by increasing production of lubricating synovial fluid, but this can lead to swelling of the joint cavity, causing pain and joint stiffness as the articular capsule is stretched
No cure for osteoarthritis (can’t reverse it): treatments may include lifestyle change, such as weight loss and low-impact exercise, and over-the-counter or prescription medications that help to alleviate the pain and inflammation; For severe cases, joint replacement surgery (arthroplasty) may be required
L15: Cartilage functions
Structural support: structure in the external ear, and the tip and septum of the nose
Protection: Acts as a shock absorber, cushioning areas where bone meets bone and preventing abrasion and damage
Movement: a joint would not be able to bend without the flexibility of cartilage
Bone growth and regeneration: Cartilage also plays a role in bone growth and repair, as in the embryo, it provides a template for ossification (process of bone formation)
Cartilage is mineralized → bone (primer for bone tissue)
*Cartilage is a tissue: cells + ECM
L15: Composition of cartilage
Specialized cells:
Chondroblasts: Produce matrix components; Eventually become chondrocytes
Chondrocytes: Immobile form of chondroblasts; Surrounded by the matrix and contained within lacunae
Structural extracellular matrix that contains:
Collagen (protein, can be of different types)
provides form
resists tension
(not able ot retain water)
Hyaluronan (Glycosaminoglycan, polysaccharide compound)
Retains water (80%)
Resists compression
(provides flexibility)
*Can change amounts of collagen and hyaluronan in cartilage
L15: Types of cartilage found in human body
Hyaline cartilage
Most common type of cartilage in your body
Translucent, slippery and smooth
Hyaline cartilage locations in your body include:
1. Synovial joints
2. Trachea
Part covering bone
Fibrocartilage:
Tough cartilage made of thick fibers; the strongest and least flexible (toughest)
Fibrocartilage locations in your body include
Tendons and ligaments
Elastic cartilage:
Most flexible cartilage; supports parts of the body that needs to bend and move to function; Elastic cartilage can bounce back to its original shape
Elastic cartilage locations in your body include:
External ears (the parts of the ear that are outside your body)
Larynx (voice box)
L15: Muscle
Functions:
Movement
Effector organs of the nervous system
Key characteristics:
High energy demanding tissue: cell metabolism is critical
Consist of excitable cells (sense electricity)
Under the control of nervous system
Types:
Skeletal
Smooth
Cardiac
Muscles store energy in the form of glycogen!!
L15: Structure of skeletal muscle
Generally connected to 2 or more bones
Connected to bones via tendons (cords of connective tissue that transmit force from muscle to bone)
*Type of cartilage!
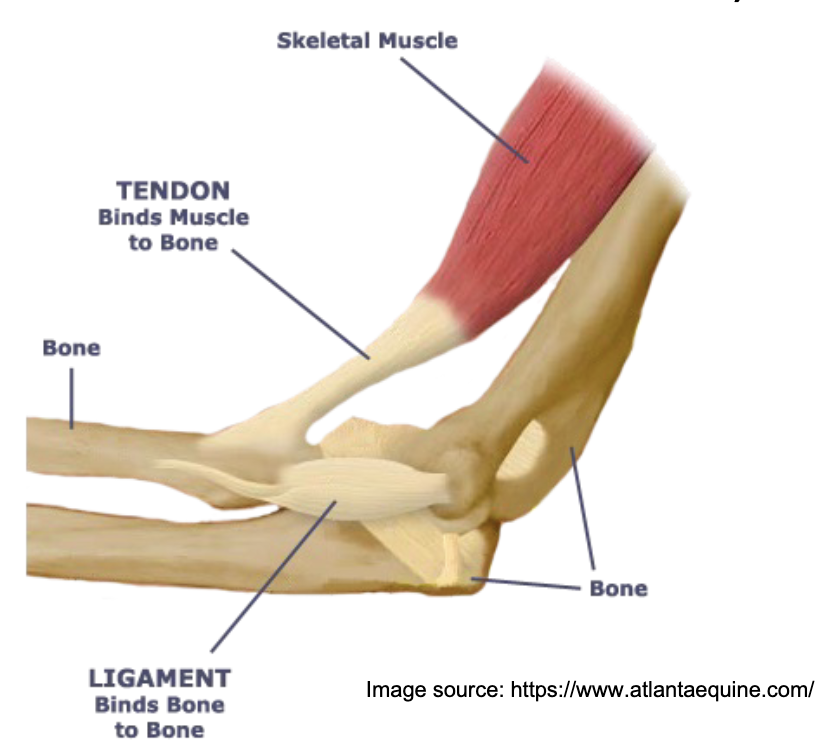
L15: Structure of skeletal muscle from macro to micro level
Muscle → fascicle → muscle fiber → myofibril → filaments (very organized)
muscle fiber is the cell, made up of myofibrils
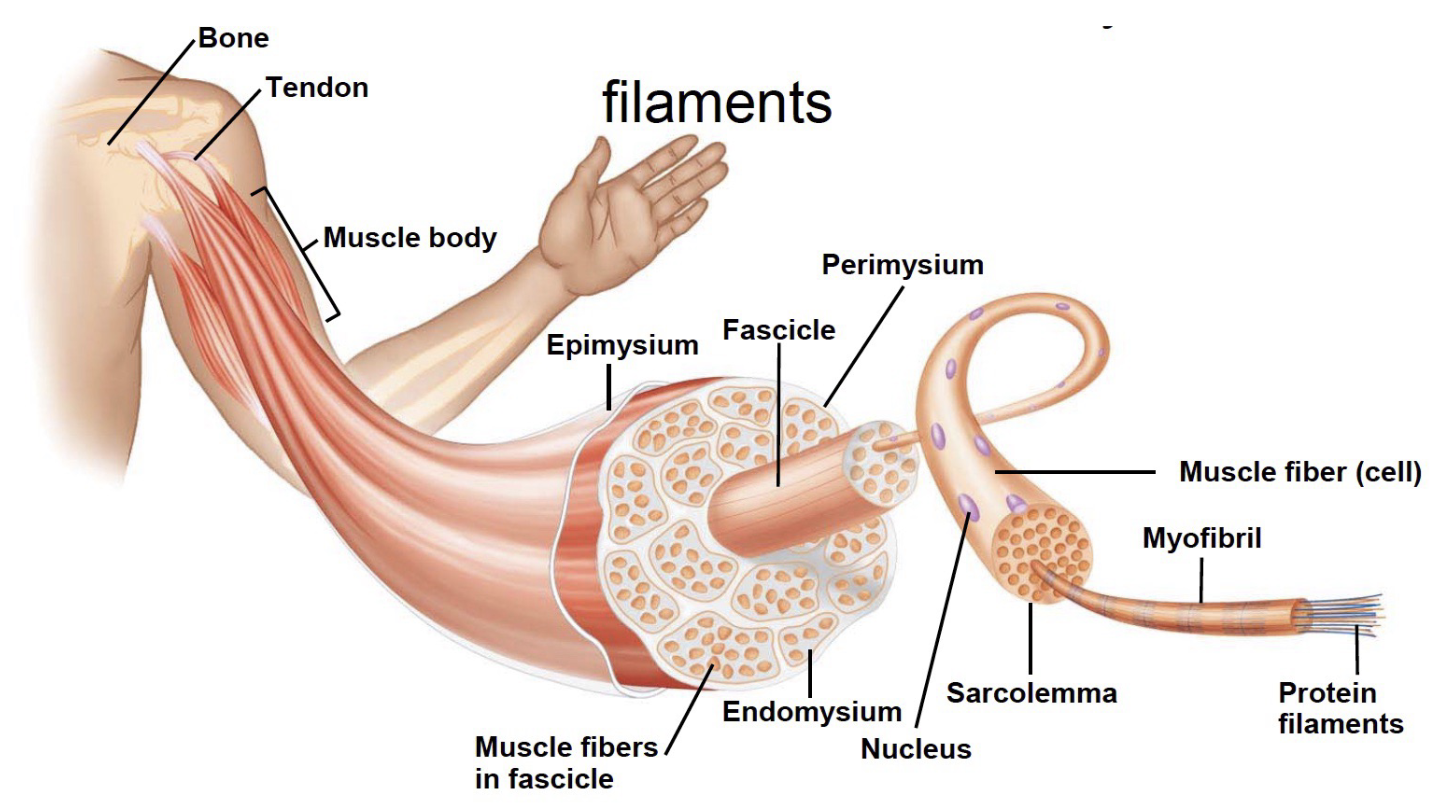
L15: Muscle fiber structure
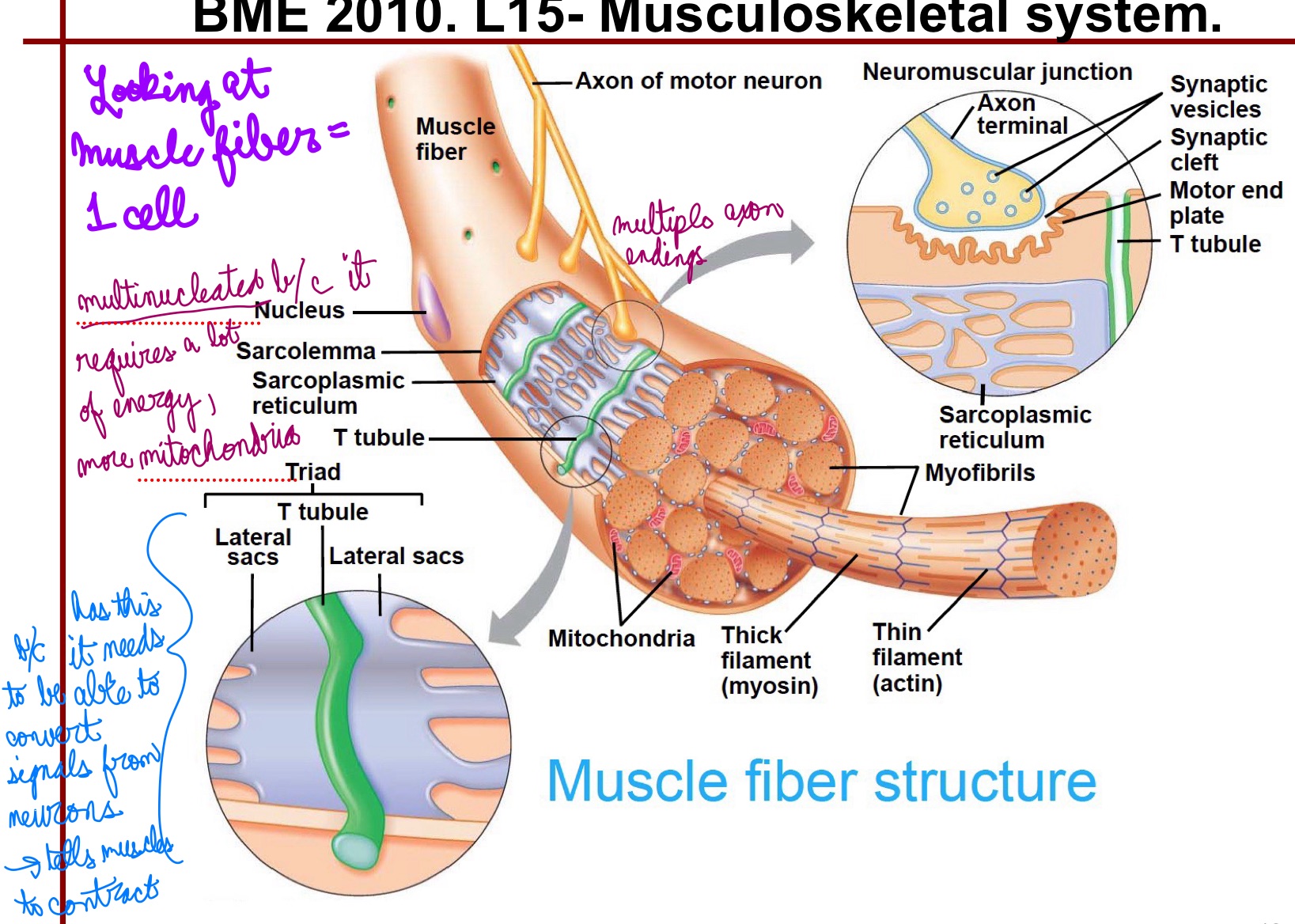
L15: Muscle fibers
Muscle Fibers = each is an individual cell
Very large cells (10-100um diamater)
Multinucleated
Innervated (one motor neuron)
Sarcolemma = Plasma membrane
Sarcolemma: Plasma membrane (it’s the cell membrane)
Sarcoplasm: semi-fluid cytoplasm
Sarcoplasmic reticulum (SR)
Specialized smooth ER
Network surrounding myofibrils
Associated with Transverse (T) tubules - like ER but also has (T) tubules
Lateral sacs are parts of the SR that store calcium ions
Triad: each tubule associated with two lateral sacs
Contain bundles of protein filaments called myofibrils
L15: Myofibrils
Makes up muscle fiber
Contractile bundles of myofilaments
Thin filament: actin
Thick filament: myosin
Thick and thin filaments together form sarcomeres (fundamental unit of myofibrils)
L15: Thin Filaments - Components
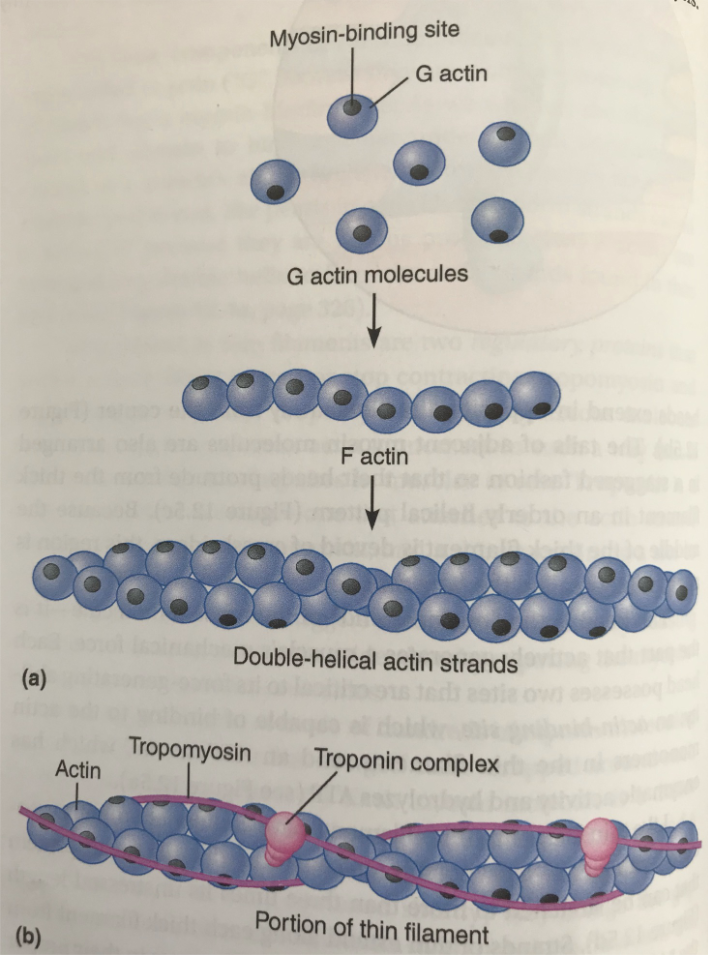
Actin
Contractile protein
Smallest funcitonal unit - G-Action (“bead”)
G-Actin has a myosin binding site (black dot)
Tropomyosin
Regulatory protein
Surrounds F-actin (“string of beads”) and covers up the myosin binding site
Different from myosin!
Troponin complex
Regulatory protein
Complex made of 3 proteins that binds to actin strang, tropomyosin, and calcium, respectively
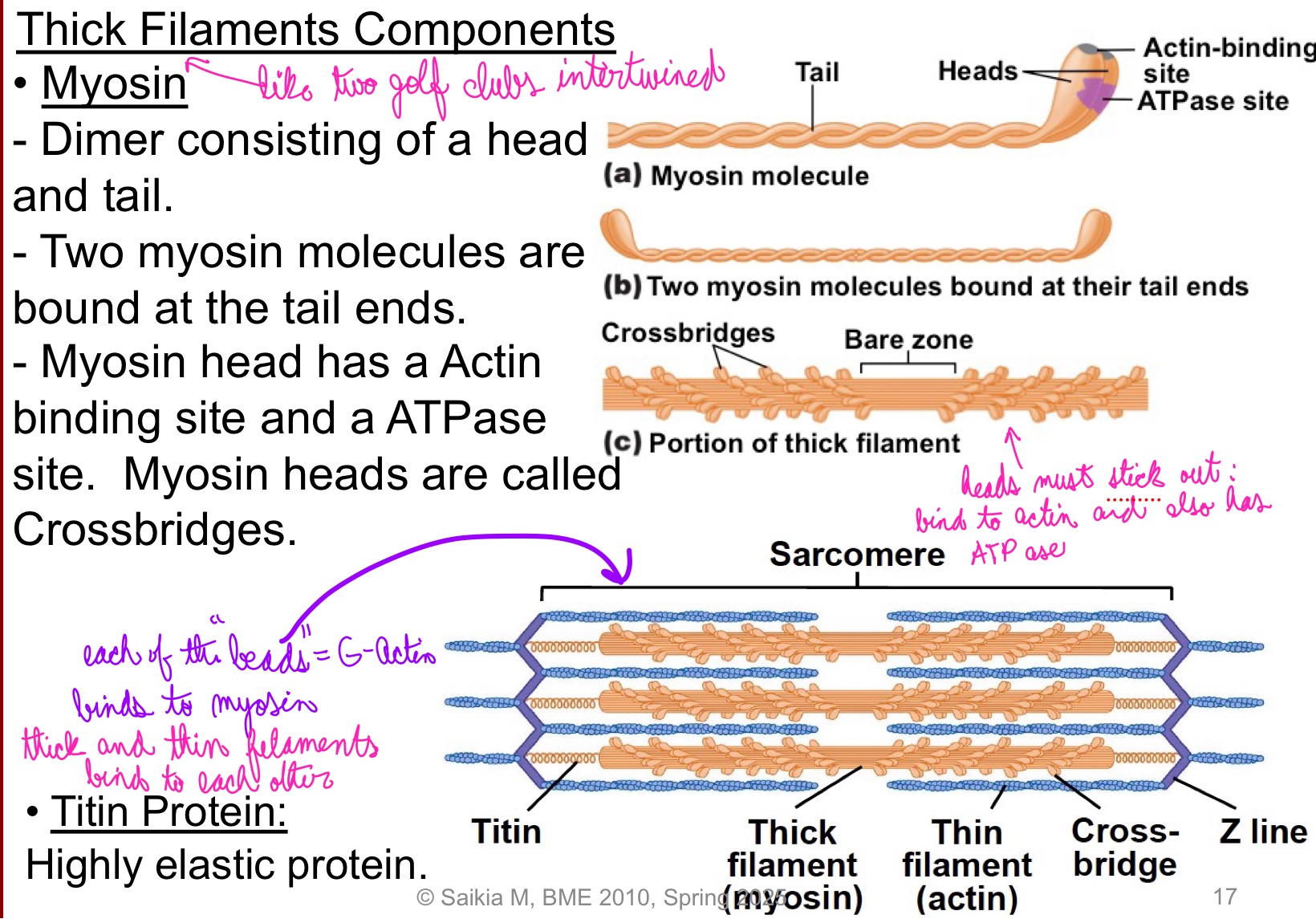
L15: Thick Filaments Components
Myosin
Myosin Dimer consisting of a head and tail
Two myosin molecules are bound at the tail ends
Myosin head has an Actin binding site and an ATPase site; Myosin heads are called crossbridges
head must stick out: bind to actin and also has ATPase
ATP is hydrolized by ATPase I think → energy is released
Titin protein:
highly elastic protein
each of the “beads” = G-actin binds to myosin
Thick and thin filaments bind to each other
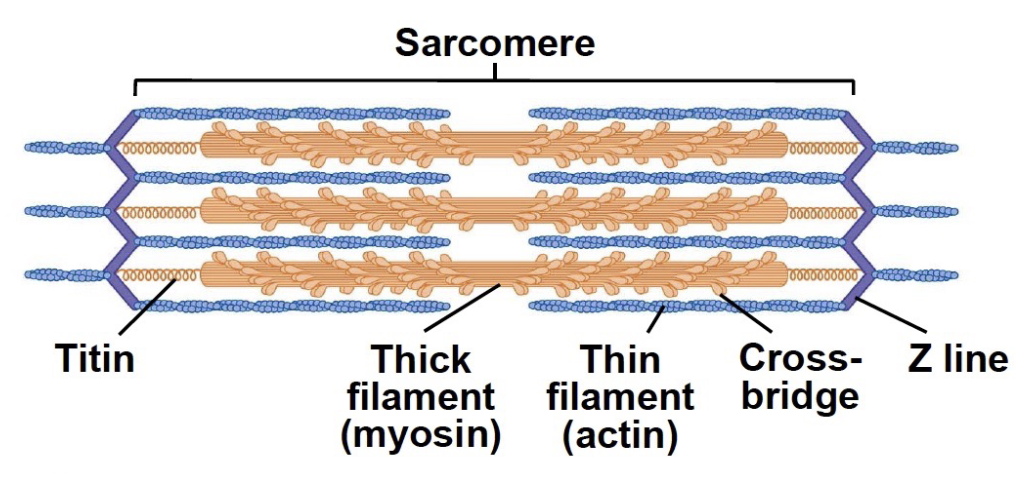
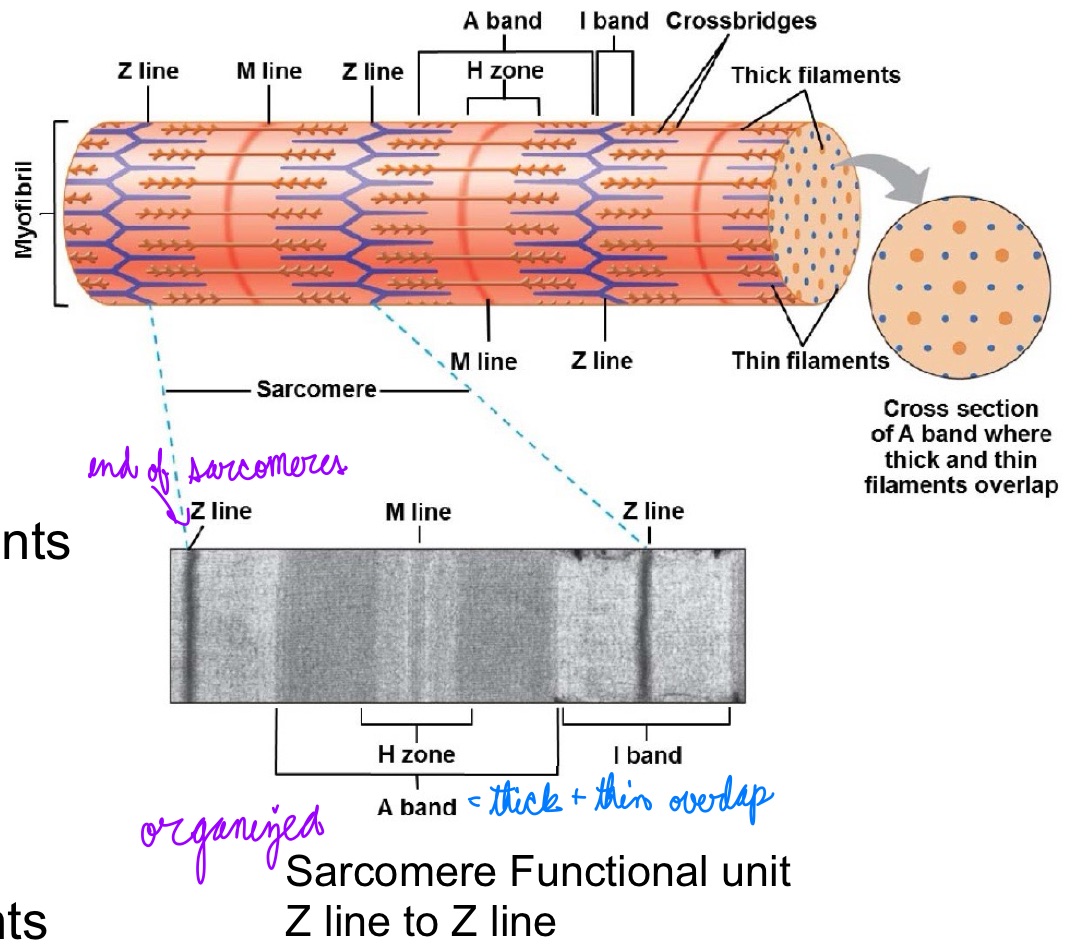
L15: Sarcomere
Sarcomere: Fundamental contractile unit of muscle fibers
A band:
Dark band
Thick filaments
Overlap with thin
H zone:
Thick filaments
No overlapping
M line:
Links thick filaments
I band:
Light band
Thin filament
No overlapping
Z line:
Links thin filaments
L16: The mechanism of muscle contraction
Upon contraction (passing electricity through):
H zone shortens
A band remains the same (as the relaxed muscle)
Z line came closer
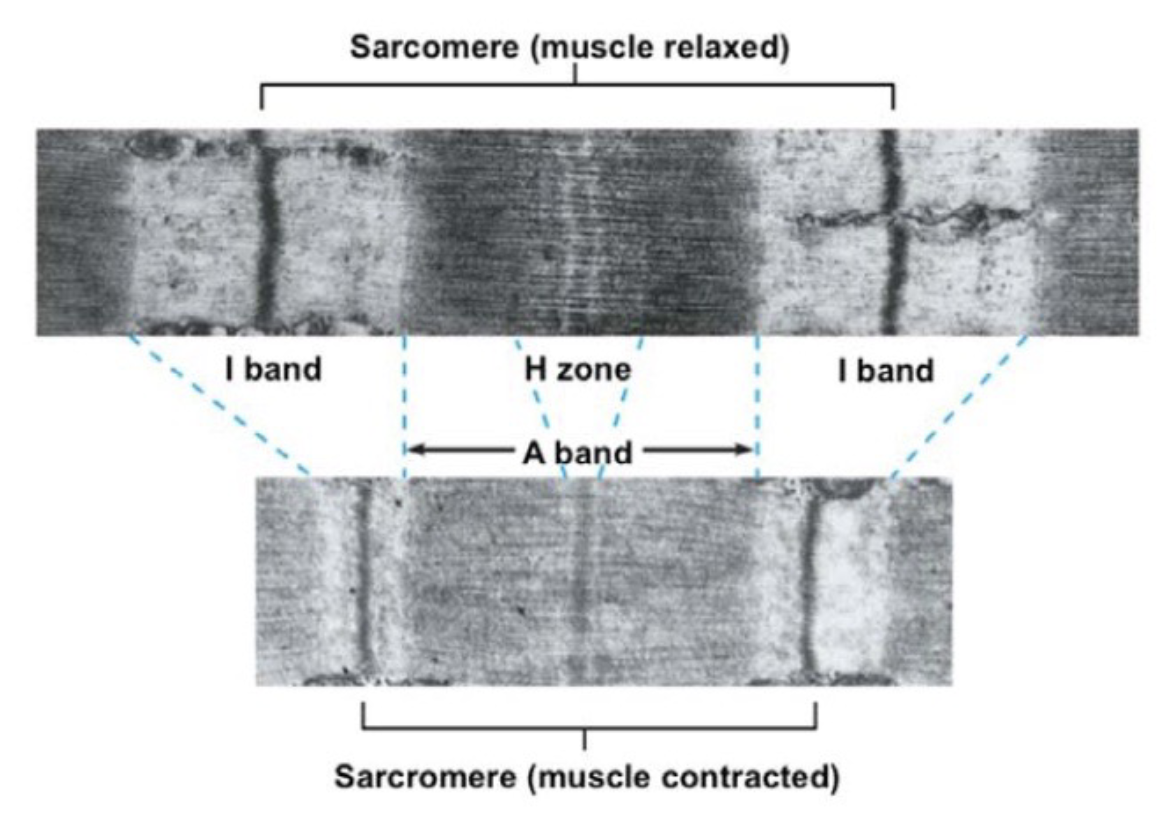
L16: Sliding filament model
During contraction, thin filaments (actin filaments) slide closer inwards)
Sliding is due to cyclical formation and breaking of crossbridges = crossbridge cycle
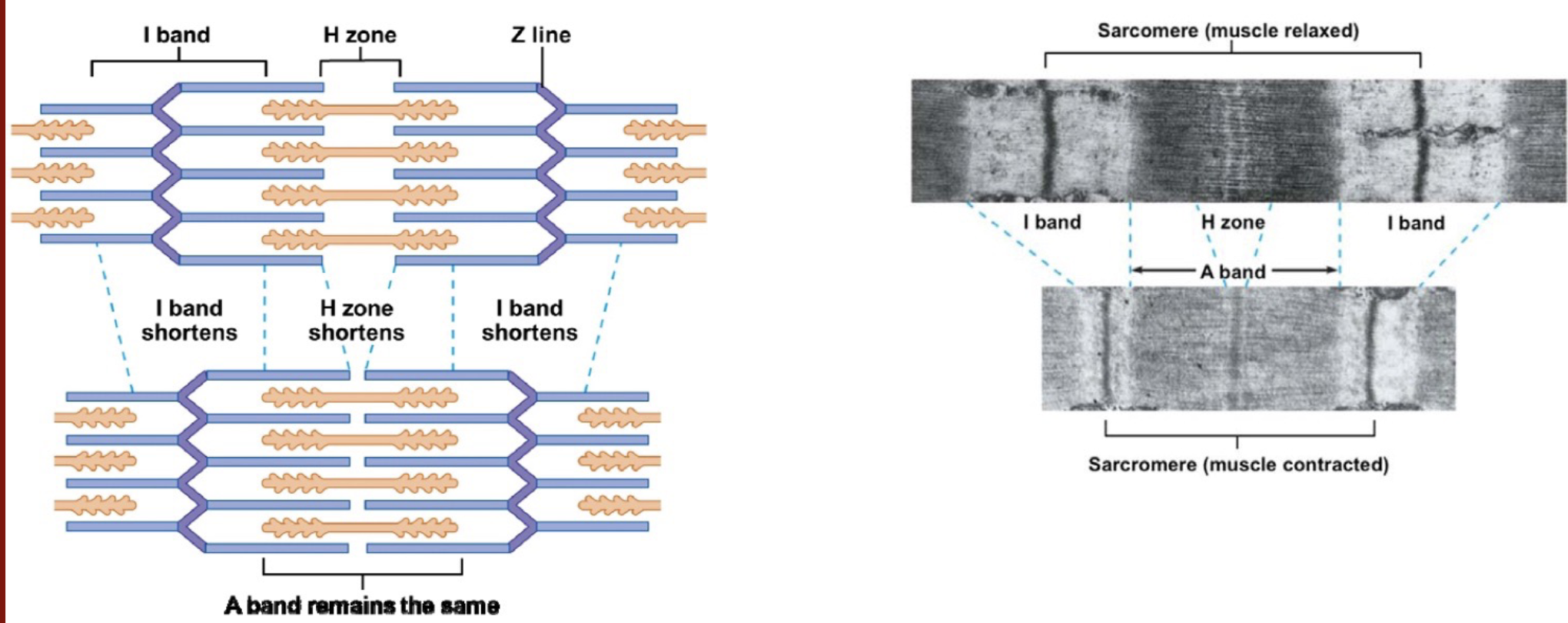
L16: Crossbridge cycle
Mechanism that drives sliding of thick and thin filaments past each other is back and forth motion of myosin crossbridges powered by ATP hydrolysis
Back and forth motion of crossbridges is due to chang ein the conformation of myosin molecules
myosin goes from high-energy form (has stored energy) to low-energy form (released energy)
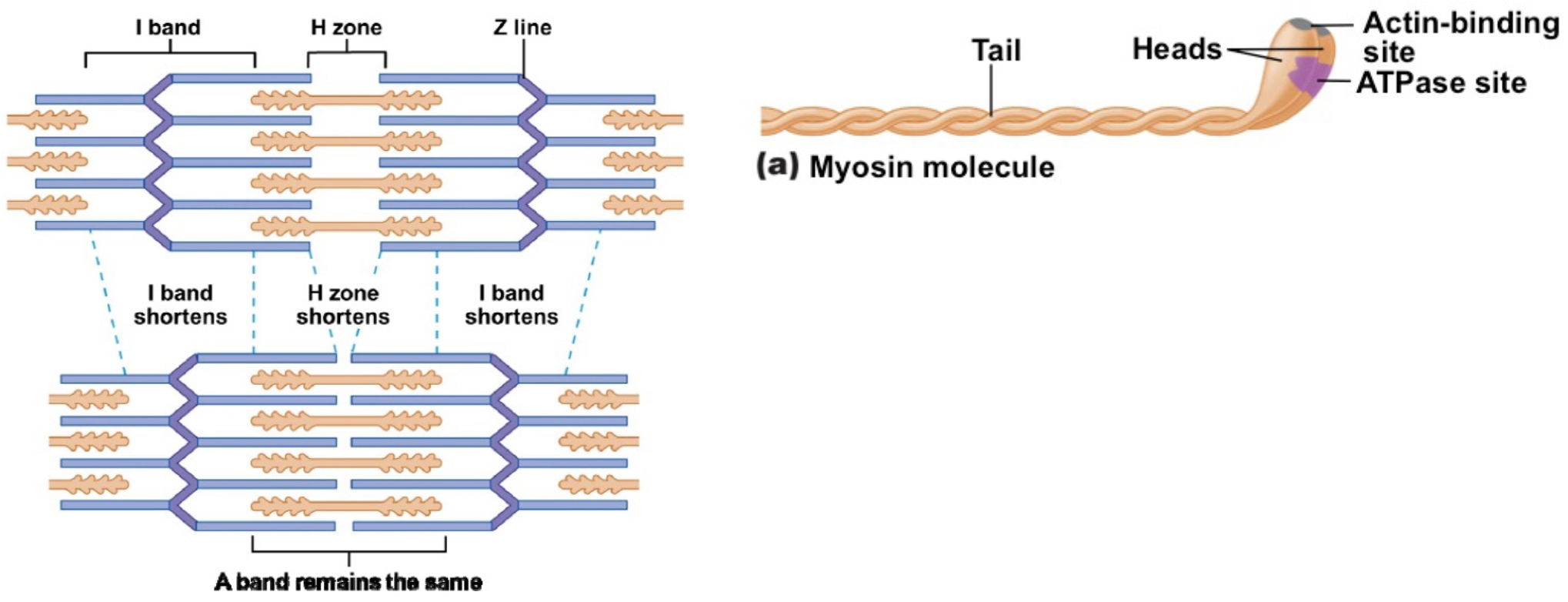
L16: The crossbridge cycle (image)
*Need Ca2+ for step 1
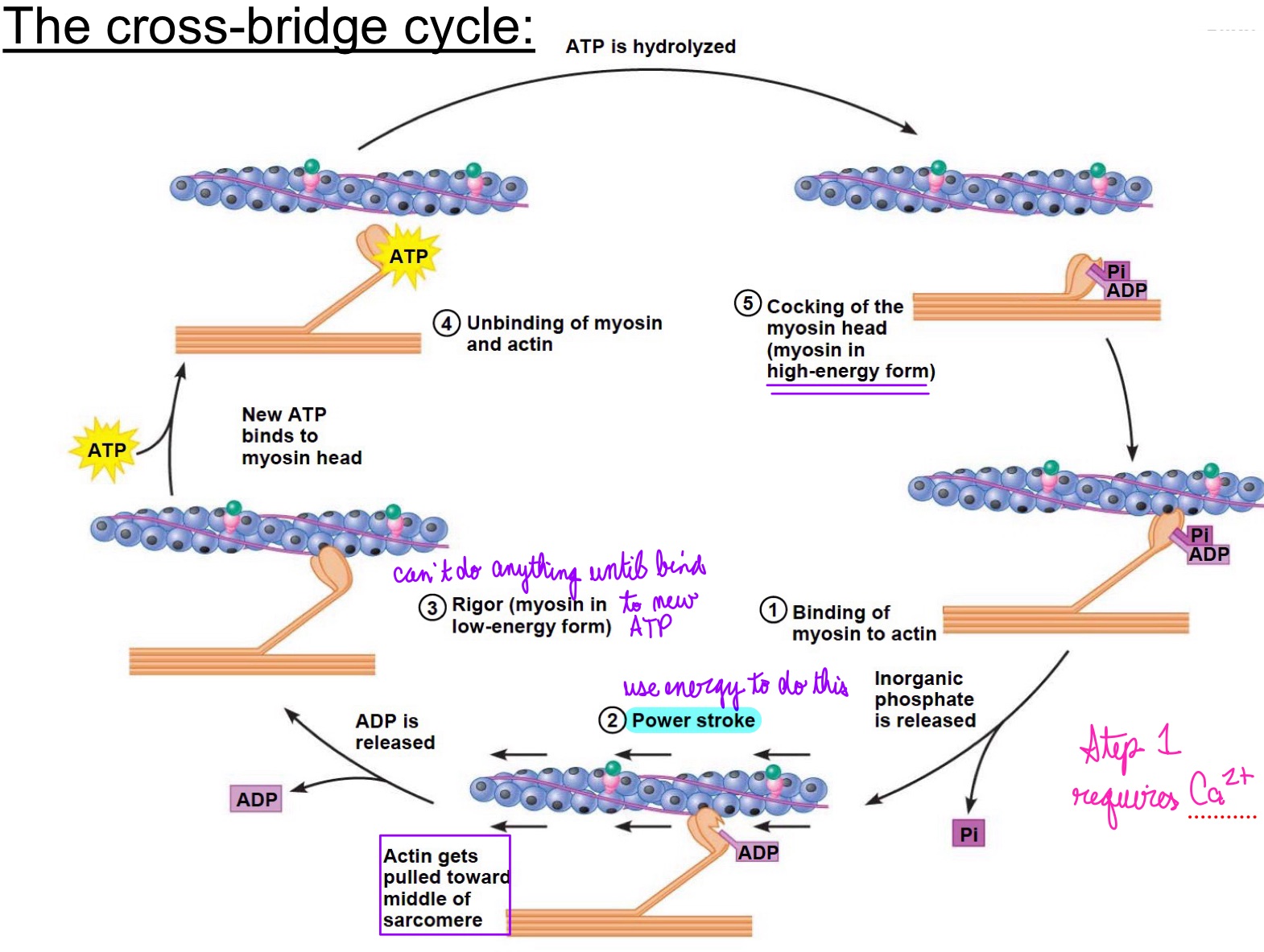
L16: The cross-bridge cycle (continued)
Crossbridges at the opposite ends of thick filaments are oriented in opposite directions from each other
power strokes of the crossbridges at the opposite ends move in opposing direction pulling the thin filaments towards the center
At the end of the crossbridge cycle, the thin filaments passively slide back to their original position
L16: Muscle fiber contraction - very fast!
Thousand sof power strokes can happen per second —> muscle fiber can contract fully in less than a tenth of a second
L16: Excitation-contraction coupling
How muscle contractions are turned on and off by the CNS via motor neurons
Want to contract only when it gets signal by nervous system; not when it has ATP
L16: CNS-mediated regulation of muscle fiber contraction
Motor neurons deliver the commands to skeletal muscles telling them when to and when not to contract
Muscle cels are excitable → capable of generating action potential (AP)
After receiving input from motor neuron → muscle cell depolarizes → fires an AP
Sequence of AP to the contraction is referred to as excitation-contraction coupling
L16: Muscle fiber stucture w/ neuromuscular junction (image)
Nerve ending binding to muscle fiber
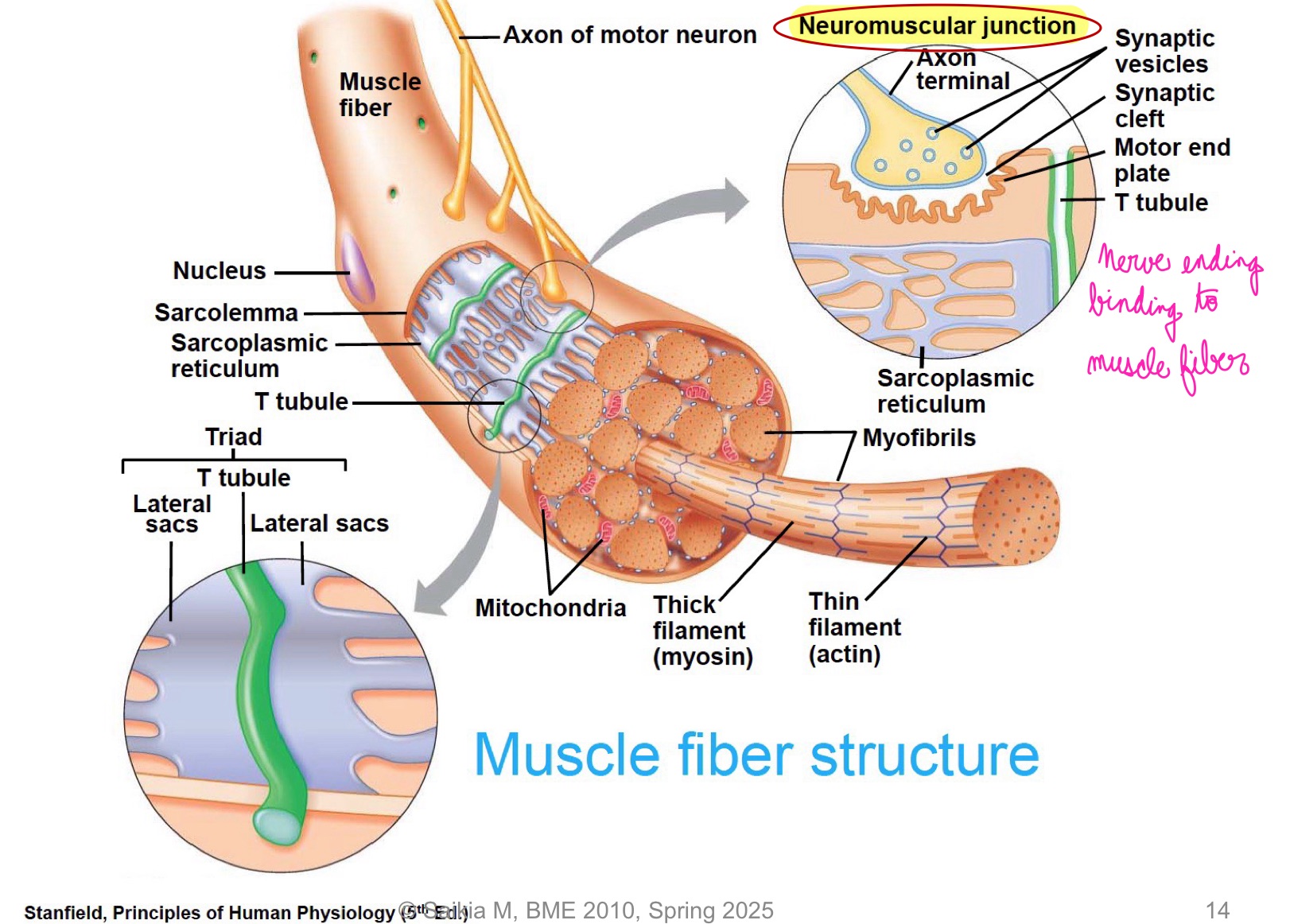
L16: Neuromuscular junction in more detail (image)
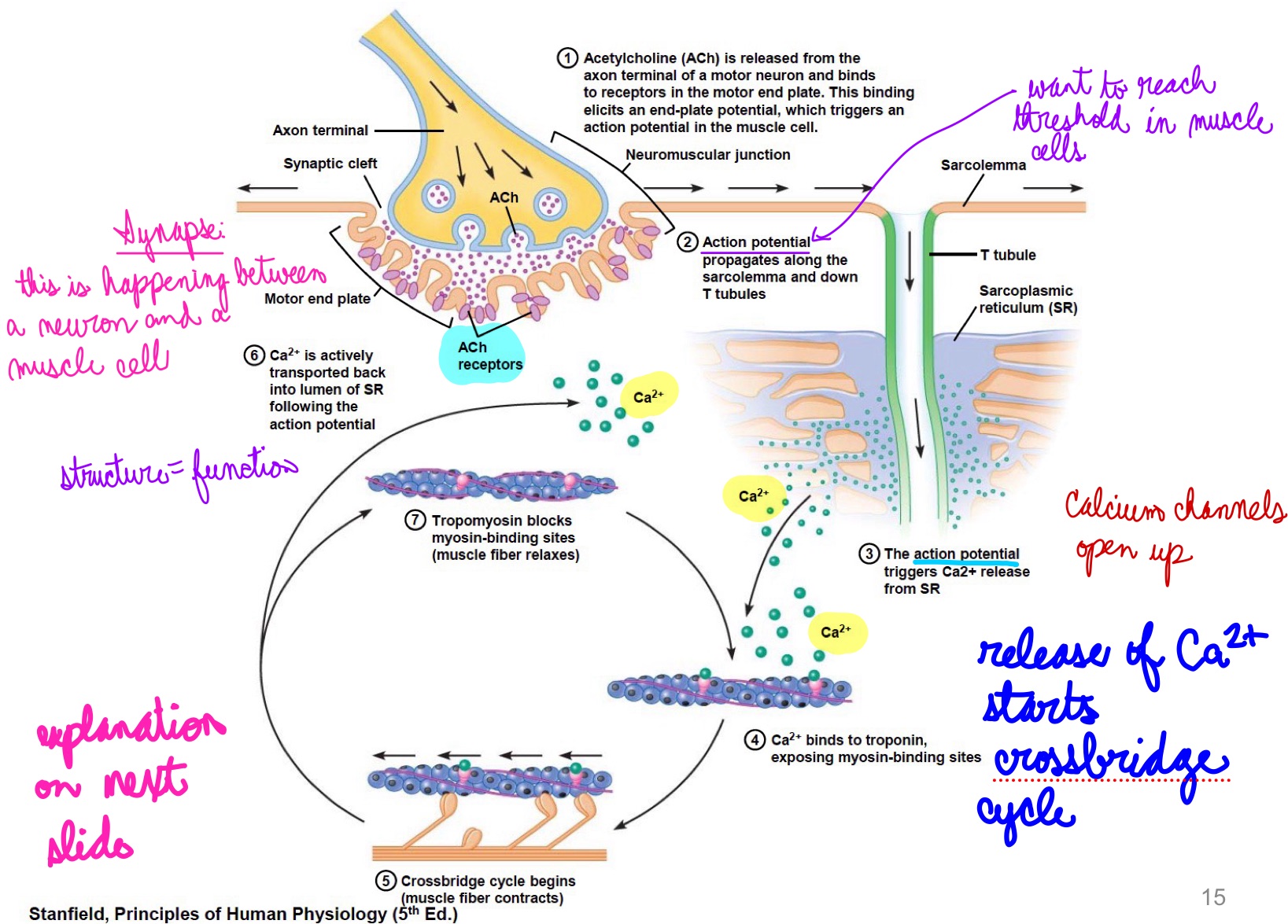
L16: Neuromuscular junction in more detail explanation; excitation-contraction coupling
Excitation-contraction coupling
Neuromuscular junction is similar to a synapse
AP travels through motor neuron (presynaptic cell) into neuromuscular junction → releases acetylcholine (ACh) → diffuses to the muscle cell (postsynaptic cell)
Specialized region of sarcolemma → motor end plates contain many ACh receptors → resulting depolarization (end plate potential) is amplified causing an AP in the muscle cell
AP propagates through the sarcolemma down the T tubules releasing calcium from sarcoplasmic reticulum (SR) that initiates the crossbridge cycle
L16: Relaxed Muscle vs. Contracting muscle
Relaxed muscle:
Concentration of calcium is low in cytosol there is no binding of calcium to troponin
Tropomyosin positioned on actin filaments blocking myosin binding sites
Note: actin filaments is like a helix, like two intertwined pearl strings
Contracting muscle
AP travels to T tubules → Calcium channels in SR open and release calcium
Calcium binds to the protein troponin complex → change in conformation of troponin complex → tropomyosin moves from resting position → Myosin binding sites exposed
Myosin heads can now bind actin and crossbridge cycle can begin
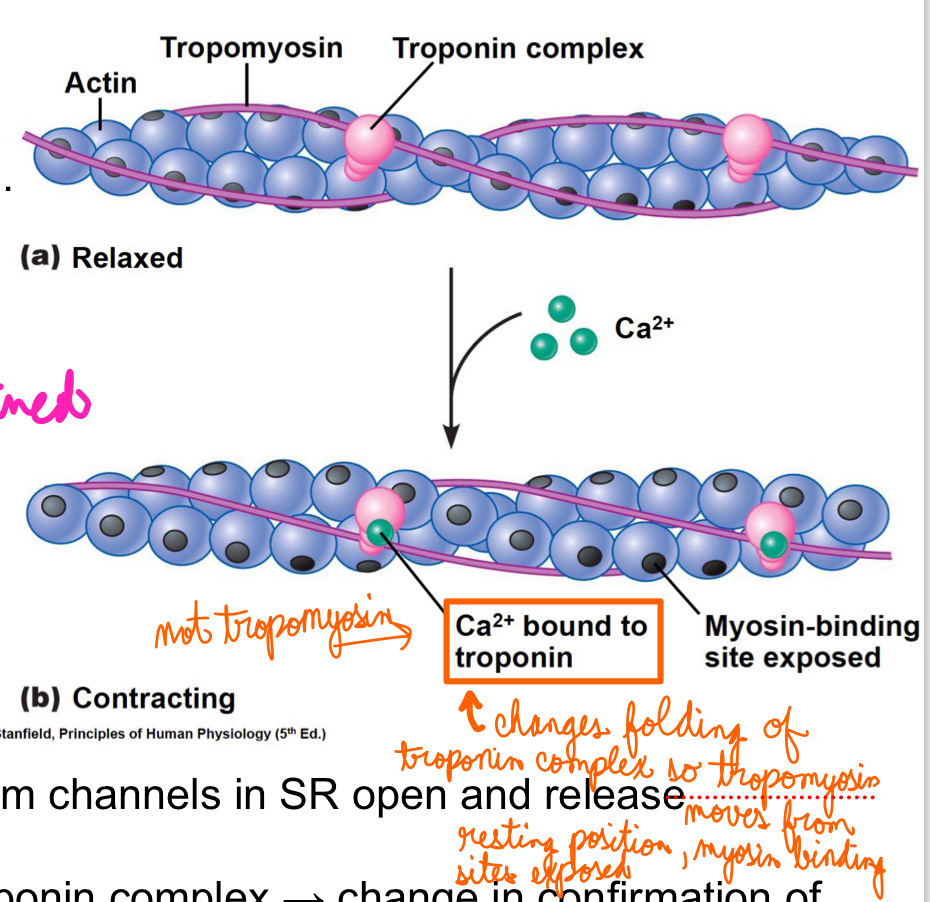
L16: Binding of Troponin to calcium
Binding of Troponin to calcium is a reversible reaction
Calcium voltage channels close when membrane potential in the SR is back to normal
Closure of calcium channels turns off release of calcium and enhances active transport back into SR → Calcium is cleared from the cytosol
the active transporters are present in the SR
Calcium dissociates from troponin → both troponin and tropomyosin revert to resting positions → Myosin binding site not exposed → decline in number of active crossbridges → eventually muscle contraction ends
L17: Purpose of Urinary (Renal) system
Waste management
Maintain blood composition
Maintain osmolarity of blood
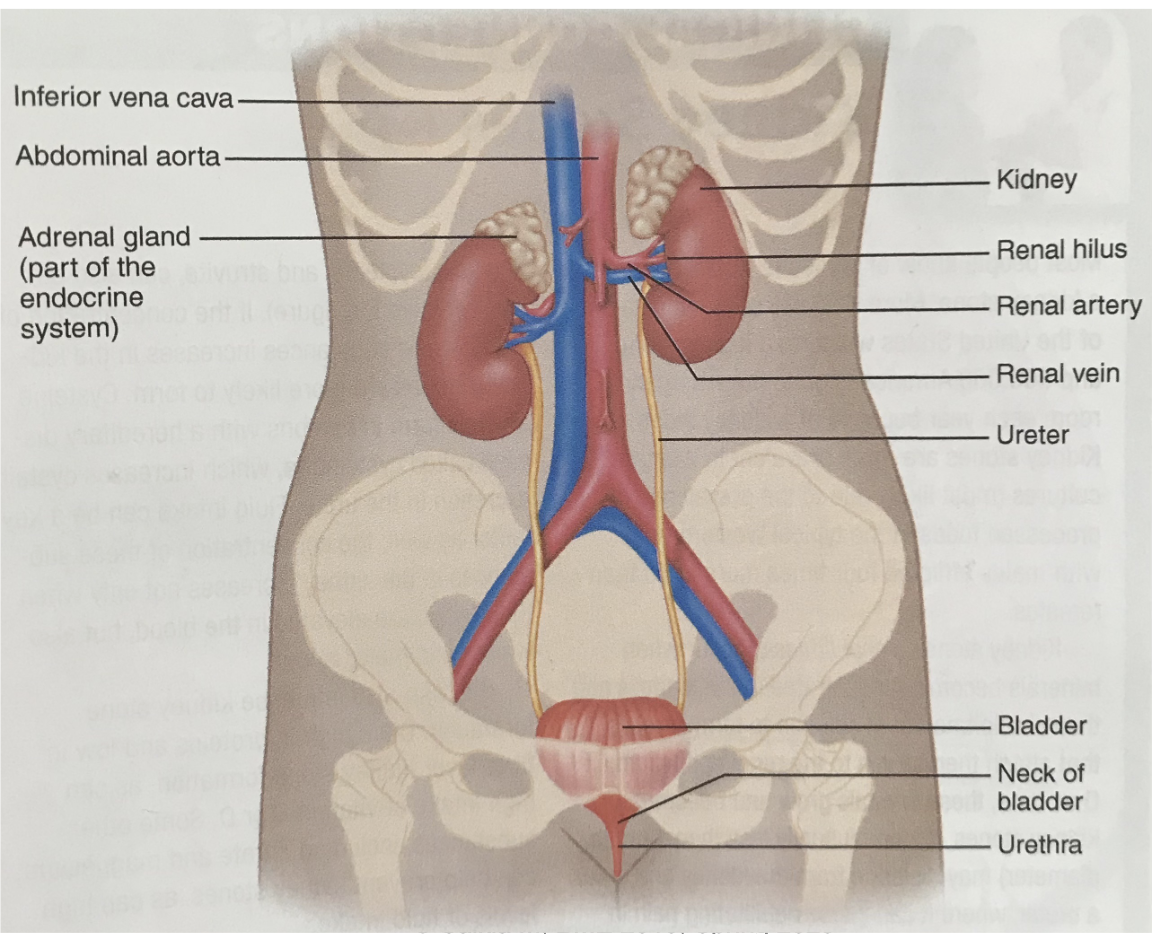
L17: Structure of the Urinary system (image)
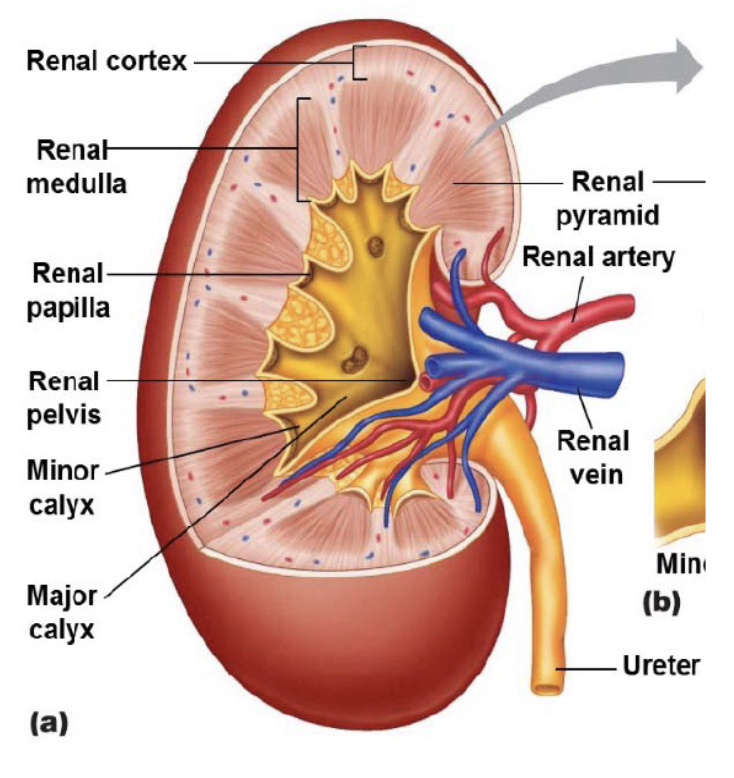
L17: Macroscopic anatomy of the kidney (image)
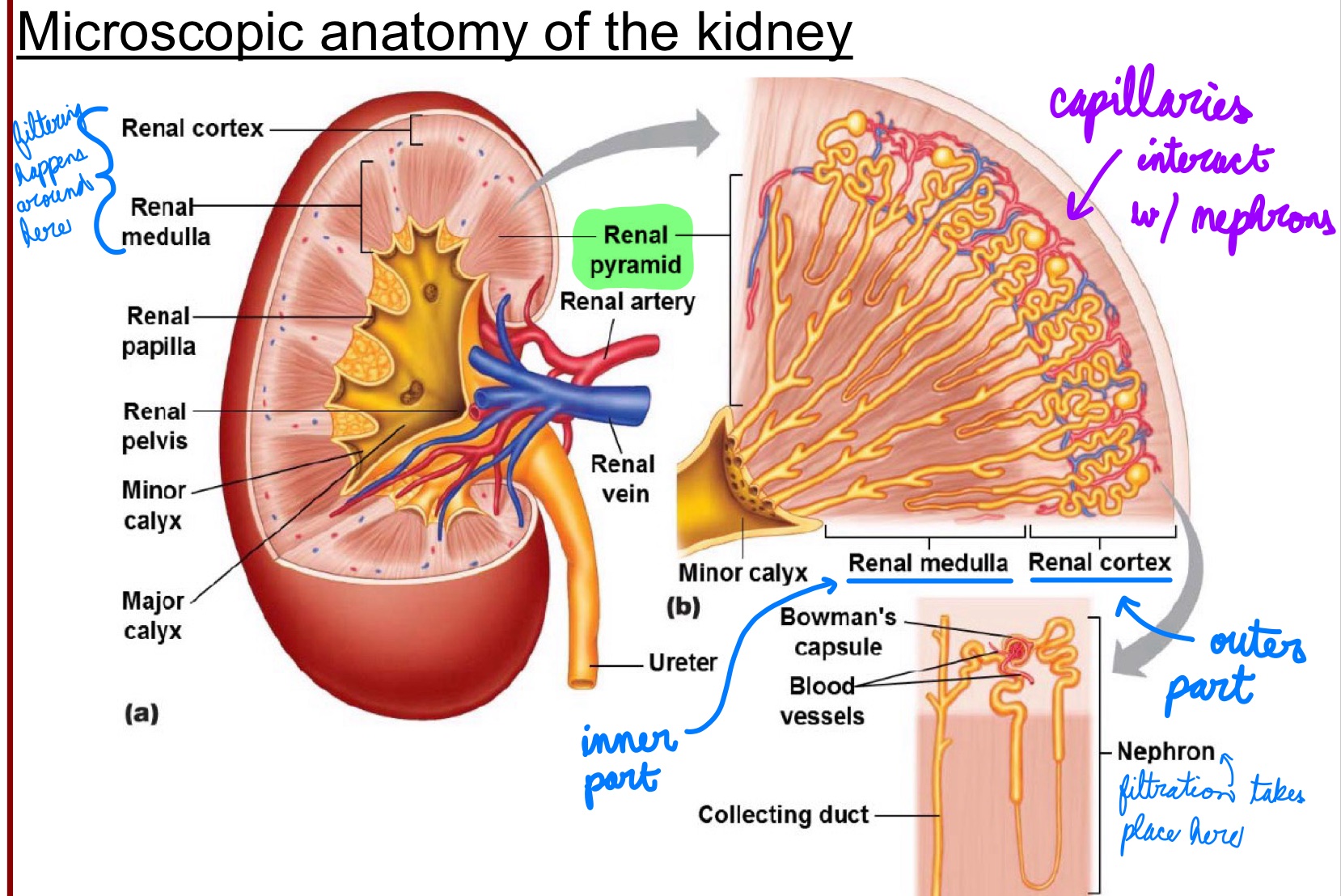
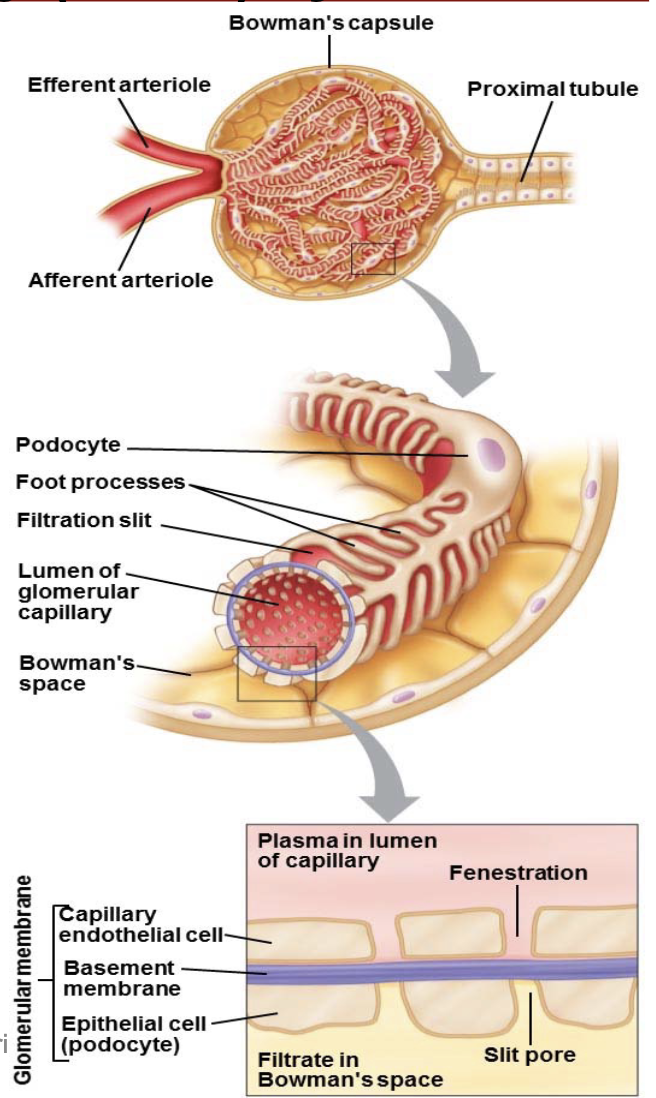
L17: Microscopic anatomy of the kidney
Capillaries interact with the nephrons
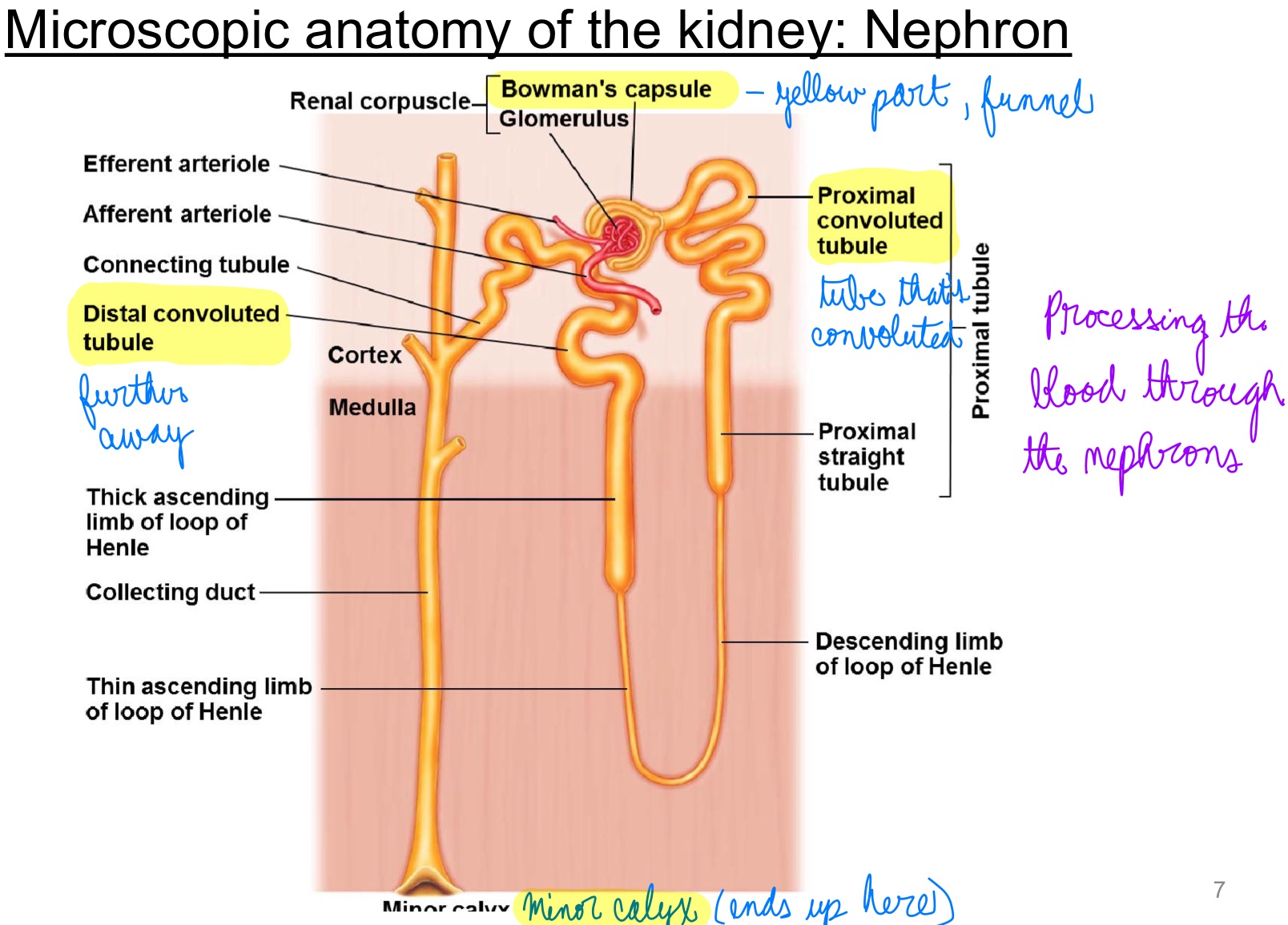
L17: Microscopic anatomy of the kidney: Nephron
the blood is processed through the nephrons
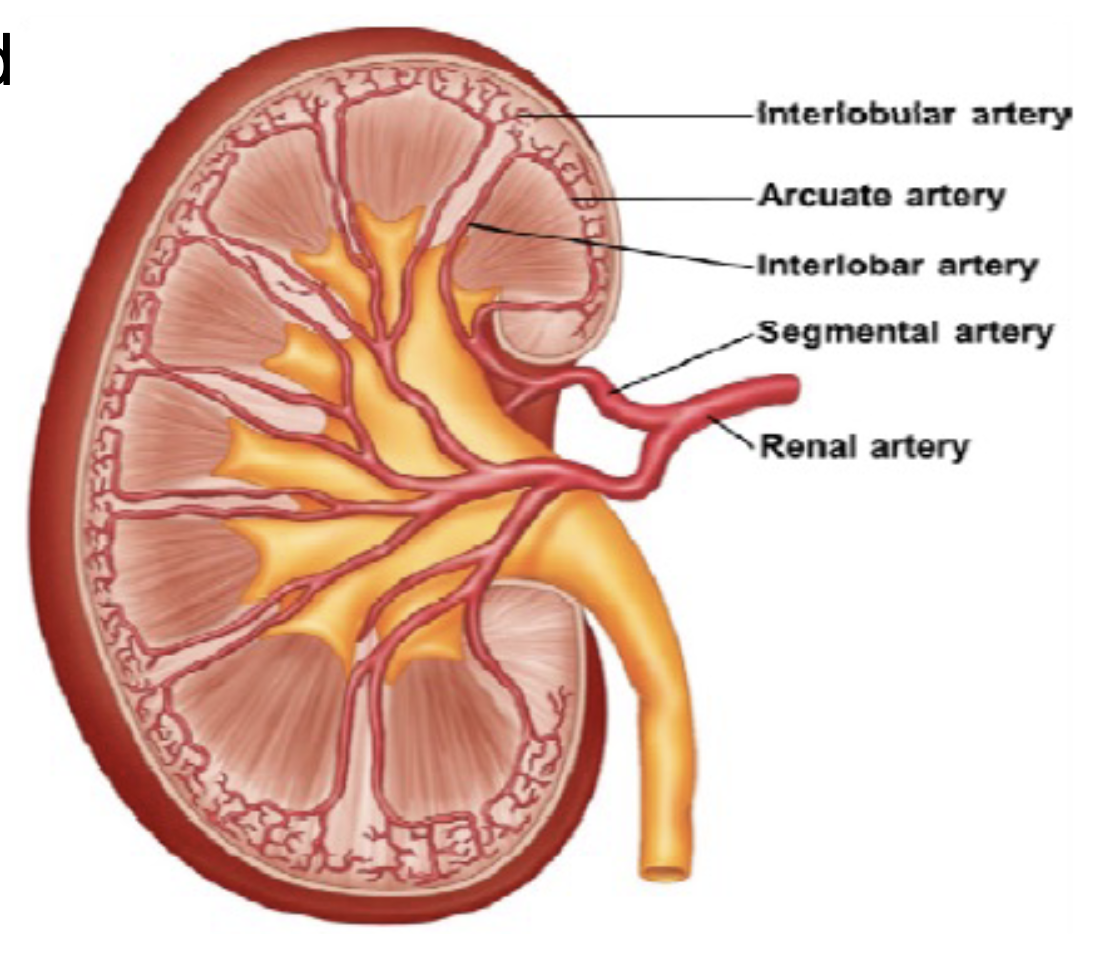
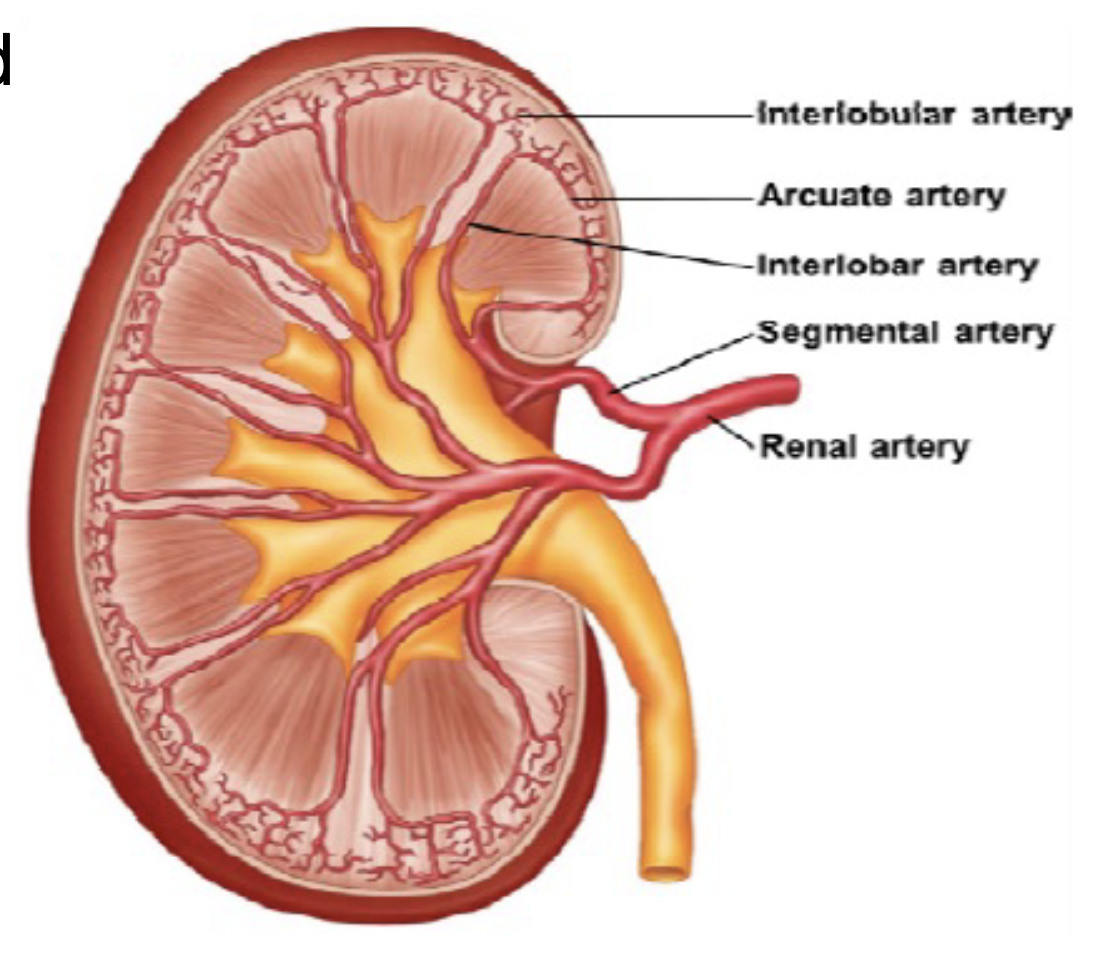
L17: Blood supply to the kidney
Renal arteries supply blood to kidneys
Within the kidney the renal artery branches into smaller arteries
*The renal arteries supply oxygenated blood
L17: Blood supply to kidney (memorize this pathway!)
Afferent arteriole → Glomerular Capillary bed → Efferent arteriole → Peritubular capillaries → Vasa Recta
L17: Blood supply to kidney (more in-depth)
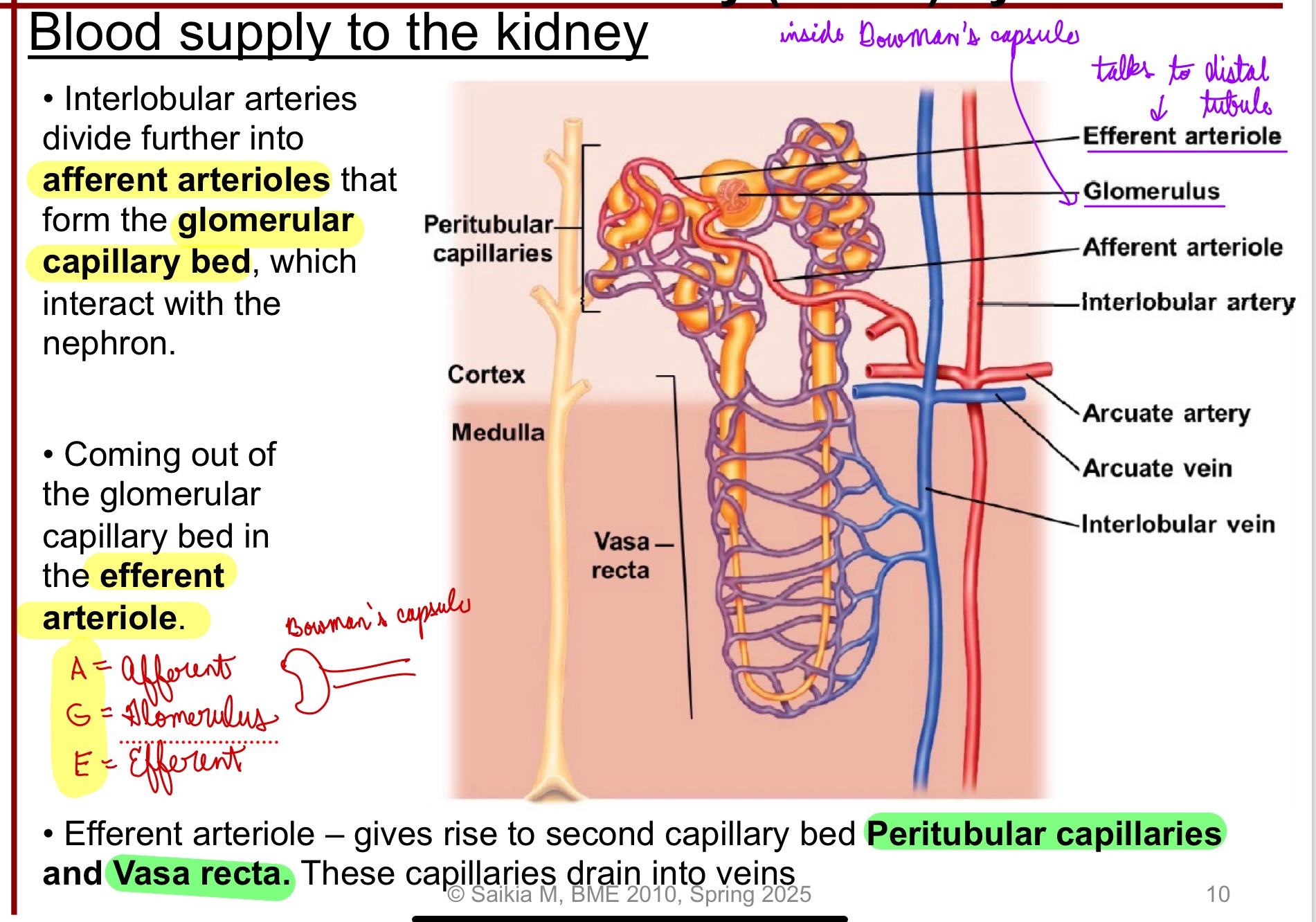
Interlobular arteries divide further into afferent arterioles that form the glomerular capillary bed, which interact with the nephron
Coming out of the glomerular capillary bed in the efferent arteriole
Efferent arteriole: gives rise to second capillary bed Peritubular capillaries and Vasa recta; these capillaries drain into veins
*Use acronym AGE:
A = Afferent
G = Glomerulus
E = Efferent
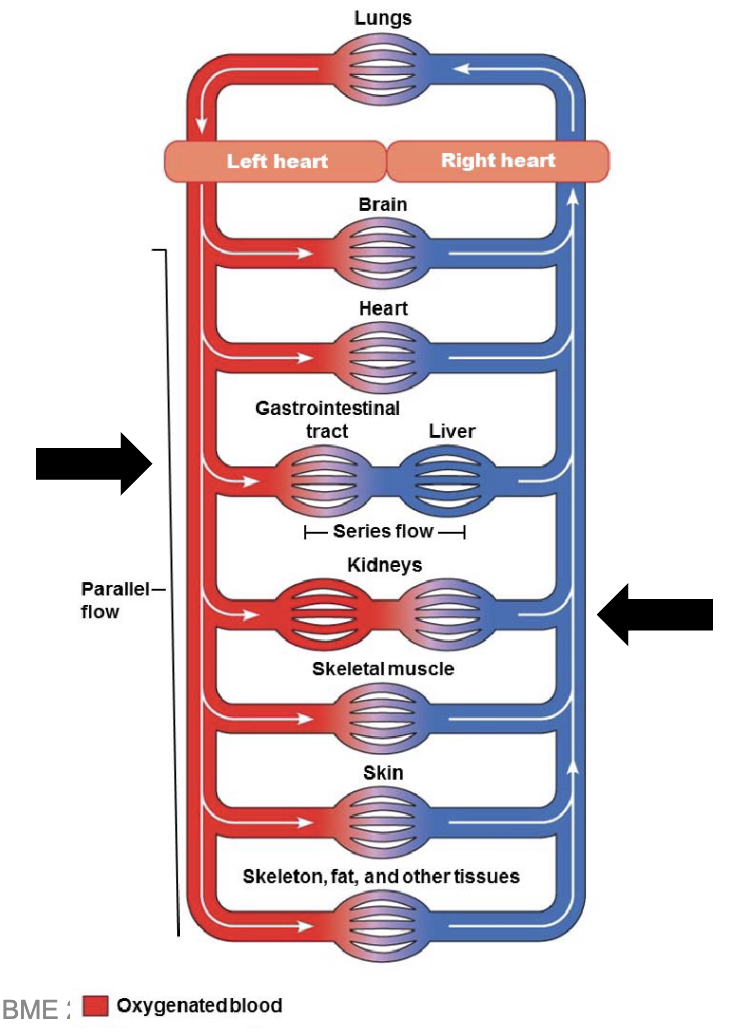
L17: Portal system
Portal system: two capillary beds joined in series
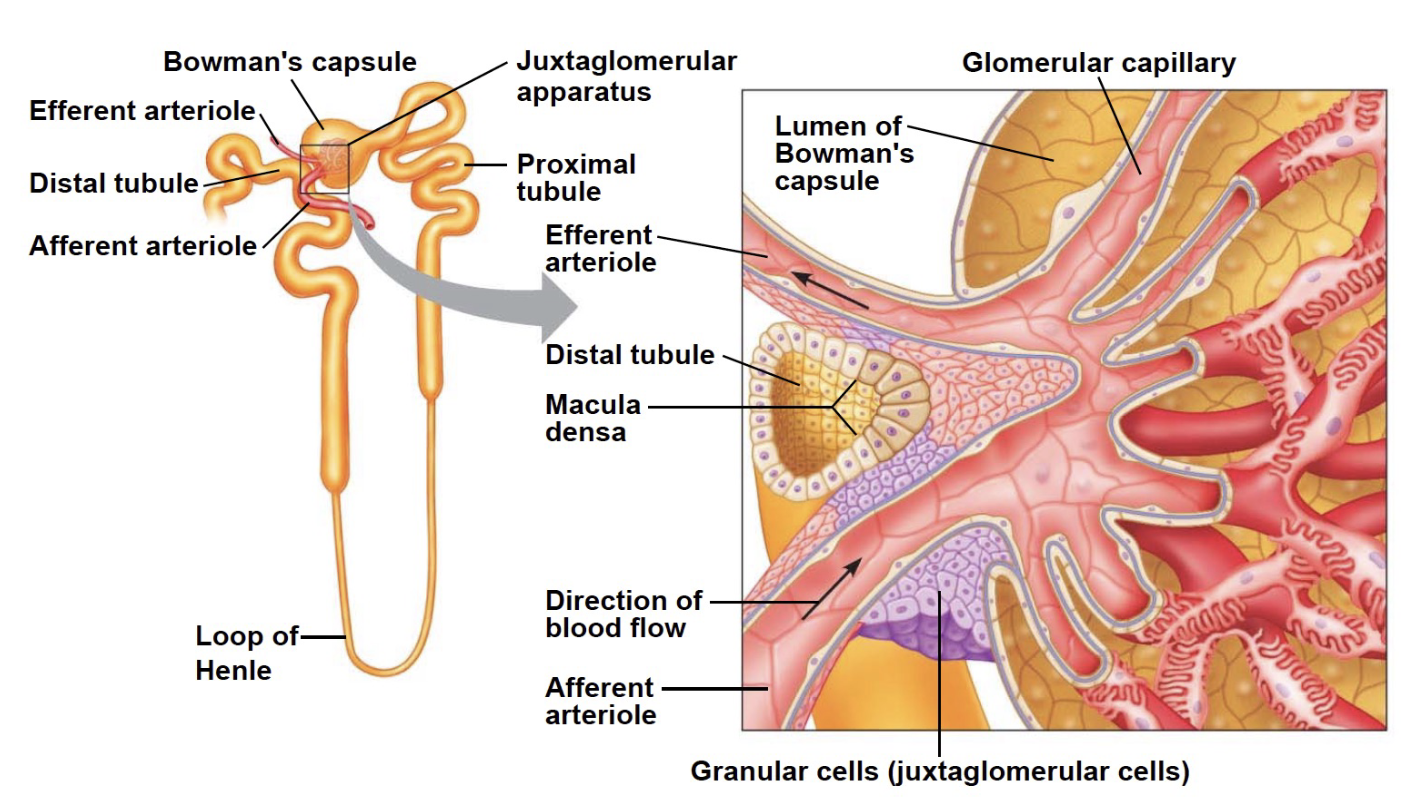
L17: Juxtaglomerular apparatus
Plays an important role in maintaining blood pressure and blood volume
Granular cells on the wall of the arteriole contain renin (an enzyme)
*The afferent arteriole looks bigger than the efferent arteriole
*In this case: in the nephron, most of interaction w/ blood happens in the bowman’s capsule
L17: Composition of blood
Plasma:
90% water
10% made up of ions, proteins (~7%), dissolved gases, nutrient molecules, and wastes
In the kidneys, water and solutes are exchanged between blood plasma and fluid in the renal tubules to regulate the composition of plasma
L17: Basic renal exchange process
3 exchange processes occur in the nephron:
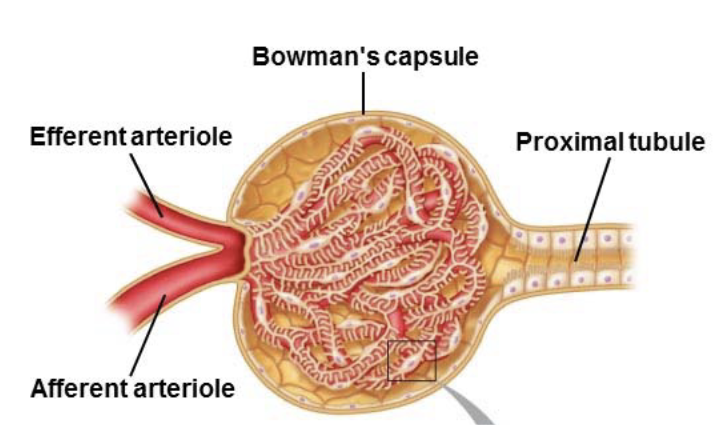
Glomerular filtration: Flow of protein free plasma from glomerular capillaries into Bowman’s capsule
Reabsorption: selective transport of molecules from renal tubules to peritubular capillaries, then returned to general circulation
Secretion: Selective transport of molecules from peritubular capillaries into renal tube
*Excretion: elimination of materials from tubules out of body
L17: Glomerular filtration
Glomerular Filtration is driven by Starling forces
Starling forces: forces that drive the movement of fluid in and out of capillaries
Determined by:
1. Hydrostatic forces (force blood exerts on its vessel wall)
2. Osmotic pressure gradients
Filtrate resembles plasma in composition except it lacks the cells and proteins found in plasma
L17: Glomerular filtration (continued)**
The sum of Starling forces in the renal corpuscle is called glomerular filtration pressure
4 forces play key role in glomerular filtration:
Glomerular capillary hydrostatic pressure (PGC): favors filtration and is equal to blood pressure of glomerular capillaries = 60 mm Hg
Hydrostatic pressure increases because efferent radius is smaller than afferent radius
Bowman’s capsule osmotic pressure (πBC): favors filtration; very little protein in the filtrate hence osmotic pressure is negligible under normal conditions (πBC) = 0 mm Hg
high protein concentration pulls plasma towards it, but there’s no protein → would have favored, but doesn’t exist
Bowman’s capsule hydrostatic pressure (PBC): Opposes filtration = 15 mm Hg
Glomerular osmotic pressure (πBC): Opposes filtration; presence of proteins in the plasma tends to draw filtrate back; Approximately 29 mm Hg
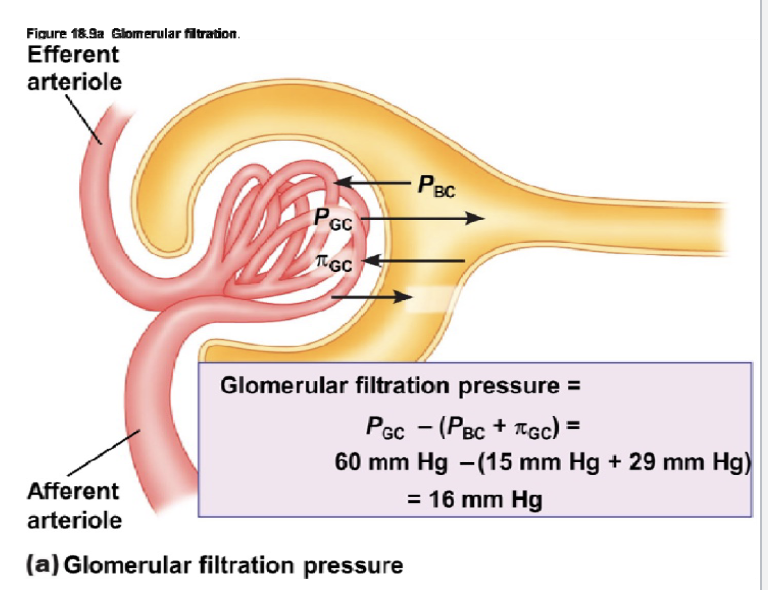
L17: Recap of Glomerular filtration pressures
Glomerular capillary hydrostatic pressure (PGC) = favors filtration, 60 mm Hg
Bowman’s capsule osmotic pressure (πBC): favors filtration = 0 mm Hg
Bowman’s capsule hydrostatic pressure (PBC): Opposes filtration = 15 mm Hg
Glomerular osmotic pressure (πBC): Opposes filtration = 29 mm Hg
Net number = 60 - (15 + 29) = 16 mm Hg
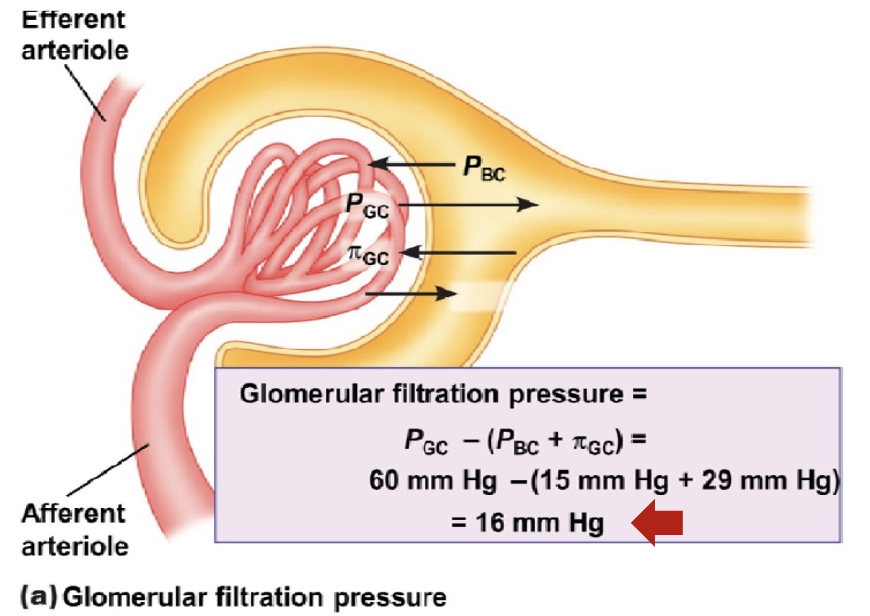
L17: Glomerular filtration (wall of bowman capsule and renal tubule)
wall of bowman capsule and renal tubule are made up of epithelial cells
In the Bowman’s capsule this epithelium folds on itself to envelope the glomerular capillaries
Glomerular filtrate must cross 3 barriers:
1. Capillary endothelial cell
2. Basement membrane
3. Bowman epithelial cell
Together this is the glomerular membrane or filtration barrier
Structure favorable for bulk flow due to presence of slit pore and fenestration
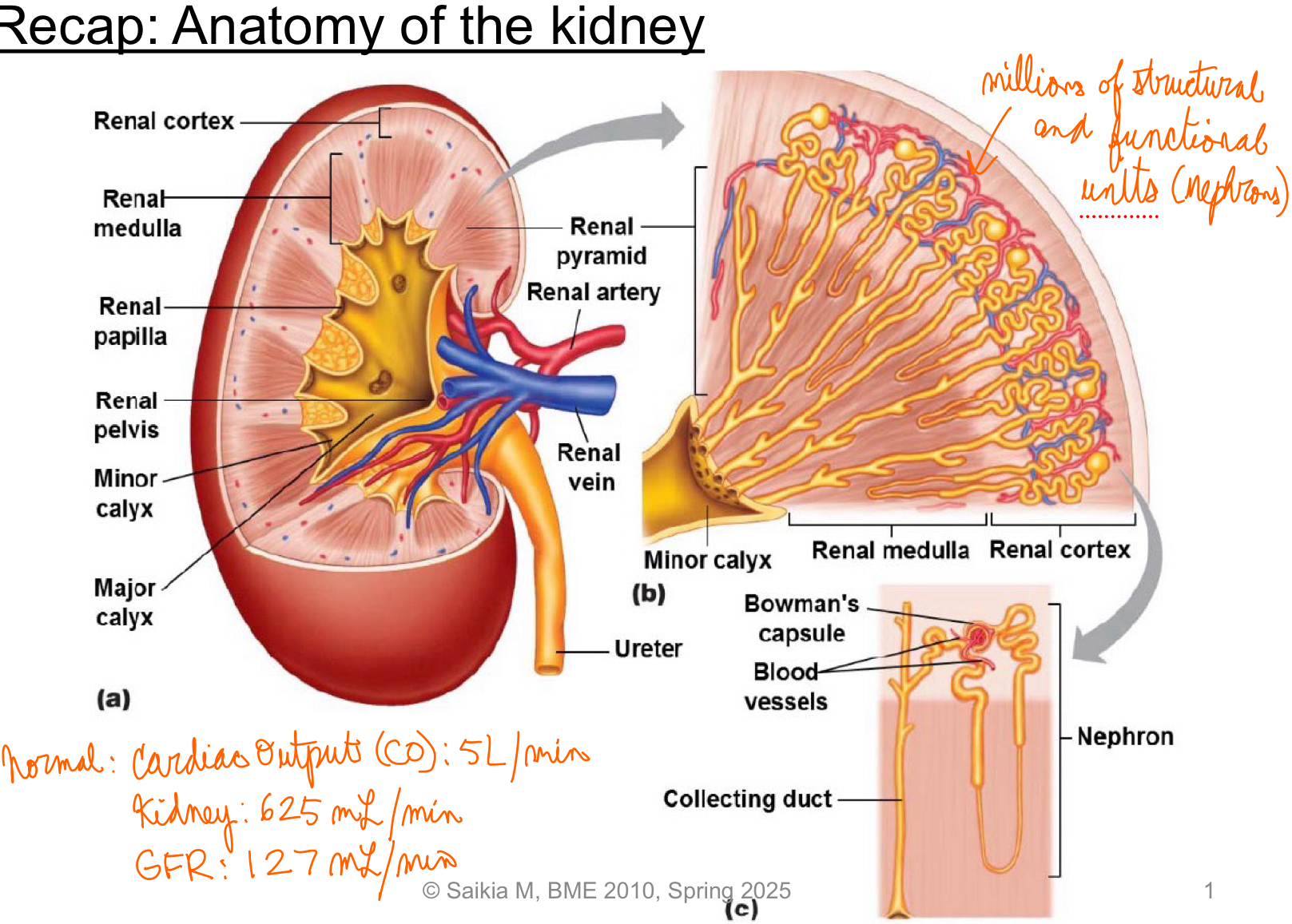
L18: Recap of anatomy of the kidney
L18: Glomerular filtration rate, filtration fraction, filtered load of a solute
Glomerular filtration rate:
Under normal conditions,
Renal plasma flow = 625 mL/min (plasma flowing through kidney/min)
Volume of plasma filtered per unit time, glomerular filtration rate (GFR) = 125 mL/min = 180 L/day!
Filtration fraction
Fraction of renal plasma volume that is filtered is the filtration fraction
Filtration fraction = GFR/renal plasma flow = 125mLmin-1/625 mLmin-1 = 0.2 = 20%
20% of the plasma that flows into the kidney is filitered into the Bowman’s capsule
Filtered load of a solute
quantity of a particular solute filtered per unit time
Filtered load = GFR x Px
Where PX is the plasma concentration of X
L18: Regulation of GFR and mechanism
Regulation of GFR
Changes in GFR are undesirable = change urine flow = interferes with kidneys’ ability to regulate plasma volume and composition
Mechanisms of GFR regulation:
Myogenic regulation of GFR
Tubuloglomerular feedback
L18: Myogenic regulation of GFR
When Mean arterial pressure (MAP) increases pressure in afferent arteriole increases causing its walls to stretch
Smooth muscle in wall of afferent arteriole contracts in response to stretch
When these walls contraction, they increase in resistance reducing blood flow
Reduced blood flow in afferent arteriole reduces the glomerular capillary pressure
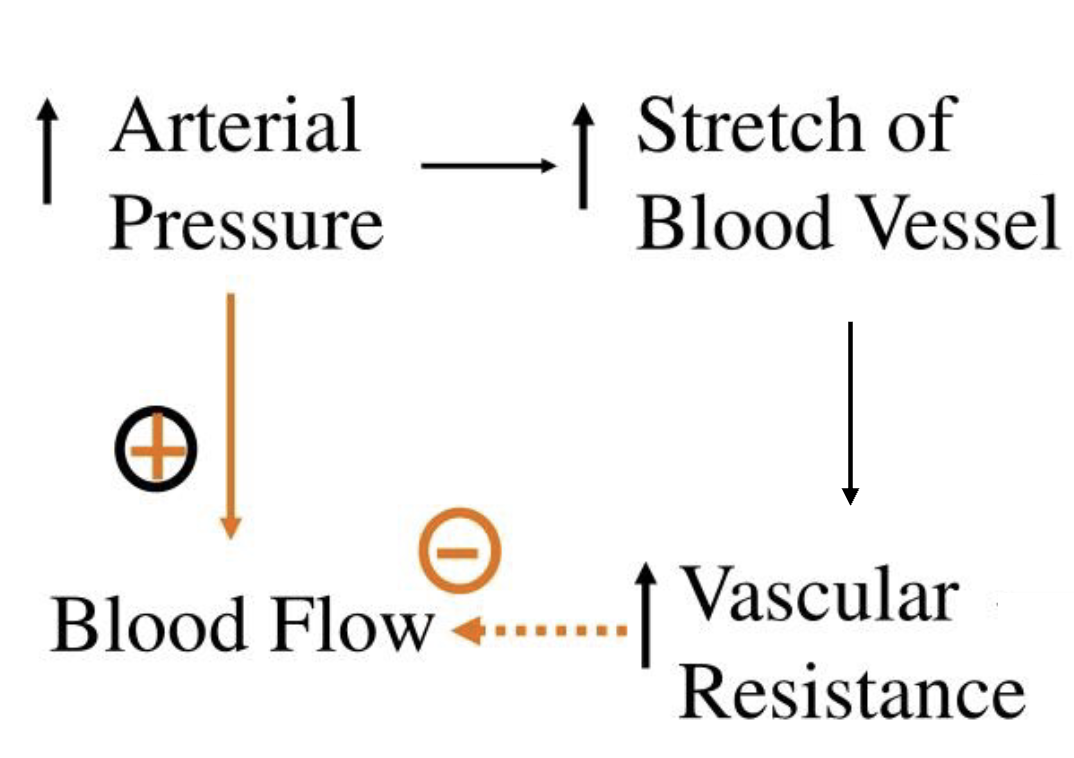
L18: Tubuloglomerular feedback
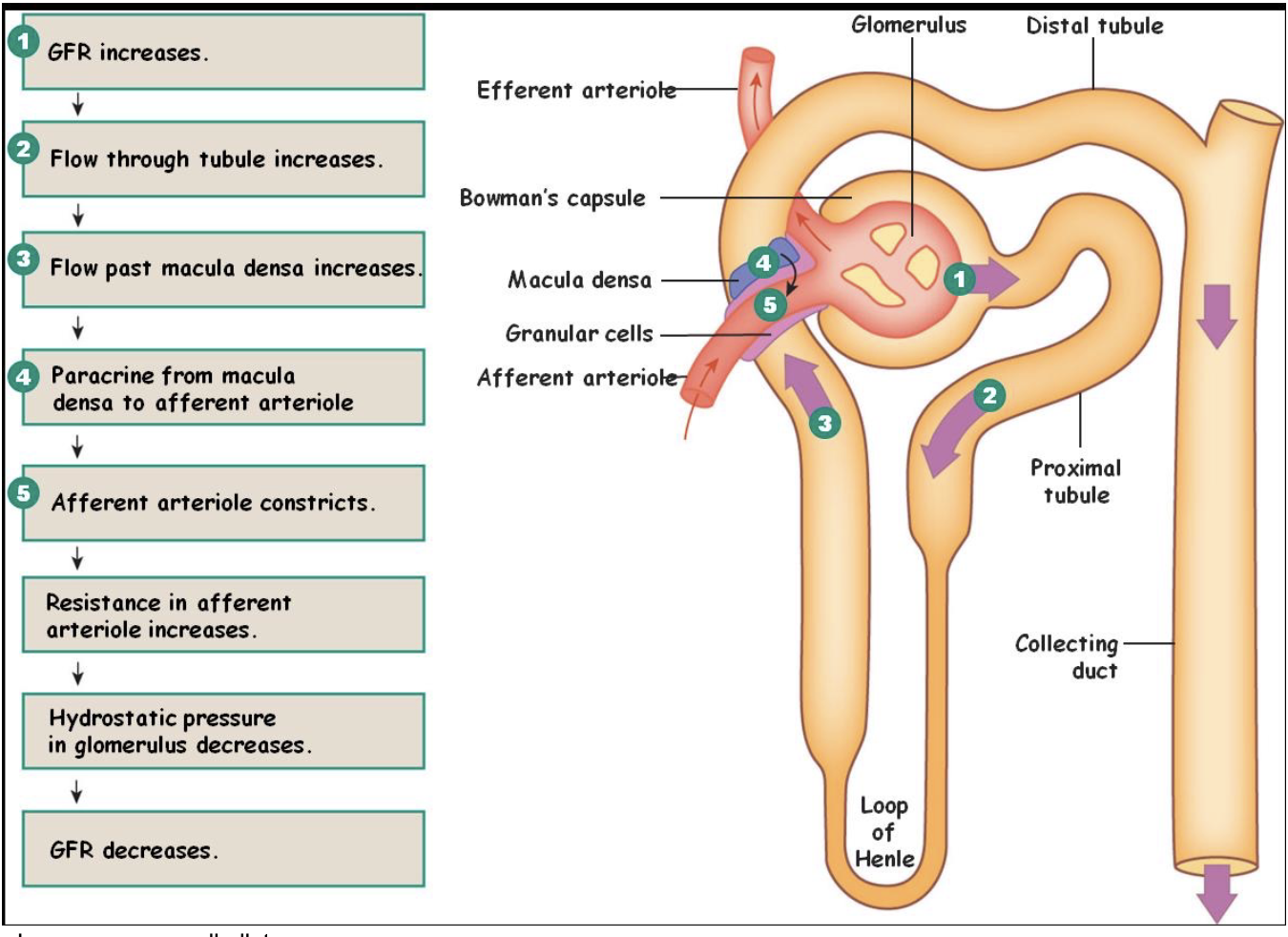
L18: Reabsorption
(refer to previous diagrams too)
Movement of solutes and water from renal tubule into blood plasma
Some solutes are reabsorbed completely
Others are regulated to vary their excretion rate
Many solutes are reabsorbed actively
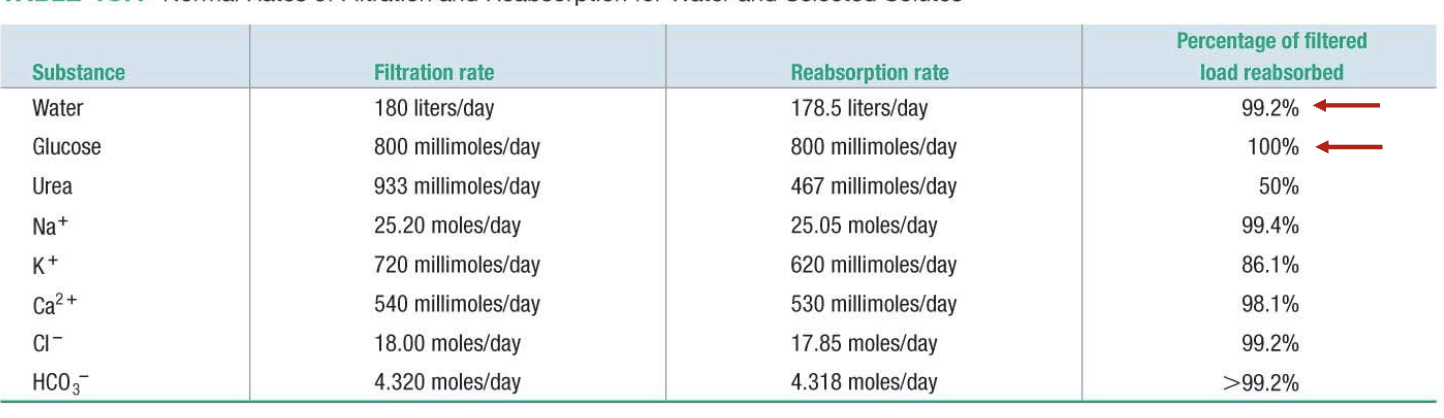
L18: Reabsorpotion (continued)
Sending things back into circulation
Solute reabsorption mostly occurs in proximal and distal convoluted tubules
Peritubular space: space between peritubular capillary and renal tubule - filled with interstitial fluid
Barrier for reabsorption
1. Tubule epithelial (primary barrier)
2. Capillary endothelial cell (barrier only to proteins, cells)
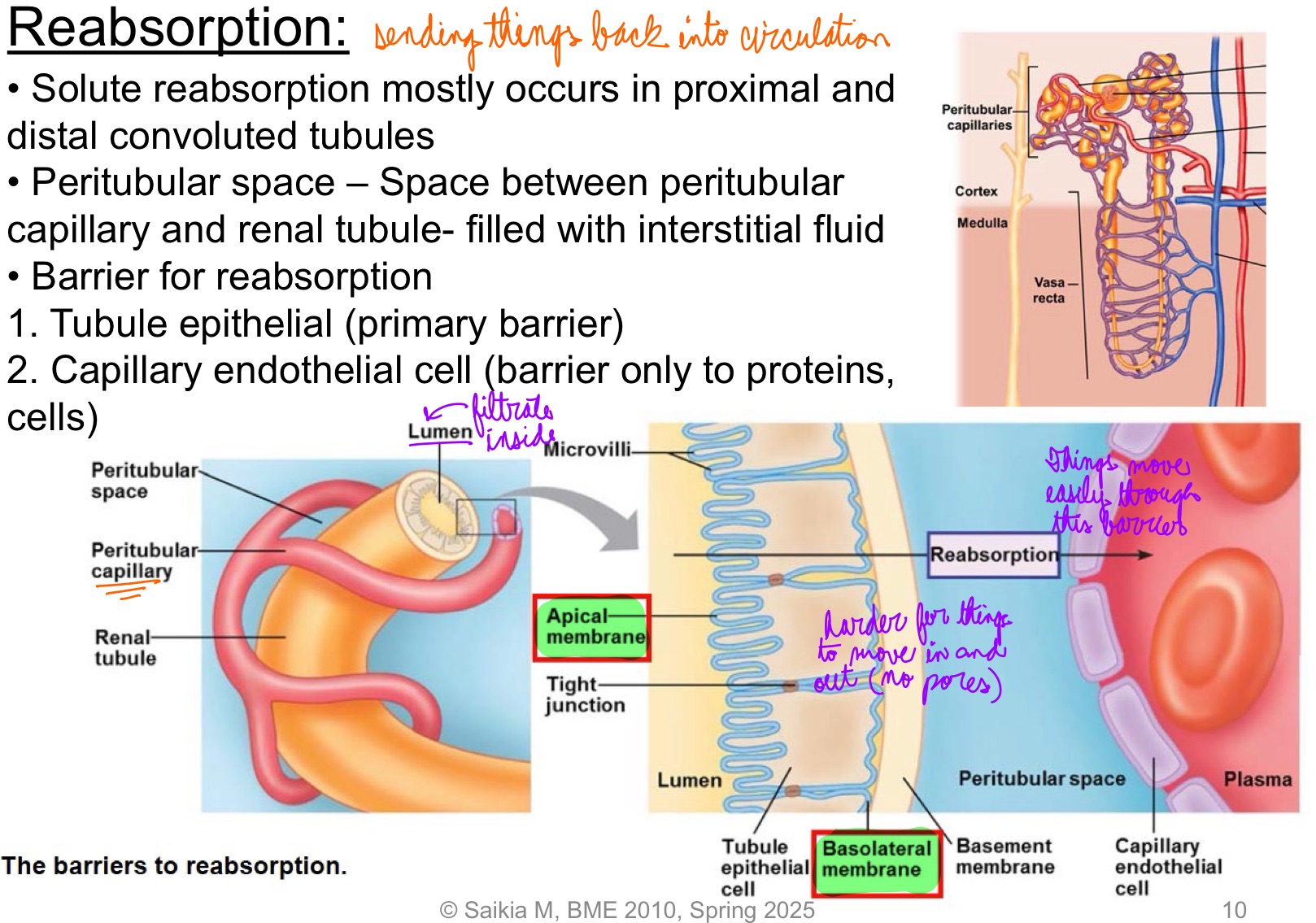
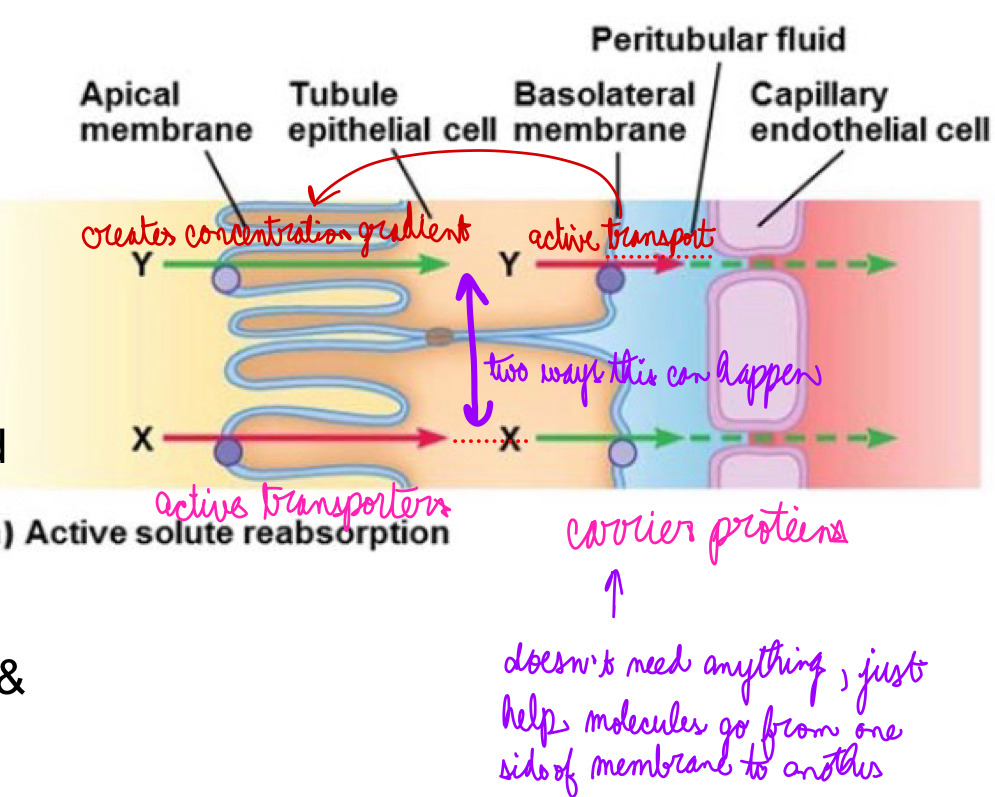
L18: Mechanism of solute reabsorption
Active reabsorption of solute
Both X and Y are actively transported. But different mechanisms
1. Active transporter for X located on apical membrane → X gets actively transported into the cell → high intracellular concentration of X → moves into peritubular space by facilitated diffusion & then diffuses into plasma
2. Active transporter for Y located on basolateral membrane → Y gets actively transported into peritubular space → diffuses into plasma; lower intracellular concentration of Y → facilitated diffusion of Y into cell
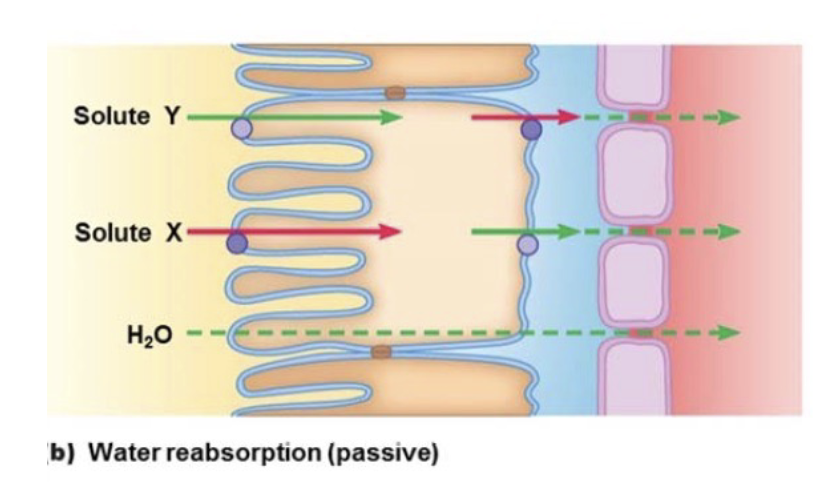
L18: Mechanism of water reabsorption
Based on osmolarity
As X and Y get reabsorbed into plasma → increase in osmolarity of plasma → water diffuses down its concentration gradient into region of high osmolarity
Passive
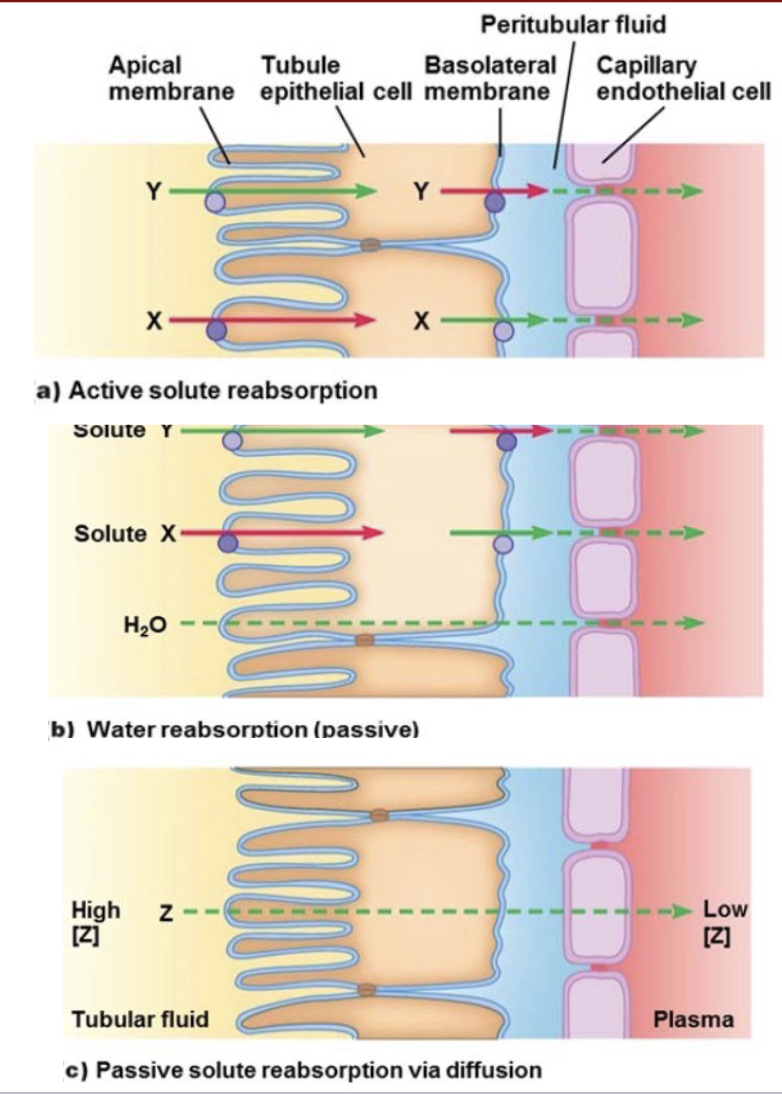
L18: Mechanism of solute and water reabsorption: Passive reabsorption
Z is passive reabsorbed
Two conditions must be met
[Z] must be greater in tubular fluid
Z must be able to permeate tubular and capillary membrane
When most of H2O is reabsorbed, [Z] ↑ in tubular fluid whereas [Z] ↓ in plasma (Remember tubular fluid came from plasma)
![<ul><li><p>Z is passive reabsorbed</p></li><li><p>Two conditions must be met</p></li></ul><ol><li><p>[Z] must be greater in tubular fluid</p></li><li><p>Z must be able to permeate tubular and capillary membrane</p></li></ol><ul><li><p>When most of H<sub>2</sub>O is reabsorbed, [Z] <strong>↑</strong> in tubular fluid whereas [Z] <span>↓ in plasma (Remember tubular fluid came from plasma)</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/8c7df3ce-3d23-4778-bf30-f6cf7b02b5d3.png)
L18: Recap: Active solute reabsorption, water reabsorption (passive), and Passive solute reabsorption via diffusion comparison
L18: Transport maximum
When solutes rae transported from filtrate to plasma, the carrier proteins and pumps can get saturated
When solute concentration is high enough, all carrier porteins and pumps are occupied, and the system is operating at transport maximum
Probably not the same as renal threshold which is the specific blood concentration of a substance (like glucose) above which the kidneys start excreting it into the urine?
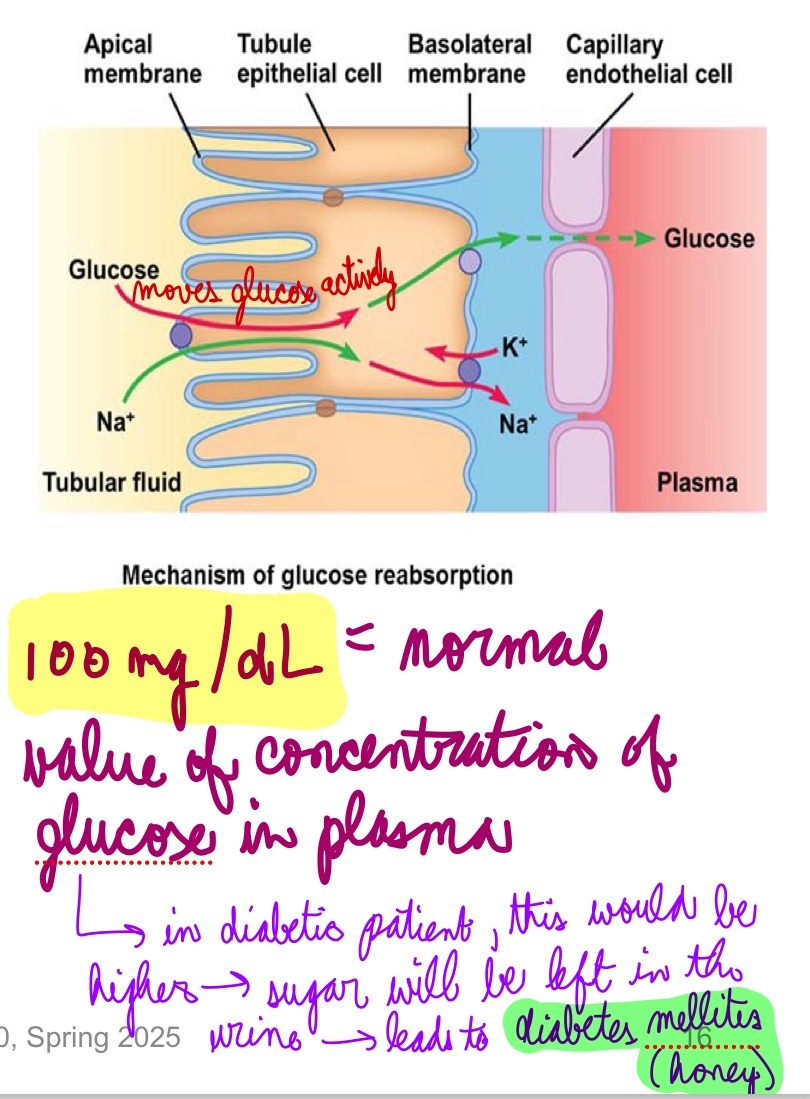
L18: Transport maximum: Relevance in Glucose reabsorption
Glucose if freely filtered at glomerulus
100% actively reabsorbed in proximal tubules
Normally no glucose appears in urine
If plasma concentration of glucose increases → filtrate concentration high→ pumps and carriers get saturated → glucose spillover into urine
1
100 mg/dL = normal value of concentration of glucose in plasma
L18: Diabetes Mellitus and Nephropathy
In Diabetes Mellitus [Glucose] in plasma is elevated (hyperglycemia) → glucose appears in the urine
Affects water reabsorption into plasma
Causes thirst and excessive urination in patients
20-30% patients of Diabetes Mellitus also develop Diabetic nephropathy
High [Glucose] damage the nephrons
L19: Secretion
Solute moves from peritubular capillaries into renal tubule
Barriers are same as reabsorption
Transport mechanisms are the same, but in the opposite direction
Secreted substances (examples)
Potassium
Hydrogen ions
Choline
Creatinine
Penicillin
Secretion increases [solute] in urine and decreases [solute] in plasma
![<ul><li><p>Solute moves from peritubular capillaries into renal tubule</p></li><li><p>Barriers are same as reabsorption</p></li><li><p>Transport mechanisms are the same, but in the opposite direction</p></li><li><p>Secreted substances (examples)</p><ul><li><p>Potassium</p></li><li><p>Hydrogen ions</p></li><li><p>Choline</p></li><li><p>Creatinine</p></li><li><p>Penicillin</p></li></ul></li><li><p>Secretion increases [solute] in urine and decreases [solute] in plasma</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3a7b9216-b077-4f99-9403-3365ab1d76d9.jpg)
L19: Regional specialization of the renal tubules
tubule epithelium varies from region to region → substance transported, and mechanism of transport differ
Proximal tubule
Non-regulated reabsorption in the proximal tubules
Highly folded apical membrane = large surface
Cells possess large no. of mitochondria = large ATP supply
Tight junctions in the epithelia are leaky
Distal tubules and collecting ducts
Regulated reabsorption and secretion in the distal tubules and collecting ducts
tight epithelium
Cells have receptors for hormones that regulate absorption
reabsorption/secretion only happen when needed
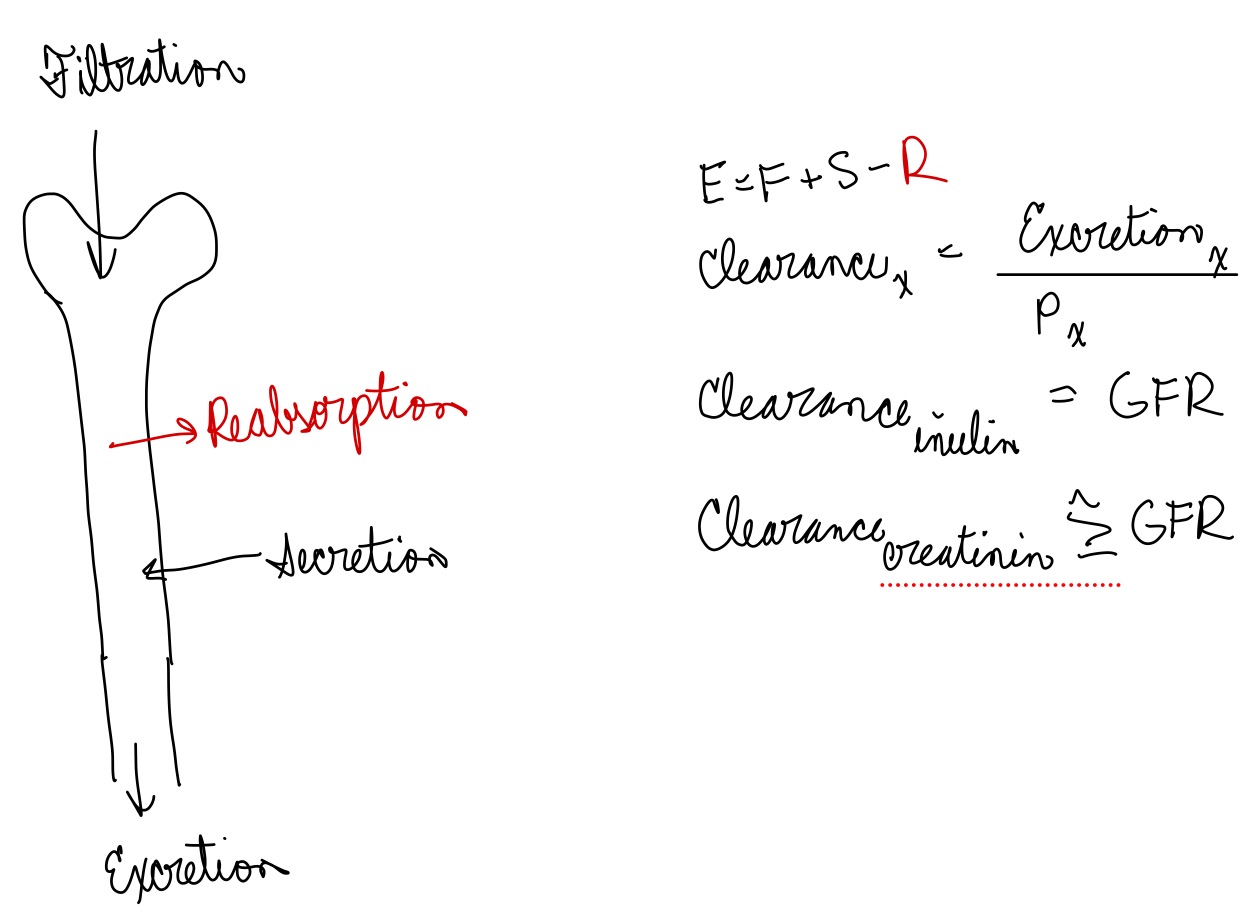
L19: Excretion rate of a solute
When filtrate is excreted = urine
Materials that enter the lumen of the renal tubules is excreted unless it is reabsorbed
**Amount excreted = Amount filtered + amount secreted - amount reabsorbed
E = F + S - R
Excretion depends on 3 factors:
Filtered load (F)
Rate of solute secretion (S)
Rate of reabsorption (R)
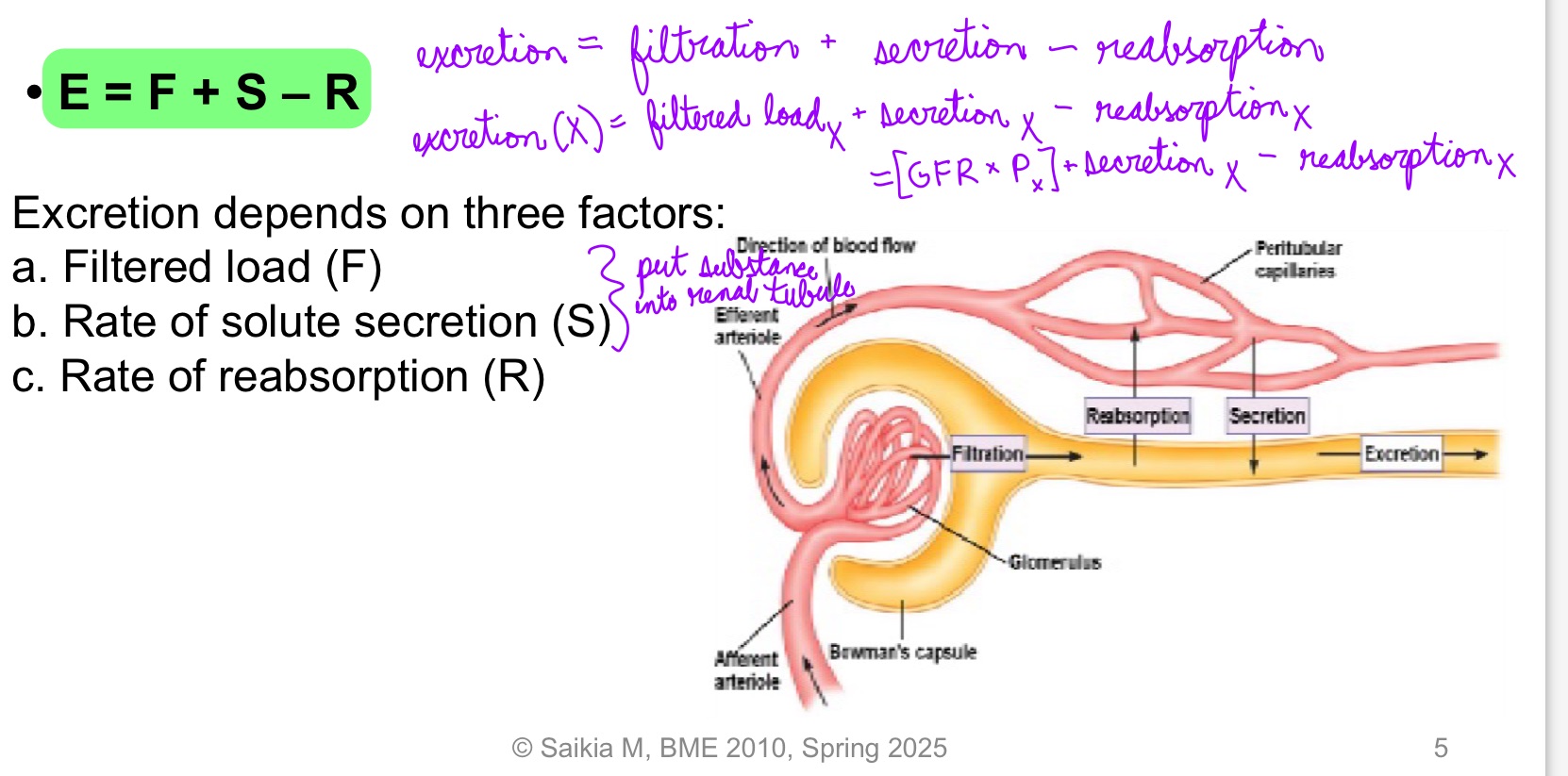
L19: Renal processing of a hypothetical solute and water (image)** pay extra attention
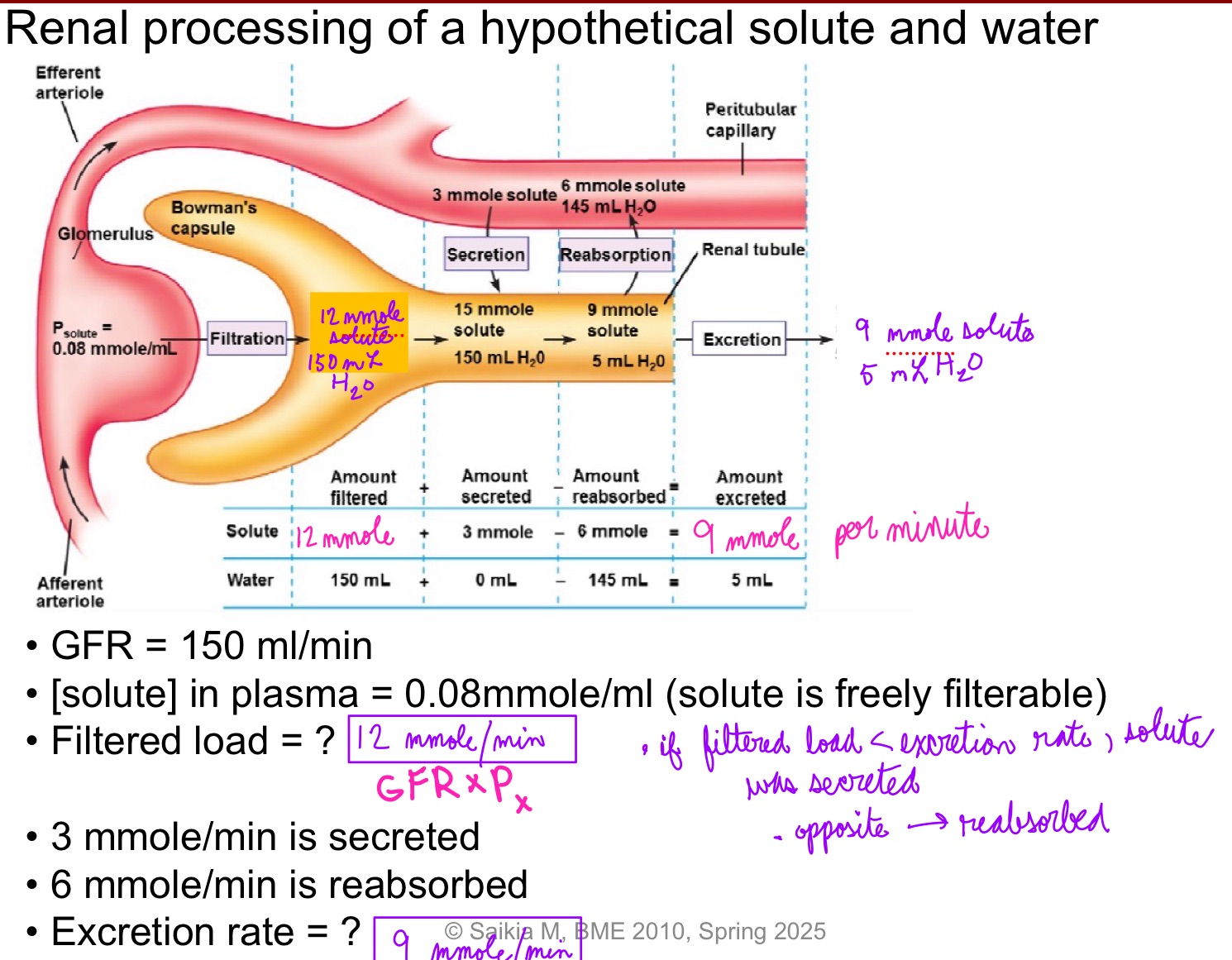
L19: Renal processing of solute
Comparing filtered load with amount of solute excreted/min → net effect of renal processing of that solute
If amount of solute excreted per minute is less than filtered load = net reabsorption of the solute occurred
If amount of solute excreted per minute is greater than filtered load = net secretion of the solute occurred
Only net effect can be determined. Cannot decouple reabsorption and secretion
Can only tell which is greater - secretion or reabsorption
L19: Clearance (of a solute)
The volume of plasma from which a substance is completely removed (cleared) by kidneys per unit tiem
Clearance depends rate of excretion of the solute and the concentration of solute in plasma
Clearance = Excretion rate/Plasma concentration
*Plasma concentration is concentration of X
L19: Clearance (calculations)
Excretion rate = 9 mmol/min = 540 mmol/hr
Px = 0.08 mmol/mL = 80 mmol/L
Clearance = 540 mmolhr-1 /80 mmolL-1= 6.75 Lhr-1
6.75 L is the hypothetical volume of plasma that is cleared of the solute in an hour
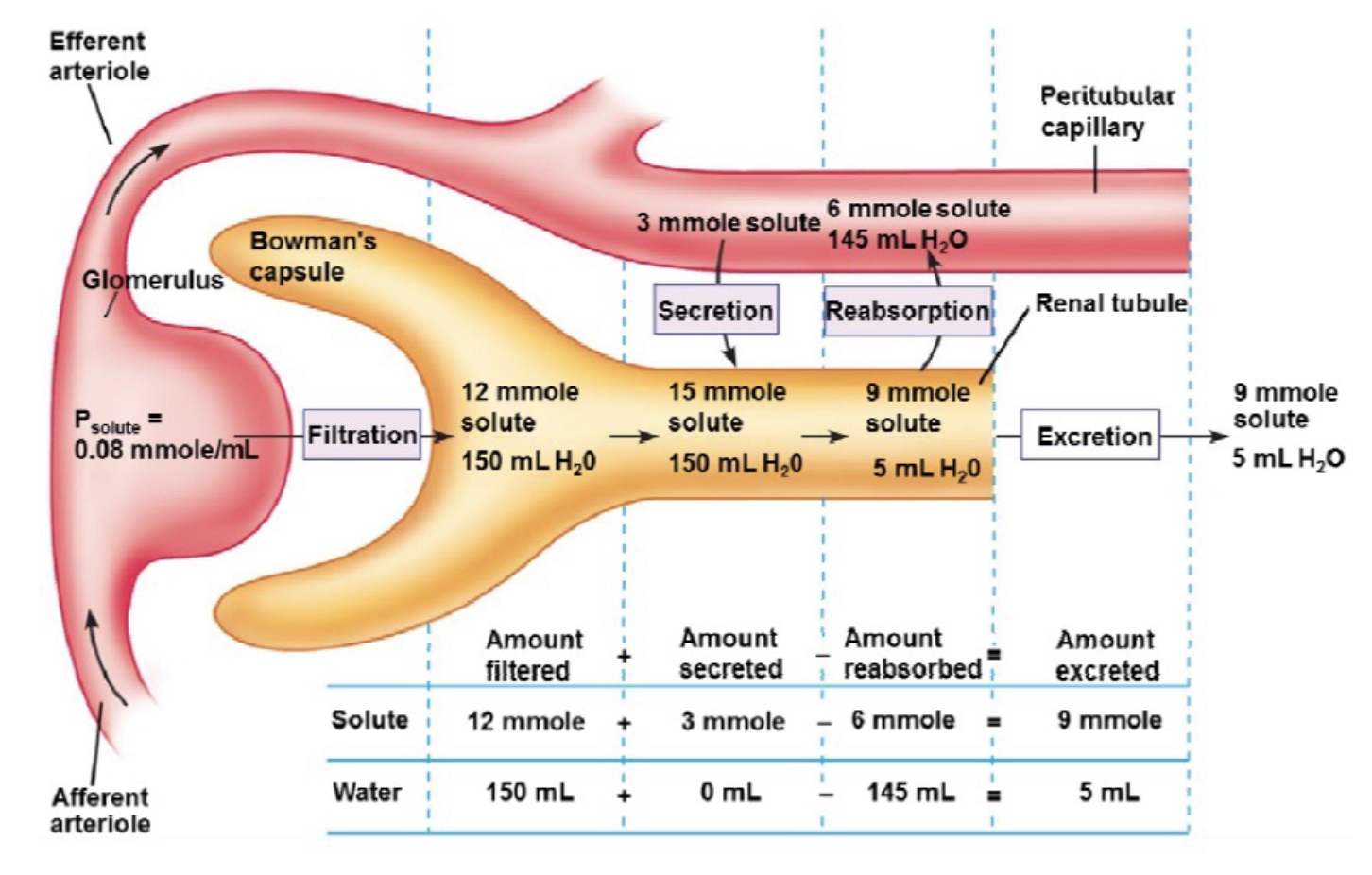
L19: Clinical use of clearance
Clearance = Excretion rate / Plasma concentration
Product of urinary concentration of a solute x, (Ux) and urine flow rate (V) gives the Excretion rate = Ux x V
Px is plasma concentration
Clearance = (Ux x V)/Px
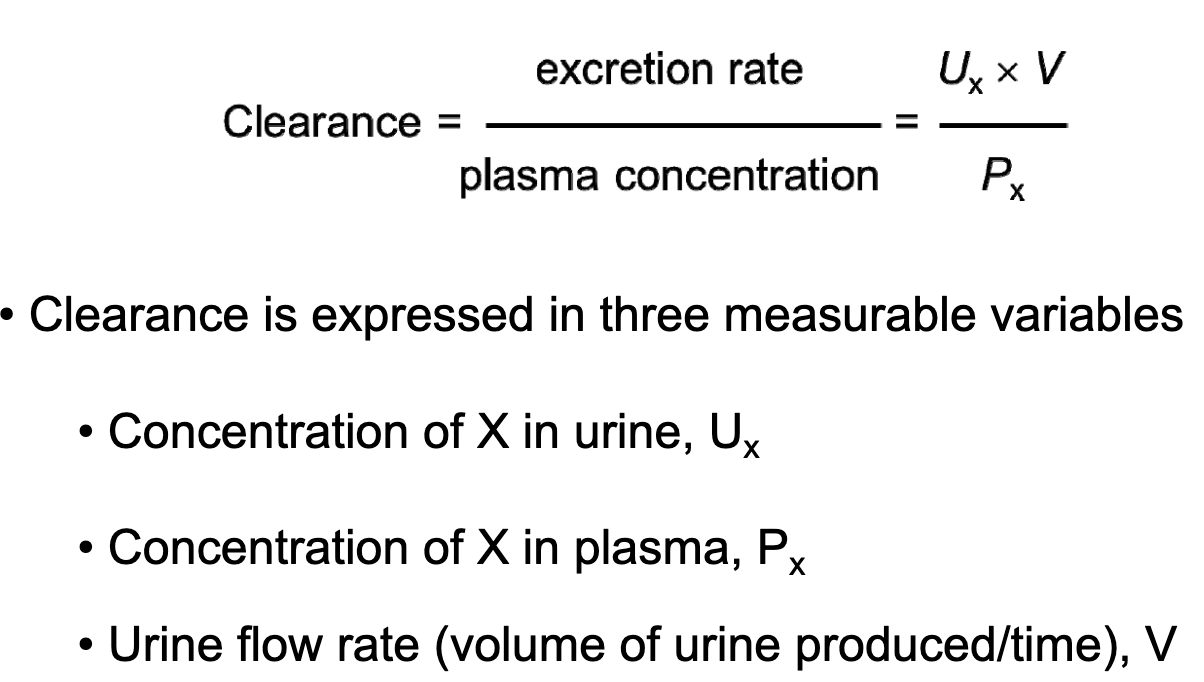
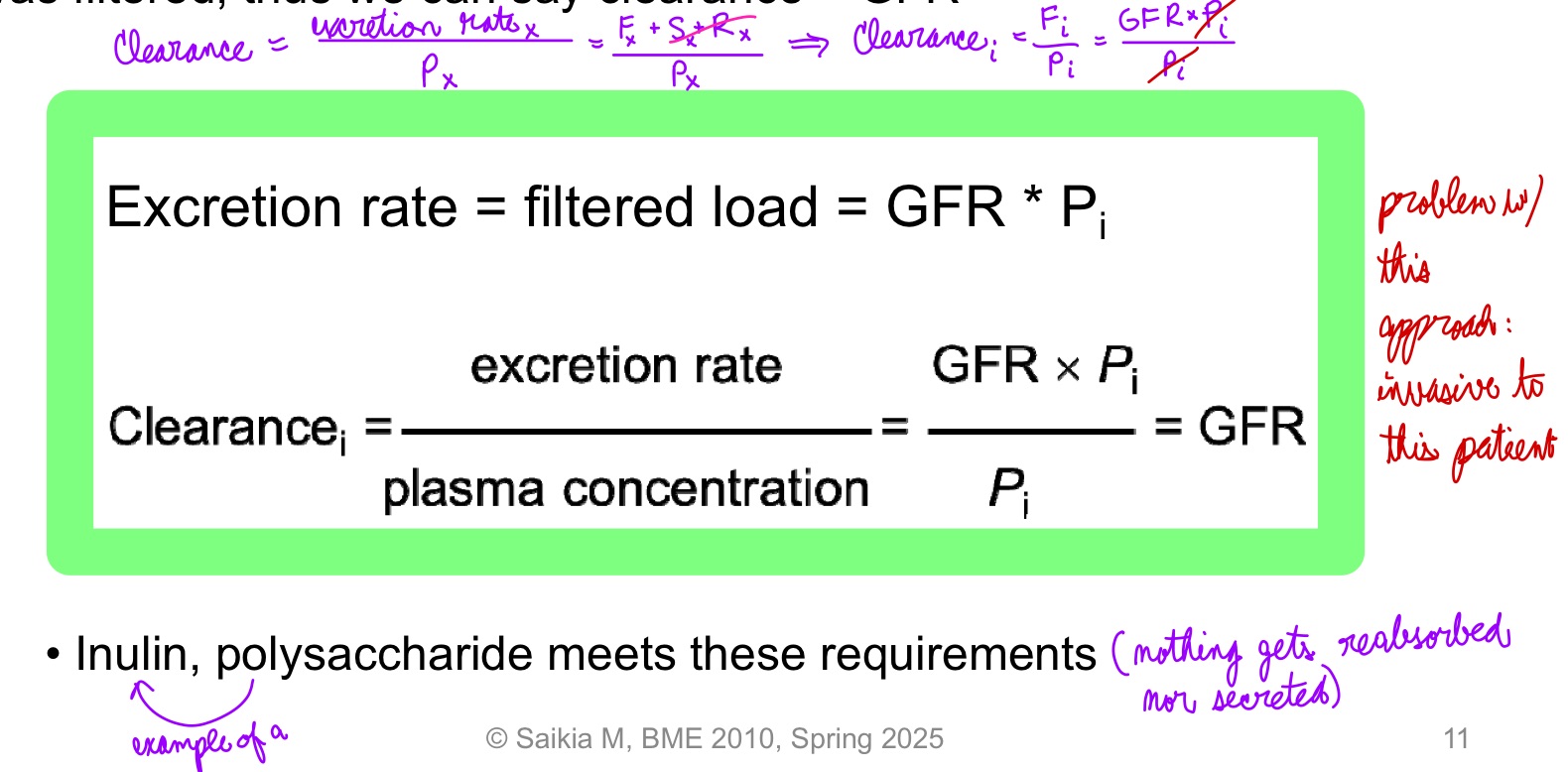
L19: In certain cases clearance provides an estimate of GFR
Clearance can be used to determien GFR if a substance is freely filtered and is neither reabsorbed nor secreted, then amount in urine is equal to amount filtered = filtered load
Under these conditions the substance is cleared from the volume that was filtered, thus we can say clearance = GFR
Inulin, a polysaccharide meets these requirements
Inulin is neither secreted nor reabsorbed
Amount of inulin excreted in urine = amount that was filtered = filtered load
So clearance of inulin = GFR
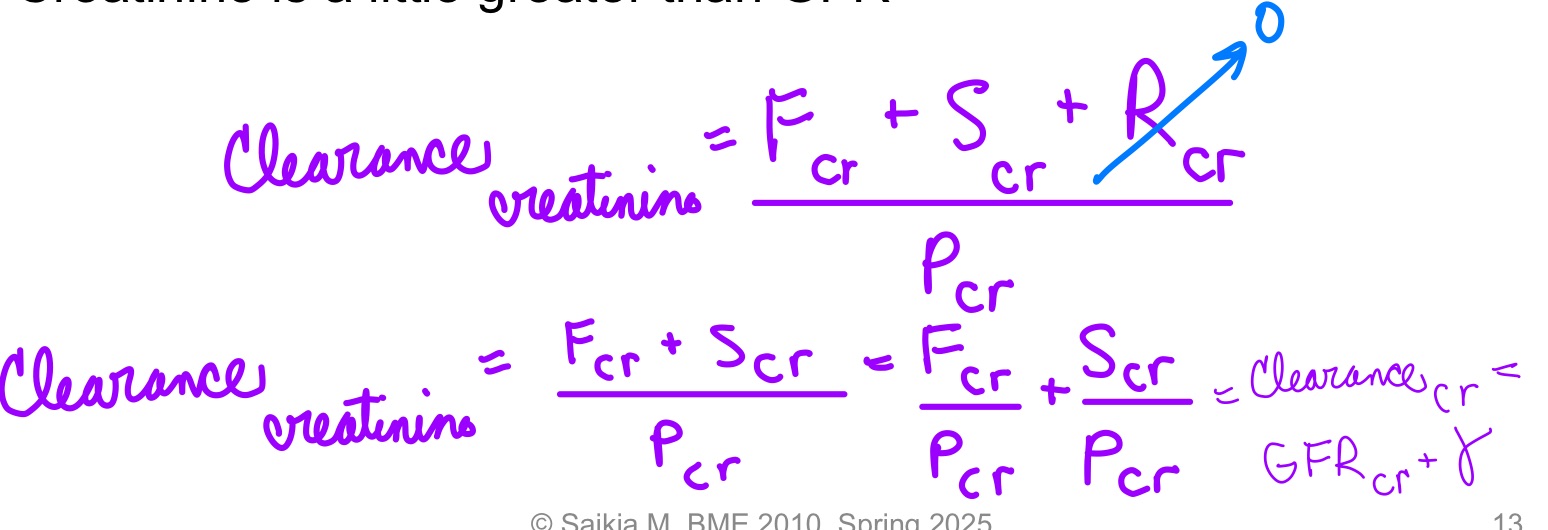
L19: Clearance estimation using Creatinine
Creatinine: product of muscle metabolism (gets secreted a little bit)
Use of creatinine to estimate GFR is non-invasive
Creatinine: by-product of muscle metabolism
Produced in body
Freely filtered
Not reabsorbed
Clearance: suitable clinical “estimate” of GFR
Because a small amount of Creatinine is secreted clearance of Creatinine is a little greater than GFR
L19: Clearance can also determine fate of solutes
If Cx > GFR, then net secretion of the solute occured
If Cx < GFR, then net reabsorprtion of the solute occurred
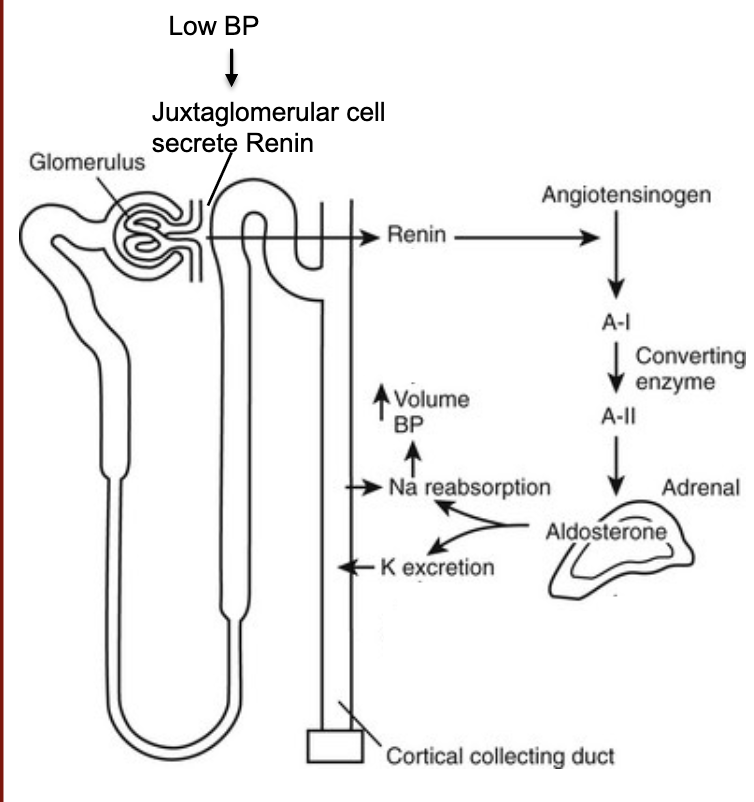
L19: Renal Endocrine system crosstalk
When blood pressure falls, juxtaglomerular cells release the enzyme Renin into your bloodstream
Renin cleaves angiotensinogen, a protein your liver makes, making angiotensin I
Angiotensin I, which is inactive, is cleaved by angiotensin-converting enzyme (ACE), into angiotensin II, an active hormone
Angiotensin II triggers Adrenal glands to release hormone Aldosterone
Aldosterone cause your kidneys to reabsorb sodium and secrete potassium. The increase in sodium in your bloodstream causes water retention. This increases blood volume and blood pressure, thus completing the renin-antiotensin-aldosterone system
L20: Recap: estimating GFR using clearance
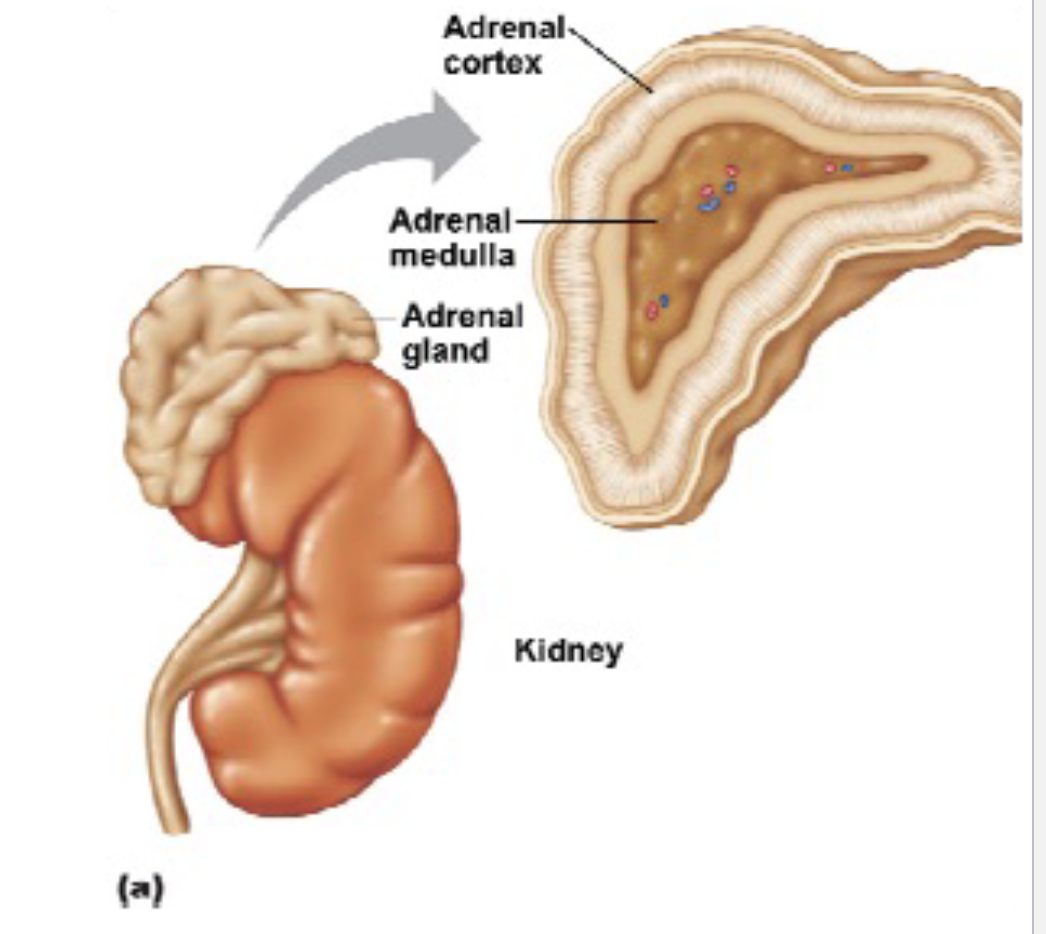
L20: Adrenal glands
Also called Suprarenal glands
Comprises of 2 layers
1. Outer layer - Cortex
2. Inner core - Medulla
Adrenal cortex secretes adrenocorticoids. example: aldosterone and cortisol
Cortisol regulates the body’s response to stress; stimulates gluconeogenesis
L20: Endocrine system (image)
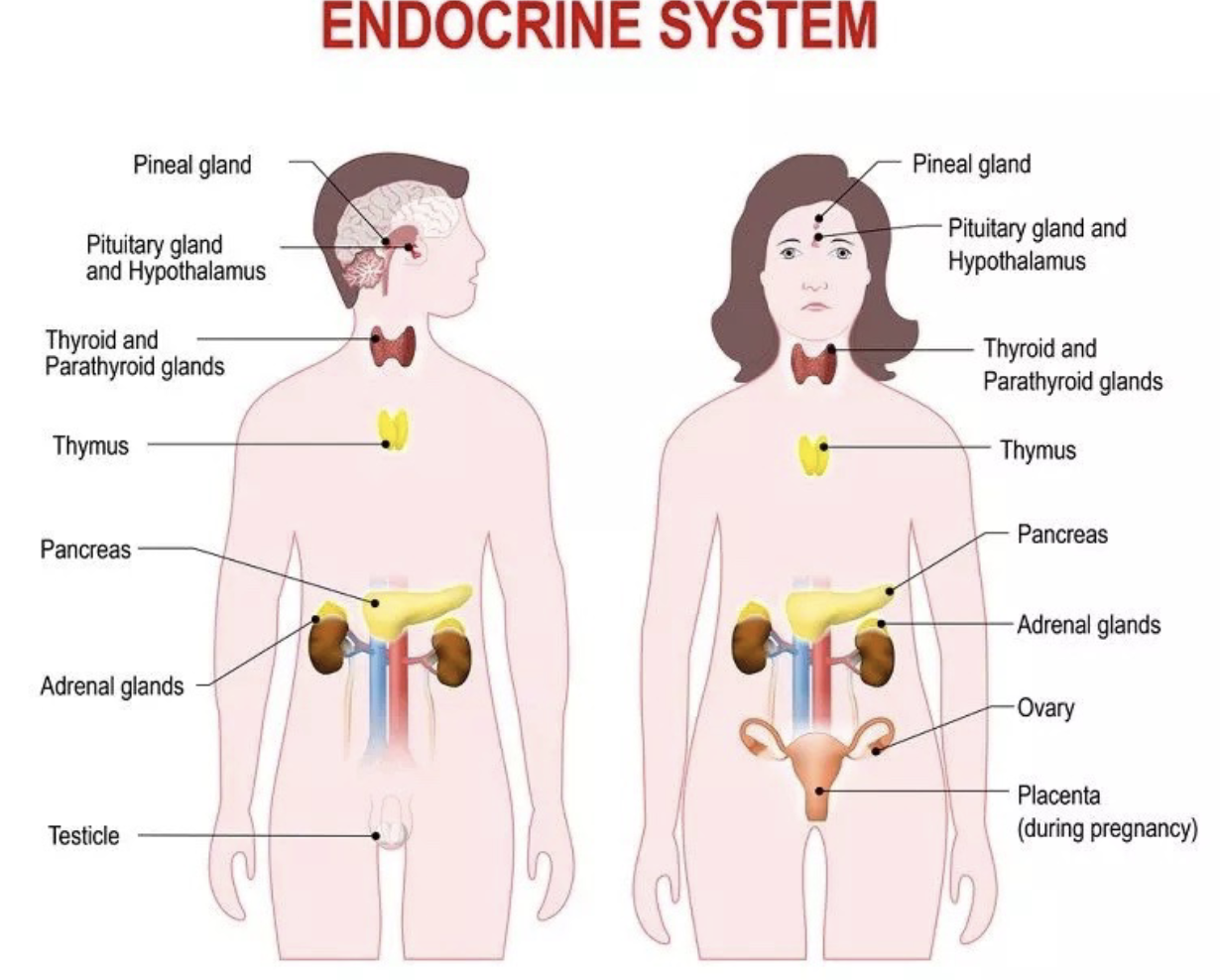
L20: Endocrine system overview
Organs of endocrine system are the endocrine glands
Endocrine glands are a group of cells that secrete hormones
Hormones allow cell to cell communication (like neurotransmitters but the signaling is slower)
Two types of endocrine glands:
Primary endocrine gland: primary function is hormone secretion
ex: Hypothalamus, thymus, pancreas, etc.
Secondary endocrine gland: hormone secretion is a secondary function
ex: stomach, kidney, skin, etc.
L20: Autocrine, Paracrine, Endocrine signaling
Autocrine: signal the same cell
Paracrine: signal nearby cells
Endocrine: signal cells far away
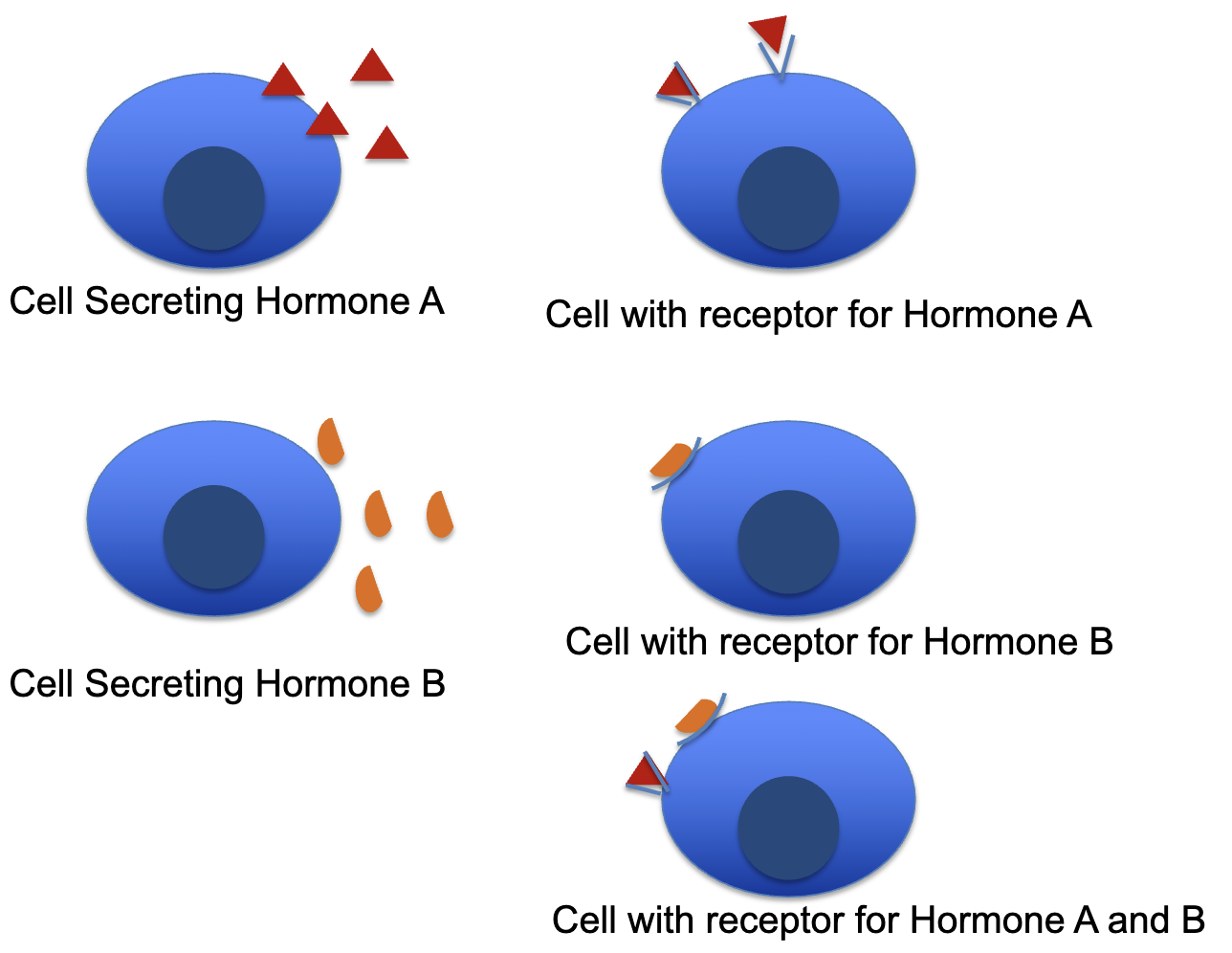
L20: Hypersecretion vs. Hyposecretion
Hyposecretion: Too little
ex: Diabetes mellitus type 1
Caused due to insufficient insulin
Hypersecretion: Too much
ex: Acromegaly
Caused due to too much growth hormone in adults
L20: List of Primary Endocrine Glands
Hypothalamus and pituitary gland
Pineal gland
Thyroid gland
Parathyroid gland
Adrenal glands
Pancreas
Gonads
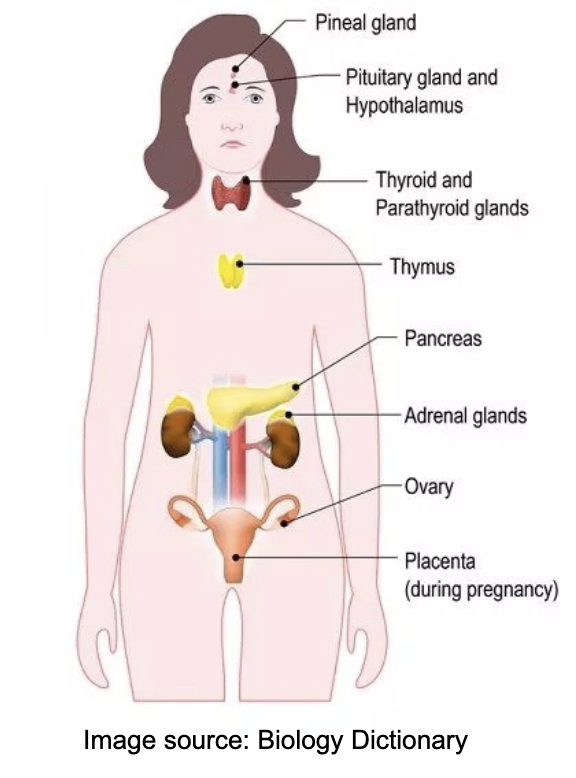
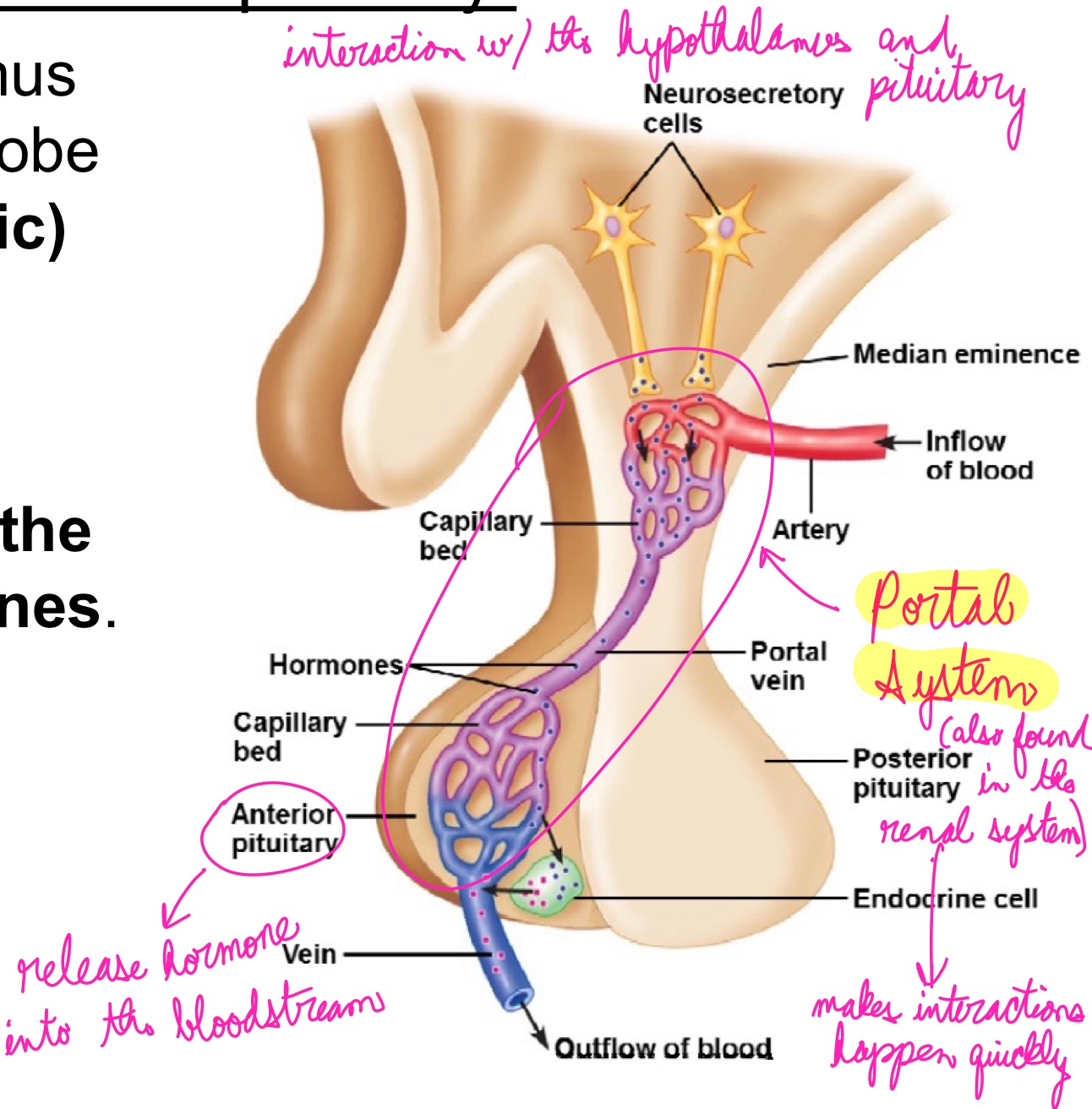
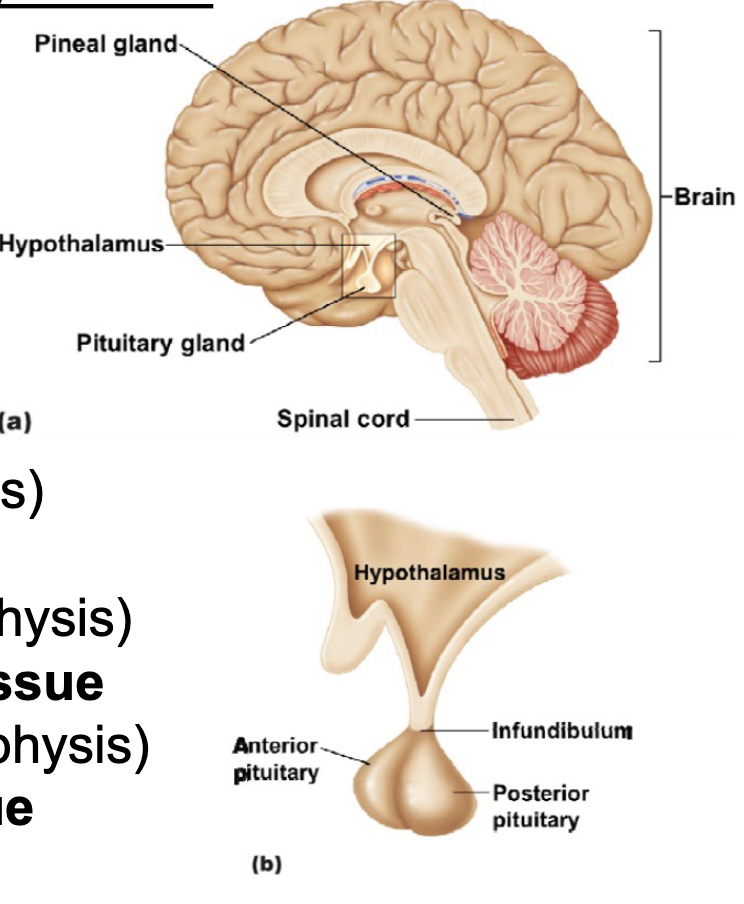
L20: Hypothalamus and Pituitary gland
Together they regulate almost every body system
Hypothalamus secretes several hormones, most of which affect the pituitary gland
Pituitary gland (aka hypophysis) consists of two parts:
1. Anterior lobe (adenohypophysis): derived of epithelial tissue
2. Posterior lobe (neurohypophysis): derived of neural tissue
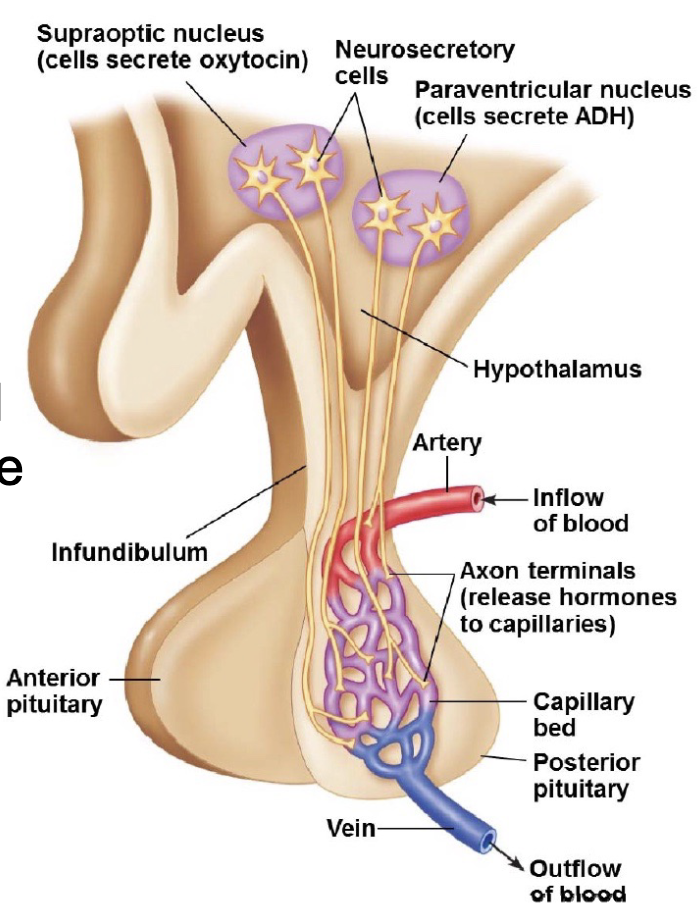
L20: Hypothalamus and the posterier pituitary gland
Two types of neurohormones secreted by hypothalamus into posterior pituitary:
1. Antidiuretic hormone (ADH): aka vasopressin
decreases urine output
ADH release stimulated by solute concentration in blood → regulates water reabsorption → targets cell in the nephron, maybe more specific parts?
2. Oxytocin
stimulated by pressure in the uterus or baby sucking → targets cells in the breast and uterus
Stimulates pressure in uterus to help birthing, also lactation
L20: Hypothalamus and anterior pituitary
Cells in the hypothalamus that control the anterior lobe secrete tropic (or trophic) hormones
Trophic hormones: hormones that regulate the control of other hormones
can be stimulatory
can be inhibitory