Protozoan Diseases
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
protozoan life cycle
trophozoite and cyst
Pathogenic properties of protozoa
-Protozoan waste products may cause symptoms
-Avoid host defenses by:
>Growing in phagocytes
>Antigenic variation
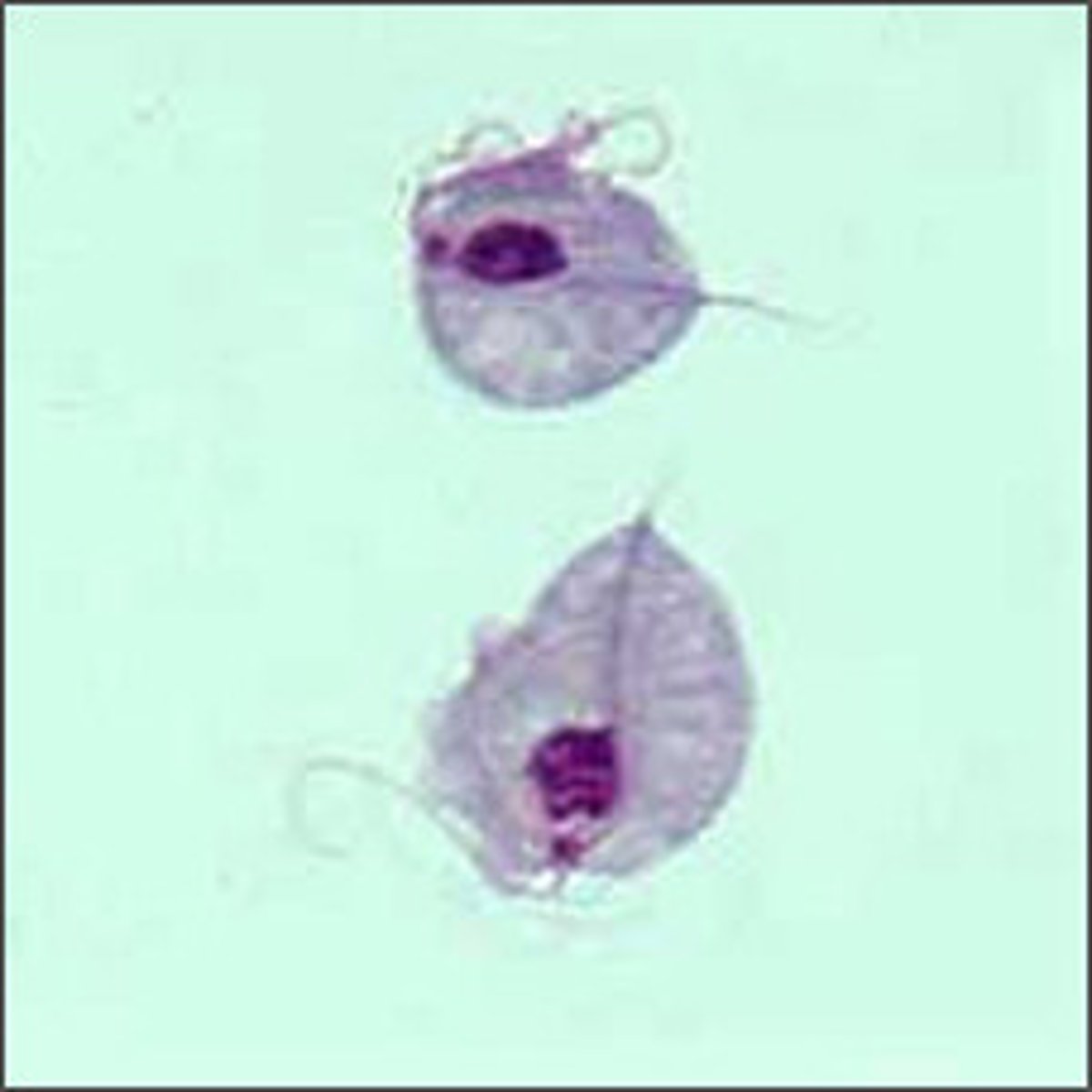
giardia lamblia
flagellate- causes GI symptoms from yucky water
Giardia lamblia transmission
cysts/fecal (human, beaver, muskrat, etc.), oral transmission
Giardia lamblia pathogenesis
protozoan's ventral sucking disk adheres to lining of duodenal wall => damage to enterocytes and loss of brush border of epithelial cells of the intestine
Giardia lamblia clinical presentation
malaise, nausea, bloating, flatulence, foul-smelling, fatty diarrhea
Giardia lamblia life cycle
1) Transmission via ingestion of food/water/fecal-oral contaminated with cysts. Cysts found in feces and are resistant forms that survive several months in cold water (individuals passed in feces cannot survive outside)
2) Trophozoites released from cyst in small intestine (2 per cyst) and they multiply by longitudinal binary fission.
3) Live in proximal small bowel, freely or attached to mucosa by a sucking disk
4) Encystation as they get near colon; pass out of body in cyst form
Giardia lamblia diagnosis
pear-shaped trophozoites with bilobed nuclei/cysts in stool, fecal antigen test
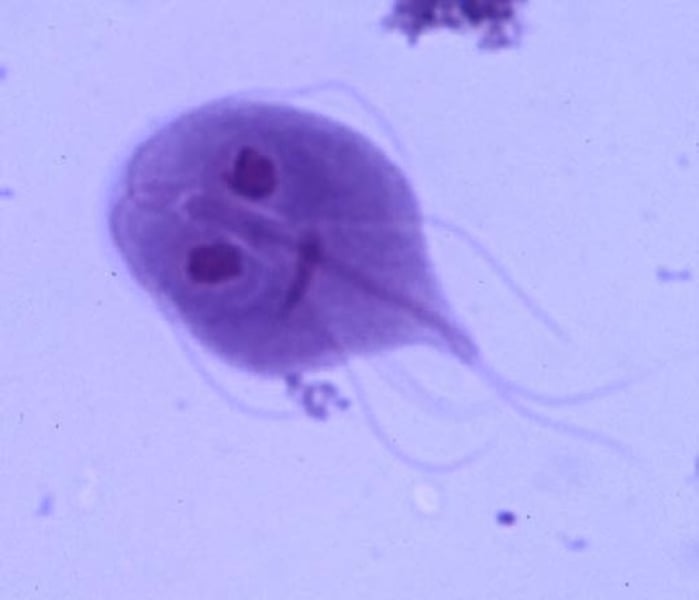
cryptosporidium
mild diarrhea from undercooked meat
cryptosporidium transmission
ingestion of oocysts in water
cryptosporidium pathogenesis
intracellular multiplication in the brush border
cryptosporidium clinical presentation
Severe diarrhea in immunocompromised AIDS patients
Mild watery diarrhea in immunocompetent host
cryptosporidium diagnosis
oocysts on acid fast stain, antigen detection
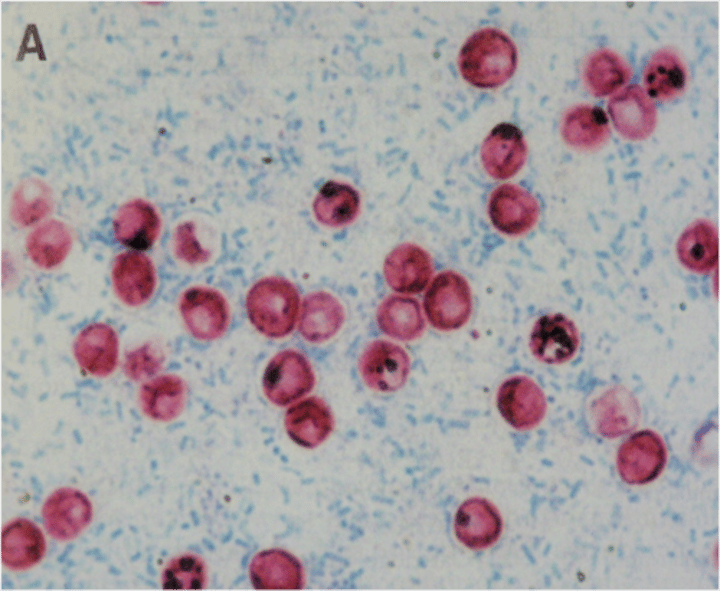
Entamoeba histolytica
amoebic dysentery from contaminated water
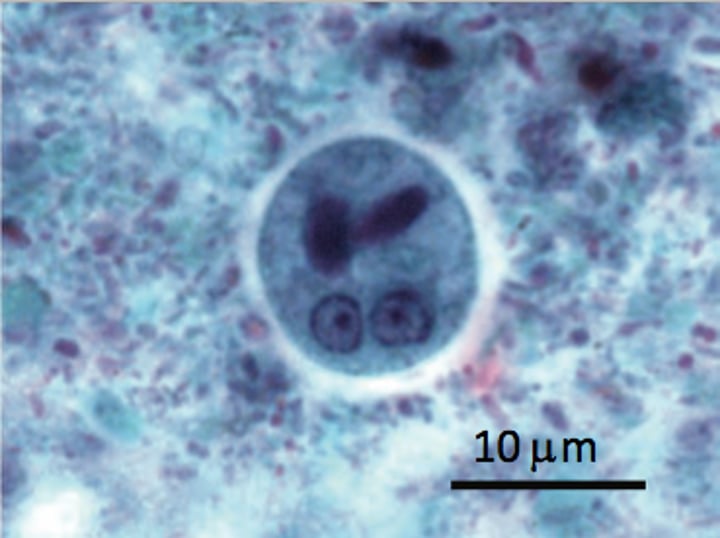
Entamoeba histolytica pathogenesis
trophozoites can invade the colonic mucosa and cause dysentery and through spreading through the bloodstream, gives rise to extraintestinal lesions (liver)
Entamoeba histolytica clinical presentation
blood and pus in stools, liver abscess, inverted flask shaped lesions in large intestine (peritoneum, liver, lungs, brains, heart)
Entamoeba histolytica diagnosis
trophozoites/cysts in stool
Entamoeba histolytica serology
nuclei have sharp central karyosome and fine chromatin "spokes"
Toxoplasma gondii
obligate intracellular parasitic protozoan
Toxoplasma gondii danger population
pregnant women, IC
Toxoplasma gondii transmission
cysts in meat, oocysts in cat feces, crosses placenta
Toxoplasma gondii disease
mononucleosis-like in IC
brain abscess in AIDS
congenital toxoplasmosis
congenital toxoplasmosis
chorioretinitis, hydrocephalus, and intracranial calcifications
Toxoplasma gondii pathogenesis
trophozoites infect brain, eyes, liver. CNS disease more common in immunocompromised patients
Naegleria fowleri
brain eating amoeba
Naegleria fowleri transmission
Swimming in freshwater lakes (think Nalgene bottle filled
with fresh water containing Naegleria); enters via cribriform plate
Naegleria fowleri disease
Rapidly fatal meningoencephalitis
Naegleria fowleri clinical presentation
severe purulent hemorrhagic inflammatory rxn
rapid onset of bifrontal headache
Naegleria fowleri pathogenesis
Trophozoite enters CNS through the cribriform plate leading to CNS infection
Naegleria fowleri diagnosis
Amoebas in spinal fluid (see picture)
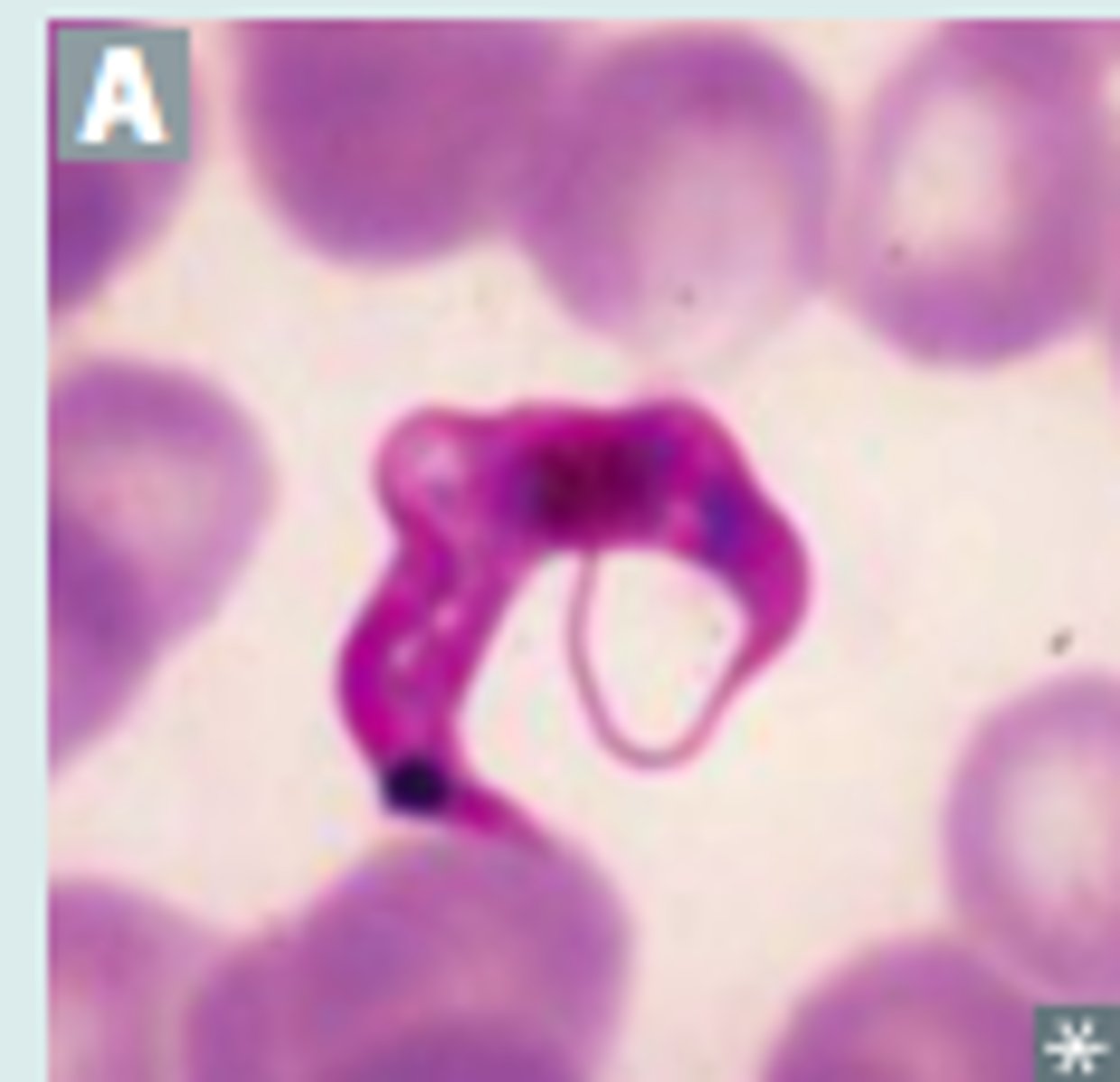
Trypanosoma brucei
African sleeping sickness
Trypanosoma brucei transmission
Tsetse fly, a painful bite
Trypanosoma brucei disease
African sleeping sickness—enlarged lymph nodes, recurring fever (due to antigenic variation), somnolence, coma
-Two subspecies: Trypanosoma brucei rhodesiense, Trypanosoma brucei gambiense
Trypanosoma brucei pathogenesis
Often fatal if left untreated:
1. Localized inflammatory reaction near entry site
2. Invasion of lymph nodes- Winterbottom's sign
3. Invasion of CNS
Trypanosoma brucei clinical presentation
African Sleeping Sickness:
Cervical and axillary LAD
Recurring fevers (antigenic variation)
Somnolence and coma (parasitic involvement of CSF and CNS)
Trypanosoma brucei diagnosis
Trypomastigote in Blood smear (see picture)
Plasmodium
causes malaria
P. falciparum
species of Plasmodium that causes the most severe cases of malaria
P. malariae
Malarial organism characteristically has a band form trophozoite stretching across the red blood cell
P. vivax
benign tertian malaria
- persistent hypnozoites (relapse)
P. ovale
Malarial organism has large, coarse, red dots within a large, pale red blood cell with fimbriated edges
- persistent hyponozoites (relapse)
Plasmodium pathogenesis
- metabolism of hemoglobin and lysis of infected RBC => anemia/agglutination of infected cells
uncomplicated malaria
Symptomatic infection with the malaria parasite manifesting as fever, body aches, headache, diarrhea, and possibly other symptoms not associated with severe illness.
Severe malaria
symptomatic malaria infection that is complicated by coma or recurrent seizures, severe anemia, respiratory distress, kidney or liver failure, systemic acidosis, or other associated life-threatening conditions
P. falciparum pathology
causes greater parasitemia b/c organism can enter reticulocytes and mature RBC.
- secreted proteins that are concentrated in "knobs" in erythrocyte membranes- adhere to vascular epithelium, causing occlusion that can lead to thrombosis and ischemia of any organ
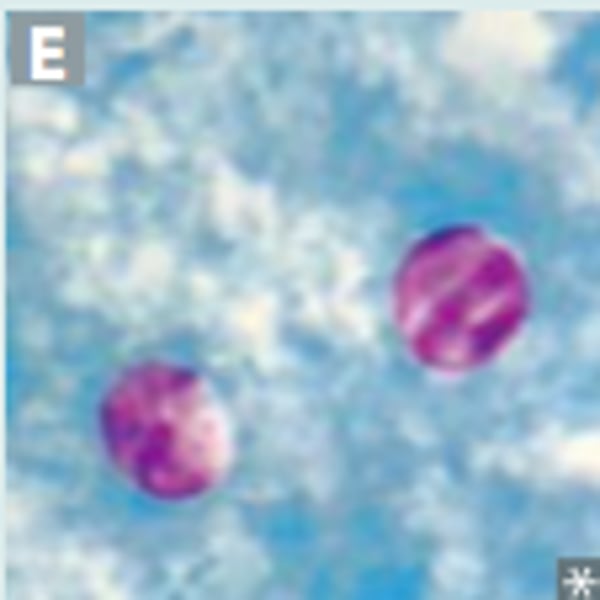
Babesia
tick bite leads to blood-borne dissiminated illness
(apicomplexa)
Babesia transmission
Ixodes tick (same as Borrelia burgdorferi)
Babesia pathogenesis
Massive destruction of erythrocytes during parasite development
Babesia clinical presentation
fever, hemolytic anemia, predominantly in northeastern US, (asplenia = danger)
Babesia diagnosis
Blood smear, ring form, Maltese cross, PCR
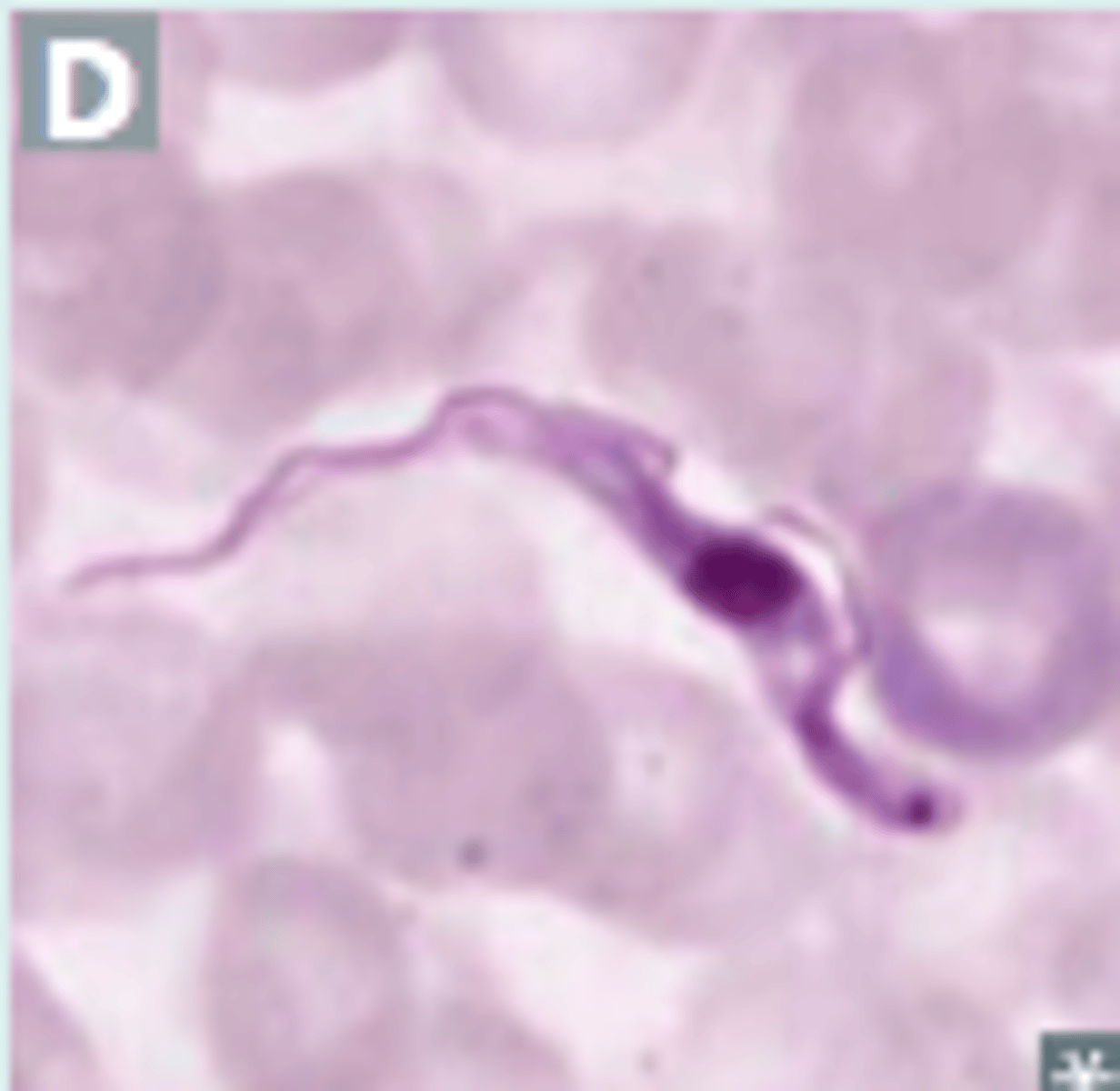
Trypanosoma cruzi
Chagas disease - blood disease from reduviid bugs (flagellates)
Trypanosoma cruzi transmission
Reduviid bug ("kissing bug") feces, deposited in a painless bite (much like a kiss)
Trypanosoma cruzi disease
-Chagas disease—dilated cardiomyopathy with
apical atrophy, megacolon, megaesophagus; predominantly in South America
-Unilateral periorbital swelling (Romaña sign) characteristic of acute stage
Trypanosoma cruzi pathogenesis
Extracellular pathogen in peripheral blood but intracellular in cardiac myocytes, smooth muscle cells and enteric neurons
Trypanosoma cruzi diagnosis
trypomastigote in blood smear
Leishmania
leishmaniasis from sand fly (volcano)
Leishmaniasis disease
cutaneous skin ulcer, spiking fever, hepatosplenomegaly, pancytopenia
Leishmaniasis pathogenesis
infective stage promastigotes reach puncture wound are phagocytized by macrophages - transform into tissue stage which divide/ infect others
Leishmaniasis diagnosis
Macrophages containing amastigotes in cutaneous lesions
Trichomonas vaginalis
exclusive human pathogen - flagellate
- STI
Trichomonas vaginalis transmission
Sexual (cannot exist outside human because it cannot form cysts)
Trichomonas vaginalis pathogenesis
adherence factors allow cervicovaginal epithelium colonization in women
Trichomonas vaginalis disease
Vaginitis—foul-smelling, greenish discharge; itching and burning; do not confuse with Gardnerella vaginalis, a gram-variable bacterium associated with bacterial vaginosis
Trichomonas vaginalis diagnosis
motile trichomonas trophozoites on a "wet prep"