Lecture 03 - Cycloalkanes
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
Alicyclic Compounds
another term for cycloalkanes
CnH2n
general formula for cycloalkanes
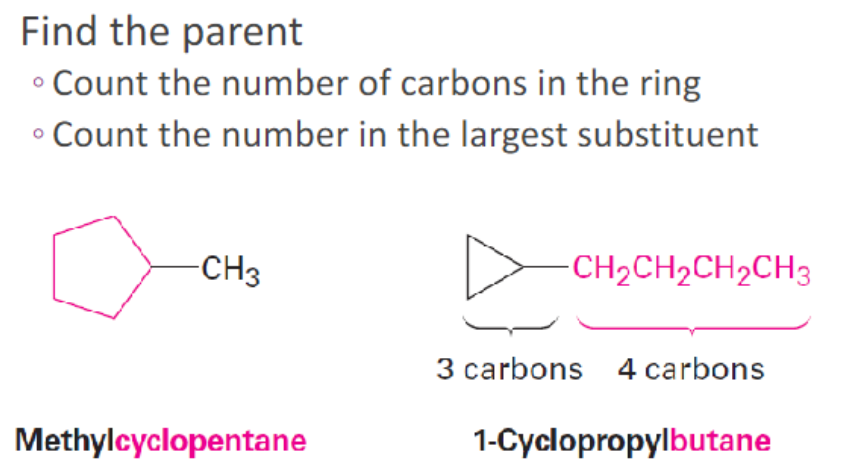
.
scanning time ** [how to find the parent chain]
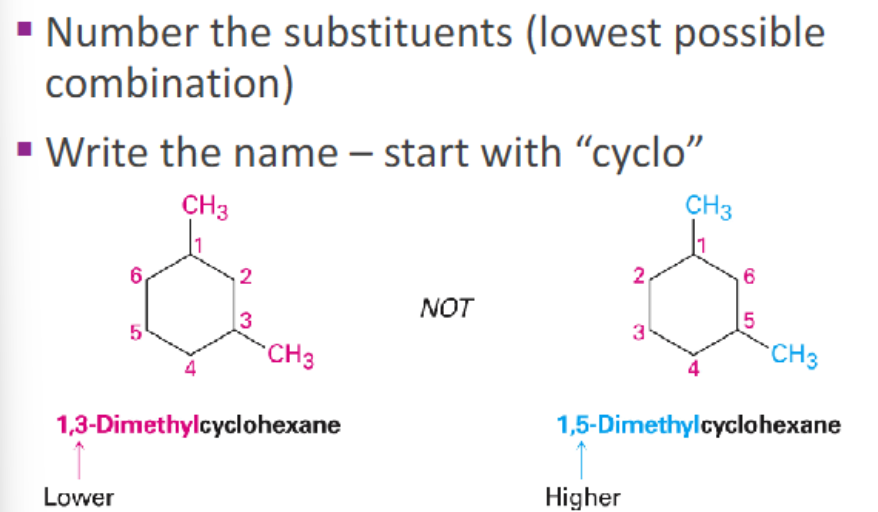
.
scanning time ** [how to find the parent chain]
less flexible
cycloalkanes are (less/more) flexible than open-chain alkanes
conformational freedom
significantly lesser - in cycloalkanes
Stereoisomerism
compounds which have their atoms connected in the same order but differ in 3D orientation
Stereochemistry
refer to the 3D aspects of chemical structure and reactivity
Constitutional Isomers
different connections between atoms
Stereoisomers
same connections but different 3D geometry
Cis-trans isomers
stereoisomers that differ in their stereochemistry about a ring or double bond
Angle Strain
induced in a molecule when bond angles are forced to deviate from the ideal 109 degrees tetrahedral value
nonplanar conformations
cyclic molecules can assume - - to minimize angle strain and torsional strain by ring-puckering
Torsional Strain
caused due to eclipsing of bonds between neighboring atoms
Steric Strain
caused due to repulsive interactions when atoms approach each other too closely
larger; smaller
- rings have many more possible conformation than - rings
Cyclopropane
most strained of all rings due to angle strain caused by its C-C-C bond angles of 60 degrees
Cyclopropane
- bonds are weaker and more reactive than typical alkane bonds
Cyclobutane
has less angle strain than cyclopropane
Cyclopropane
has considerable torsional strain and bent bonds
Cyclobutane
more torsional strain due to larger number of ring hydrogens & slightly bent
Cyclopentane
no angle strain and large torsional strain
.
learning reminder: conformation drawing (cis and trans) **
Cyclohexane
colorless, mobile liquid w/ mild, sweet odor
water; alcohol, acetone, benzene etc.
cyclohexanes are slightly soluble in - and soluble fully in -
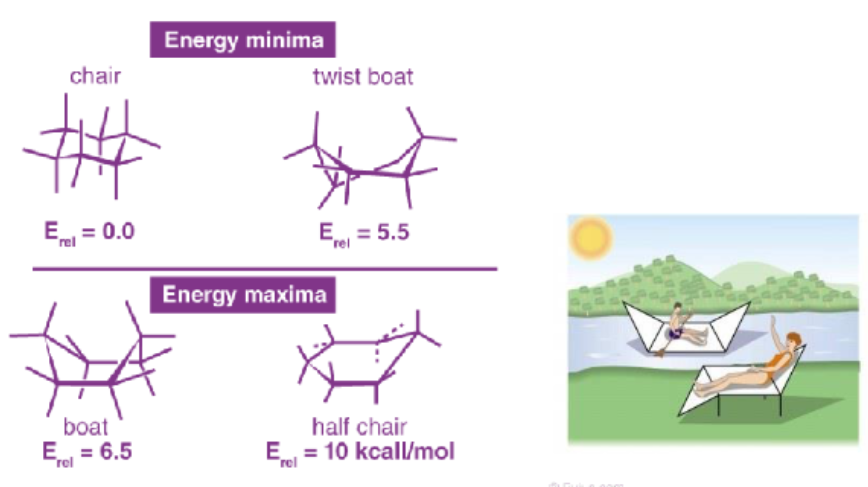
.
scanning time ** (conformations of cyclohexane)
Chair Conformation
strain-free, 3D shape
Boat Conformation
no angle strain, large number of eclipsing interactions
Twist-boat Conformation
nearly free of angle strain
Axial; Equatorial
each carbon atom in cyclohexane has one - and one - hydrogen
Axial
six - bonds, parallel and alternate up and down
Equatorial
6 - bonds, one on each carbon
Ring-flip
interconversion of chair conformations, resulting in the exchange of axial and equatorial positions
Steric Strain
causes difference between axial and equatorial conformers
Gauche; Anti
- Butane is less stable than - Butane
Trans isomer
methyl groups are on opposite faces of the ring
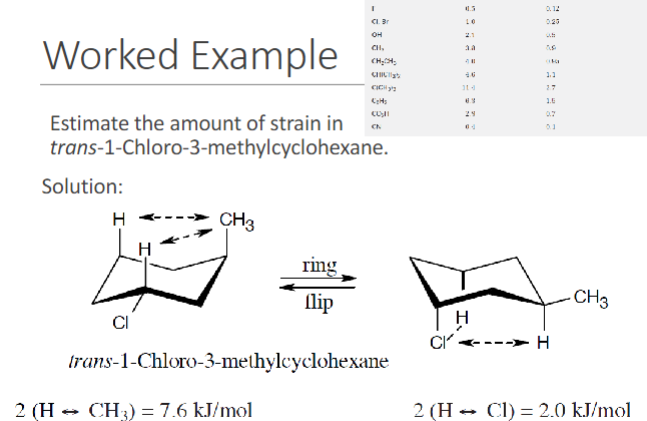
.
learning time: (estimating the amount of strain)