Bonding, structure and the properties of matter
1/74
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
What are the three types of strong chemical bonds?
1. Ionic Bonding – Between oppositely charged ions.
2. Covalent Bonding – Atoms share electron pairs.
3. Metallic Bonding – Atoms share delocalized electrons.
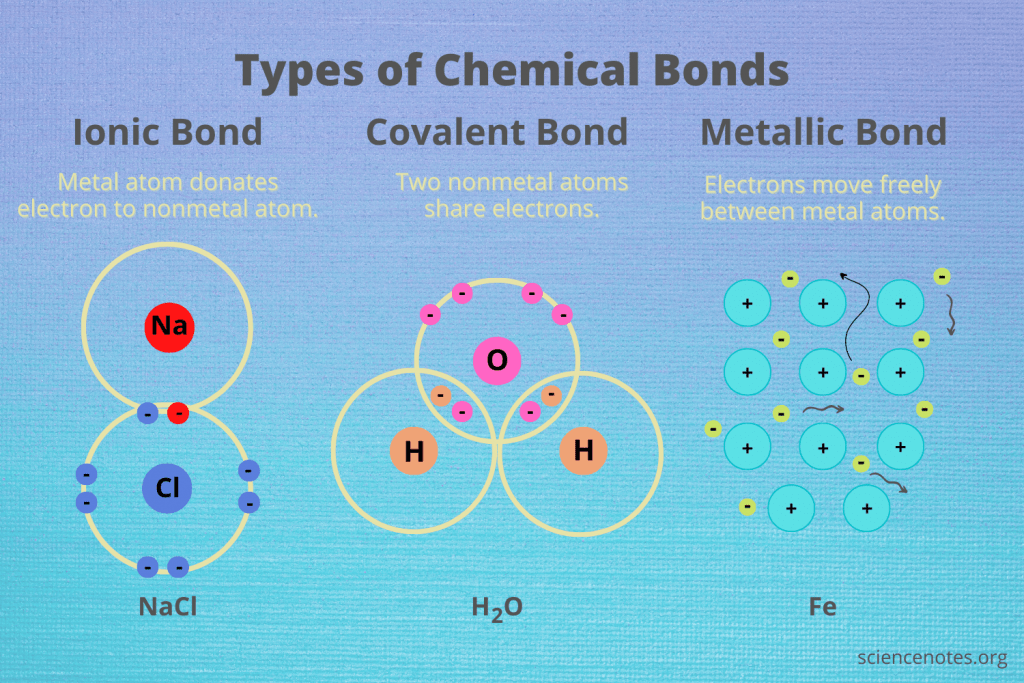
What is ionic bonding?
Ionic bonding occurs when metals transfer electrons to non-metals, forming oppositely charged ions that attract each other.
• Example: Sodium chloride (NaCl) – Sodium (Na⁺) donates an electron to chlorine (Cl⁻).
• Electrostatic forces hold the ions together in a giant ionic lattice.
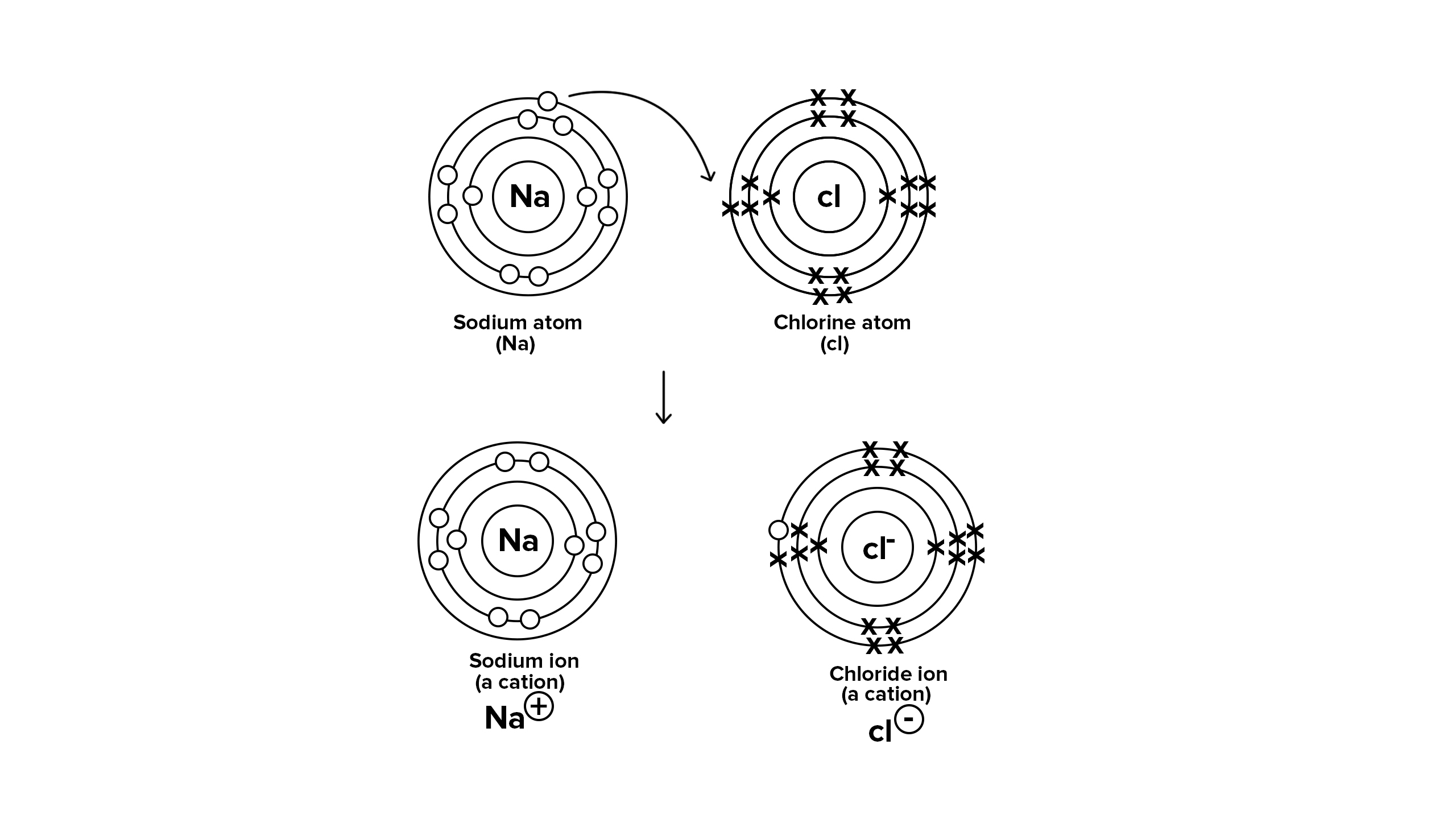
What is covalent bonding?
Covalent bonding occurs when non-metal atoms share pairs of electrons to achieve a full outer shell.
• Example: Water (H₂O) – Oxygen shares electrons with two hydrogen atoms.
• Forms molecules or giant covalent structures (e.g., diamond).
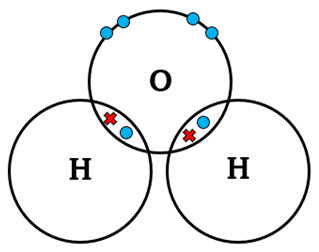
What is metallic bonding?
Metallic bonding occurs in metals and alloys, where atoms share delocalized electrons, creating a ‘sea of electrons’ that allows conductivity and malleability.
• Example: Copper (Cu) – Strong electrostatic forces between metal ions and delocalized electrons.
How do electrostatic forces play a role in bonding?
• Ionic bonding: Strong electrostatic attraction between oppositely charged ions.
• Covalent bonding: Strong attraction between shared electron pairs and nuclei.
• Metallic bonding: Electrostatic attraction between metal ions and delocalized electrons.
What happens to electrons when a metal reacts with a non-metal?
The metal atom loses electrons to become a positively charged ion, while the non-metal gains electrons to become a negatively charged ion.
Why do ions formed from Group 1, 2, 6, and 7 elements have a noble gas electronic structure?
Because they gain or lose electrons to achieve a full outer shell, like the noble gases in Group 0.
What diagram is used to show electron transfer in ionic bonding?
A dot and cross diagram shows the transfer of electrons from a metal to a non-metal.
How can you predict the charge of an ion from its group number?
• Group 1 metals → +1 charge
• Group 2 metals → +2 charge
• Group 6 non-metals → -2 charge
• Group 7 non-metals → -1 charge
How can we visualize the structure of ionic compounds?
• Dot and cross diagrams show 2D electron transfer.
• Lattice structures show 3D ionic arrangements.
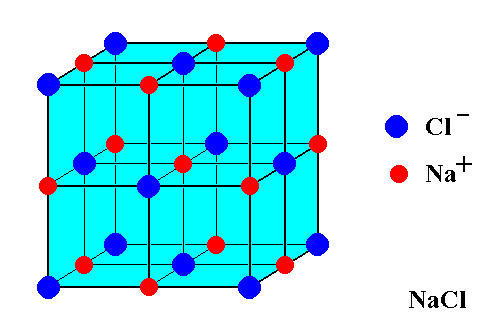
How are ionic compounds held together?
Ionic compounds are held together by strong electrostatic forces of attraction between oppositely charged ions
What happens when atoms share pairs of electrons?
They form covalent bonds, which are strong bonds between atoms.
What are the three types of covalently bonded substances?
1. Small molecules (e.g., H₂O, CO₂)
2. Polymers (e.g., polyethene)
3. Giant covalent structures (e.g., diamond, silicon dioxide)
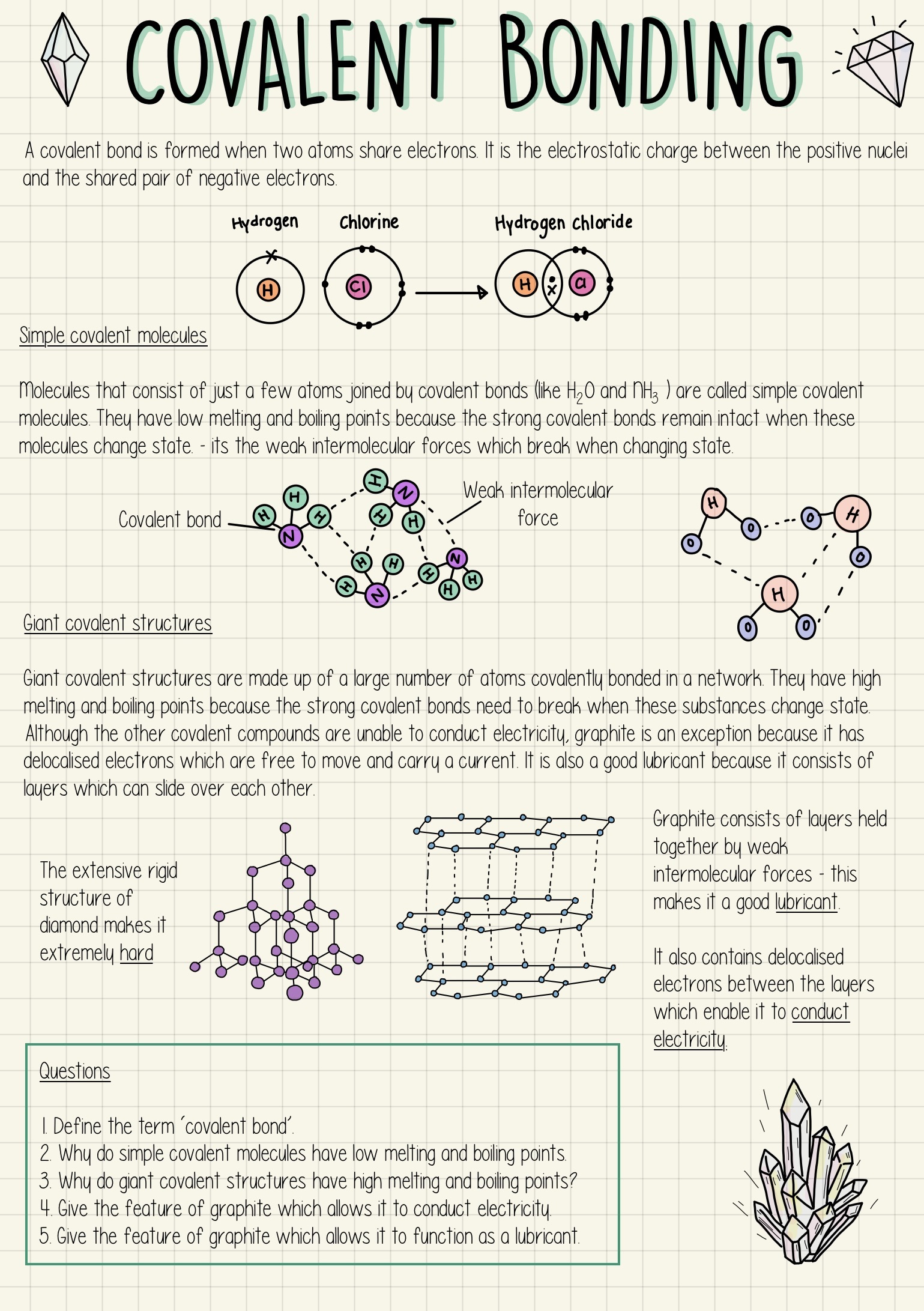
How can covalent bonds be represented?
Using dot and cross diagrams to show shared electron pairs.
How are polymers represented?
As repeating units in a structure, often written as:
-(monomer unit)-ₙ
where n is a large number.
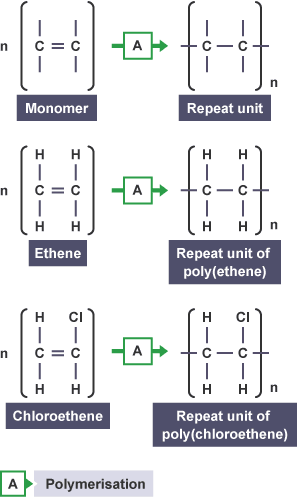
Name two substances with giant covalent structures.
Diamond (pure carbon) and silicon dioxide (SiO₂).
What symbols can represent covalent bonds?
1. Dot and cross diagrams (electrons shown)
2. Lines for single bonds (e.g., H—H for H₂)
3. Ball and stick models (3D representation)
What are the limitations of different molecular representations?
• Dot and cross diagrams don’t show bond angles.
• Ball and stick models exaggerate bond lengths.
• 2D diagrams don’t represent 3D structures.
How can you find the molecular formula from a diagram?
Count the number of each type of atom in the structure.
Example:
• Methane model → CH₄
• Water model → H₂O
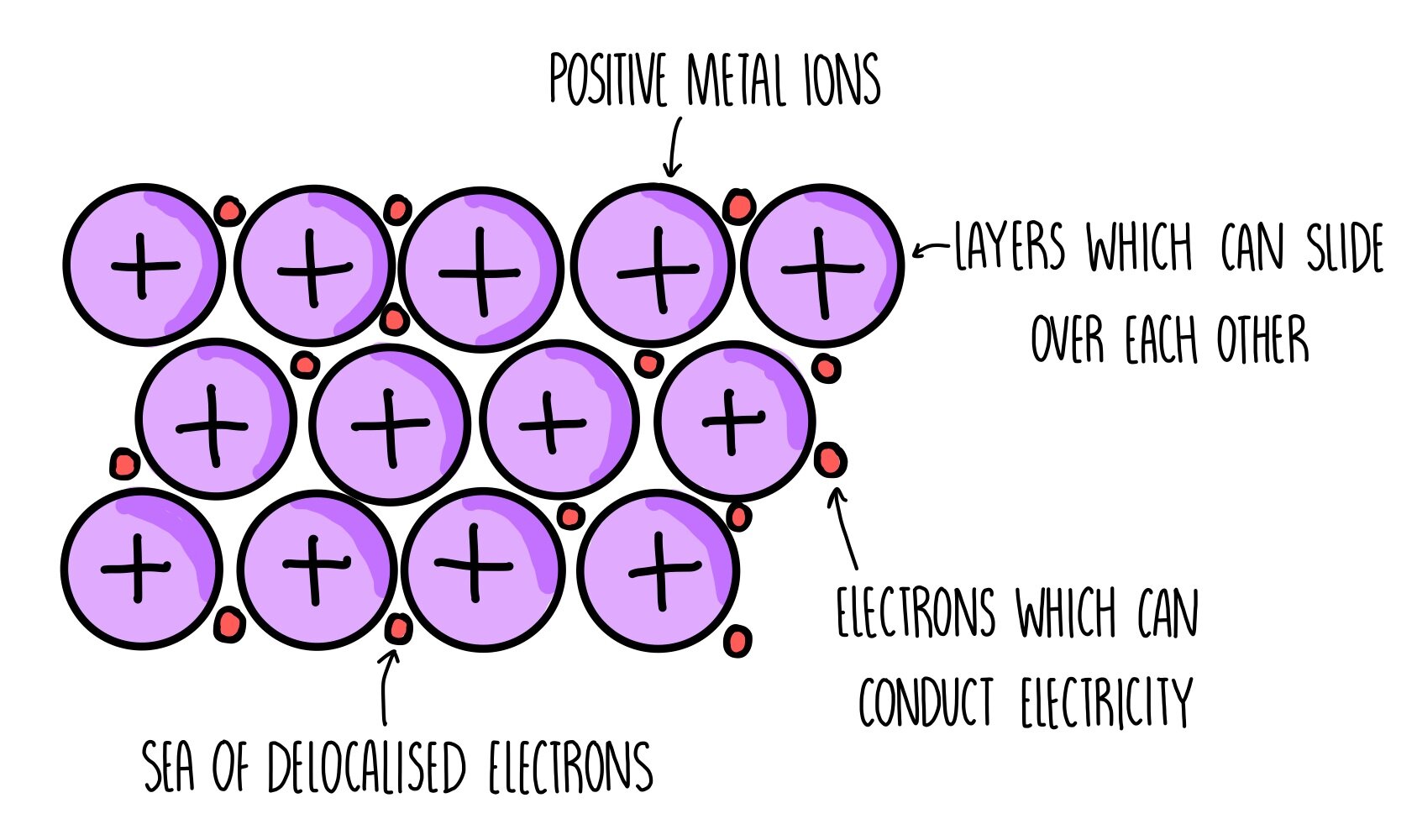
What is the structure of metals?
Metals consist of giant structures of atoms arranged in a regular pattern (lattice structure).
What happens to the electrons in the outer shell of metal atoms?
The electrons in the outer shell are delocalised (free to move) throughout the entire structure.
What gives rise to strong metallic bonds?
The sharing of delocalised electrons between metal atoms creates strong metallic bonds.
How does metallic bonding affect the properties of metals?
✅ Good electrical conductivity – Due to free-moving delocalised electrons.
✅ High melting & boiling points – Strong metallic bonds require a lot of energy to break.
✅ Malleability & Ductility – Layers of atoms can slide over each other.

JAKE BREAK 💝
JAKE BREAK 💝

What are the three states of matter?
Solid, liquid, and gas.
What happens at the melting and boiling points of a substance?
• Melting & Freezing occur at the melting point.
• Boiling & Condensing occur at the boiling point.
How are the states of matter represented in the particle model?
Particles are represented as small solid spheres, arranged differently in solids, liquids, and gases.
What determines the amount of energy needed for a substance to change state?
The strength of the forces between particles. Stronger forces = higher melting and boiling points.
What are the limitations of the simple particle model?
❌ It does not show forces between particles.
❌ It assumes particles are solid spheres (not true).
❌ It does not show particles moving or their interactions.
How can we predict the state of a substance at a given temperature?
By comparing the temperature to its melting and boiling points:
• Below melting point → Solid
• Between melting and boiling points → Liquid
• Above boiling point → Gas
What are the 3 states of matter shown as?
In chemical equations, the three states of matter are shown as (s), (l) and (g), with (aq) for aqueous solutions.
What type of structure do ionic compounds have?
Giant ionic lattices with strong electrostatic forces of attraction between oppositely charged ions.
Why do ionic compounds have high melting and boiling points?
Because large amounts of energy are needed to break the many strong ionic bonds.
Why do ionic compounds conduct electricity when dissolved in water or melted?
Because the ions are free to move, allowing charge to flow.
What state are substances with small molecules usually in, and why?
They are usually gases or liquids because they have low melting and boiling points due to weak intermolecular forces.
When a small molecule substance melts or boils, what forces are broken?
Intermolecular forces are broken, not covalent bonds.
How does molecule size affect melting and boiling points?
Larger molecules have stronger intermolecular forces, leading to higher melting and boiling points.
Why don’t small molecule substances conduct electricity?
Because they do not have an overall electric charge, meaning no free-moving charged particles to carry current.
How do intermolecular forces explain the properties of molecular substances?
1. Low melting/boiling points – Weak forces break easily.
2. Gases or liquids at room temp – Most small molecules have very weak forces.
3. Do not conduct electricity – No free-moving charged particles.
What are polymers, and what makes them different from small molecules?
Polymers are substances made of very large molecules where atoms are linked by strong covalent bonds.
What type of bonding exists in polymers?
✔ Covalent bonds – Strong bonds between atoms in a polymer chain.
✔ Intermolecular forces – Hold polymer molecules together and are stronger than in small molecules.
Why do polymers have higher melting points than small molecules?
Because their intermolecular forces are stronger, requiring more energy to break.
How can you identify a polymer from a diagram?
1⃣ Repeating units – Enclosed in brackets (….)n.
2⃣ Long chains of covalently bonded atoms.
📌 Example:
• Polypropene (used in food containers, carpets).
What are giant covalent structures, and why do they have high melting points?
Giant covalent structures are solids where all atoms are linked by strong covalent bonds in a continuous network. These bonds must be broken to melt the substance, requiring a lot of energy.
📌 Examples:
• Diamond (pure carbon) – Extremely hard, high melting point.
• Graphite (pure carbon) – Conducts electricity, slippery.
• Silicon Dioxide (SiO₂) – Found in sand, very strong.
What is the structure of diamond, and how does it affect its properties?
✔ Each carbon atom bonds to 4 others → Strong structure.
✔ No free electrons → Does not conduct electricity.
✔ Hardest natural substance → Used in cutting tools.
✔ High melting/boiling points → Strong covalent bonds throughout.
Why is graphite soft and able to conduct electricity?
✔ Each carbon bonds to only 3 others → Layers of hexagonal rings.
✔ Weak forces between layers → Layers slide, making it slippery.
✔ Free-moving electrons → Conducts electricity.
✔ Used as a lubricant and in pencils.
Why do metals have high melting and boiling points?
Metals have giant structures of positive ions surrounded by delocalised electrons. The strong electrostatic forces between the ions and electrons require a lot of energy to break, leading to high melting/boiling points.
📌 Key Property: Good conductors of electricity and heat because delocalised electrons can move freely.
Why can pure metals be bent and shaped?
In pure metals, atoms are arranged in layers, which can slide over each other, making them soft and easy to shape.
📌 Example: Pure gold is too soft for jewelry, so it’s mixed with other metals.
How do alloys differ from pure metals in structure?
In alloys, different-sized atoms disrupt the layers, preventing them from sliding easily, making alloys harder than pure metals.
📌 Example:
• Steel (iron + carbon) → Stronger and more durable than pure iron.
• Bronze (copper + tin) → Used in statues and medals.
What are the key differences between Giant covalent structures, Metals and alloys?
Property | Giant Covalent Structures | Metals | Alloys |
Melting Point | Very High | High | High |
Electrical Conductivity | Only graphite conducts | Conducts | Conducts |
Hardness | Hard (except graphite) | Soft (layers slide) | Harder than pure metals |
Uses | Cutting tools, lubricants | Wires, pipes, coins | Machinery, jewelry |

JAKE BREAK 💝
JAKE BREAK 💝

What is the structure of diamond?
In diamond, each carbon atom forms four covalent bonds in a giant covalent structure, creating a rigid 3D network.
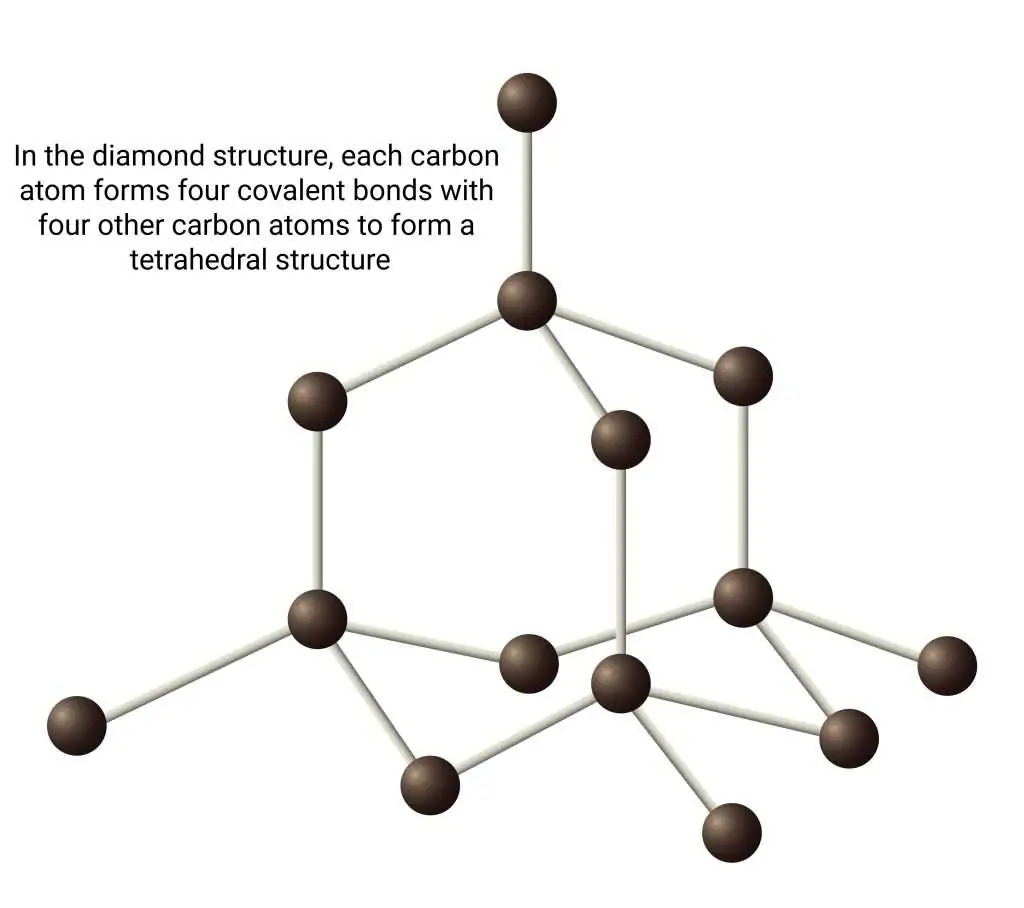
How does the structure of diamond relate to its properties?
✔ Very hard → Strong covalent bonds in a rigid lattice.
✔ High melting/boiling points → Large energy needed to break bonds.
✔ Does not conduct electricity → No free-moving electrons.
✔ Used in cutting tools and jewelry.
How are carbon atoms bonded in graphite?
✔ Each carbon forms three covalent bonds, creating layers of hexagonal rings.
✔ The layers are held together by weak forces, allowing them to slide.
✔ One electron per carbon is delocalised, making graphite similar to metals.
How does the structure of graphite relate to its properties?
✔ Soft and slippery → Weak forces between layers allow them to slide.
✔ High melting point → Strong covalent bonds within layers.
✔ Conducts electricity → Delocalised electrons move freely.
✔ Used in lubricants and pencils.
What are the key Differences Between Diamond and Graphite?
Property | Diamond | Graphite |
Bonding | 4 covalent bonds per carbon | 3 covalent bonds per carbon |
Structure | Rigid 3D network | Layers of hexagonal rings |
Hardness | Very hard | Soft, layers slide |
Melting Point | Very high | High |
Electrical Conductivity | Does not conduct (no free electrons) | Conducts (delocalised electrons) |
Uses | Cutting tools, jewelry | Lubricants, pencils, electrodes |
What is graphene?
Graphene is a single layer of graphite, composed of carbon atoms arranged in hexagonal rings.
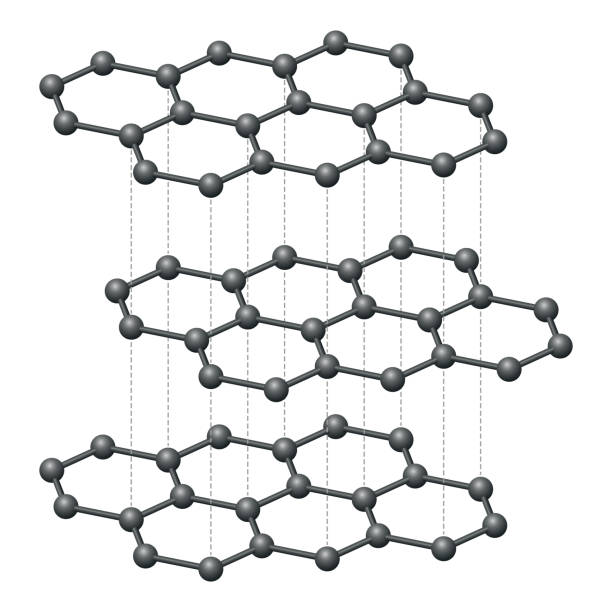
How does the structure of graphene relate to its properties?
✔ Extremely strong → Each carbon atom forms three covalent bonds.
✔ Very light and flexible → Single layer of atoms.
✔ Excellent electrical conductor → Delocalised electrons move freely.
✔ High thermal conductivity → Efficient heat transfer.
What are the uses of graphene?
✔ Electronics → Used in touchscreens, transistors, and sensors.
✔ Composites → Strengthens materials while keeping them lightweight.
✔ Energy storage → Used in batteries and supercapacitors.
✔ Medical applications → Potential use in drug delivery and biocompatible materials.
What are fullerenes?
Fullerenes are molecules of carbon atoms with hollow shapes.
✔ Their structure is based on hexagonal rings, but may also include pentagonal or heptagonal rings.
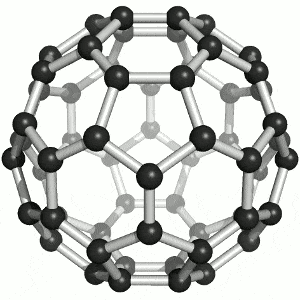
What is special about Buckminsterfullerene (C₆₀)?
✔ First discovered fullerene.
✔ Spherical shape resembling a football.
✔ Contains 60 carbon atoms in hexagonal and pentagonal rings.
What are carbon nanotubes?
✔ Cylindrical fullerenes with very high length-to-diameter ratio.
✔ Strong and lightweight.
✔ Excellent electrical and thermal conductivity.
What are the uses of fullerenes and carbon nanotubes?
✔ Nanotechnology → Used in drug delivery and medical imaging.
✔ Electronics → Used in miniature circuits and transistors.
✔ Materials → Reinforcing composites to create strong, lightweight materials.
✔ Lubricants → Buckminsterfullerene is used as a molecular lubricant.
✔ Catalysts → Fullerenes can act as catalysts due to their high surface area.

JAKE BREAK 💝
JAKE BREAK 💝

What is nanoscience?
Nanoscience is the study of structures 1–100 nm in size, approximately the size of a few hundred atoms.
How do nanoparticle sizes compare to fine and coarse particles?
✔ Nanoparticles: <100 nm.
✔ Fine particles (PM2.5): 100–2500 nm (1 × 10⁻⁷ m to 2.5 × 10⁻⁶ m).
✔ Coarse particles (PM10, dust): 1 × 10⁻⁵ m to 2.5 × 10⁻⁶ m.
How does decreasing particle size affect surface area to volume ratio?
✔ When the side of a cube decreases by a factor of 10, the surface area to volume ratio increases by a factor of 10.
✔ Higher surface area to volume ratio → Greater reactivity and effectiveness.
Why do nanoparticles behave differently from bulk materials?
✔ Higher surface area to volume ratio → Increases reactivity.
✔ Different optical, electrical, and chemical properties than bulk materials.
✔ Smaller quantities needed for the same effect as larger materials.
How do you calculate surface area to volume ratio?
✔ Surface area of a cube = 6 × (side length)².
✔ Volume of a cube = (side length)³.
✔ Ratio = Surface Area / Volume.
📌 Example Calculation:
• Cube with 10 nm sides:
• Surface area = 6 × 10² = 600 nm².
• Volume = 10³ = 1000 nm³.
• Ratio = 600/1000 = 0.6 nm⁻¹.
How do nanoparticle sizes compare to atoms and molecules?
✔ Atoms and small molecules → ~0.1 nm.
✔ Nanoparticles → 1–100 nm (10-1000× bigger than atoms).
✔ Typical human hair diameter → ~80,000 nm.
In which fields are nanoparticles commonly used?
Nanoparticles are used in:
✔ Medicine – Targeted drug delivery, cancer treatment.
✔ Electronics – Smaller, faster circuits.
✔ Cosmetics & Sun Creams – UV protection, better absorption.
✔ Deodorants – Odor control with antibacterial properties.
✔ Catalysts – Speed up chemical reactions.
What makes nanoparticles useful for different applications?
✔ High surface area to volume ratio → More reactive.
✔ Unique optical, electrical, and chemical properties.
✔ Can interact at a cellular level (important in medicine).
📌 Example:
• Silver nanoparticles in bandages kill bacteria.
What factors should be considered when evaluating the use of nanoparticles?
✔ Effectiveness – Does it work better than other materials?
✔ Cost – Is it expensive to produce?
✔ Safety – Are there any health or environmental risks?
✔ Longevity – Does it last a long time?
✔ Environmental impact – Is it biodegradable or toxic?
📌 Example:
• Titanium dioxide nanoparticles in sunscreen – Effective at blocking UV rays but may cause environmental harm.
What are the possible risks of nanoparticles?
✔ Health Risks – May be absorbed into the bloodstream, unknown long-term effects.
✔ Environmental Risks – Can accumulate in water and soil, affecting ecosystems.
✔ Lack of Research – Long-term effects are not fully understood.
✔ Potential Toxicity – Some nanoparticles can be harmful if inhaled or ingested.
Why is ongoing research on nanoparticles important?
✔ New applications are constantly being developed.
✔ Need to ensure safety before widespread use.
✔ Finding alternatives if risks outweigh benefits.
✔ Regulations may be needed to control nanoparticle use.
📌 Example:
• Carbon nanotubes are useful in electronics, but studies show they may act like asbestos in the lungs.