x ray crystal structures (copy) 3
1/21
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
amorphous material properties (3)
broad melting point
globular
isotropic - no order
no diffraction
yield n flow under pressure
crystalline material properties (4)
narrow melting point
regular faces and edges
anisotropic - very ordered
diffracts light
fracture/cleave under pressure
what are the three crystallisation steps
supersaturation
formation of nuclei
crystal growth
why do crystals diffract
because molecules are arranged in an ordered fashion
what happens when an x ray beam strikes a crystal surface at theta θ?
part of the beam is scattered by the layer of atoms at the surface
why do x rays scatter
the electron clouds of the atoms in the crystal
what does amount of scatter depend on?
size of atom
why are x rays scattered in an identical way
as a crystal has atoms in identical position
what is visible light between
400 - 700 nm
what do lattice points define?
unit cells
what is a unit cell
smallest repeating internal unit that has the symmetry characteristic of the solid- atom, ion or molecule
what are the seven basic crystal systems
cubic
tetragonal
orthorhombic
rhombohedral
hexagonal
monoclinic
triclinic
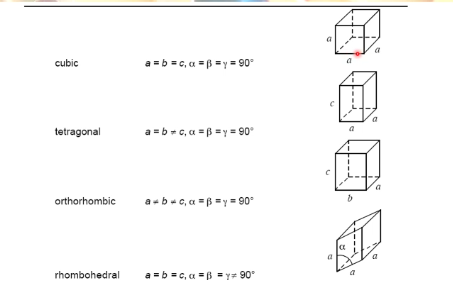
cubic unit cells properties
all sides equal
all sides equal 90
whats a primitive cubic cell (PC)
atom in each corner of a cube
what is the net number of atoms of pc?
1
whats a body centered cube (BCC)
atom in each corner and the centre
what is the net number of atoms of bcc?
2
whats a face centred cube (FCC)
atom in every corner and on each face
what is the net number of atoms of fcc?
4
what causes diffraction spots
x rays striking a single crystal
what does each diffraction spot correspond to?
single point in lattice
Formula you should know
2d sinθ=nλ