triesters, acyl chlorides
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
features of carbonyl group
strongly polar
Dipole-dipole forces
boiling points are high
big difference in electronegativity
why do shorter chain aldehydes and ketones mix completely with water ?
because hydrogen bonds form between oxygen of carbonyl group and water molecule
what is used to make soap from triesters
concentrated NaOH
show reaction between triester and naoh to make soap
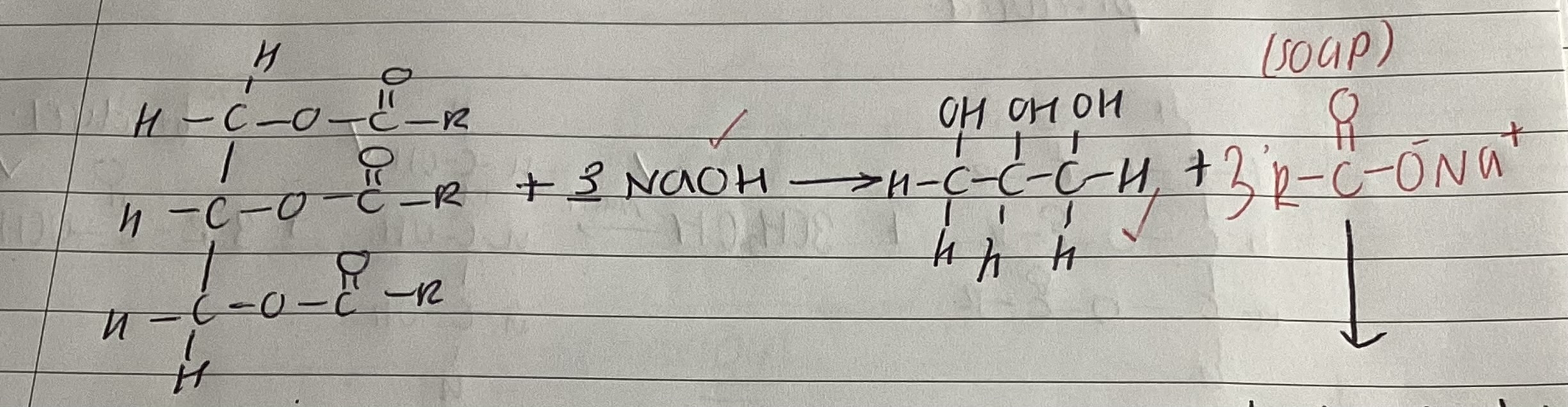
what is used to make biodiesel from triester?
methanol in presence of base catalyst, CH3OH
what is acylation
process by which an acyl group is introduced into another molecule
group of compounds are referred to as
acid derivatives
two carbons would read as,
ethanoyl
what does the nucleophile attack
the slightly positive C atom
Groups which strongly attract electrons tend to form the most
stable negative ions
which is more reactive acyl chlorides or acid anhydrides
acyl chlorides
what is the reaction mechanism called?
addition elimination
advantages of ethanoic anhydride as an acylating agent
cheaper
less corrosive
reacts less readily with water
safer as produced ethanoic acid, not HCl
ethanoic anhydride is used in the production of
aspirin
show mechanism with water
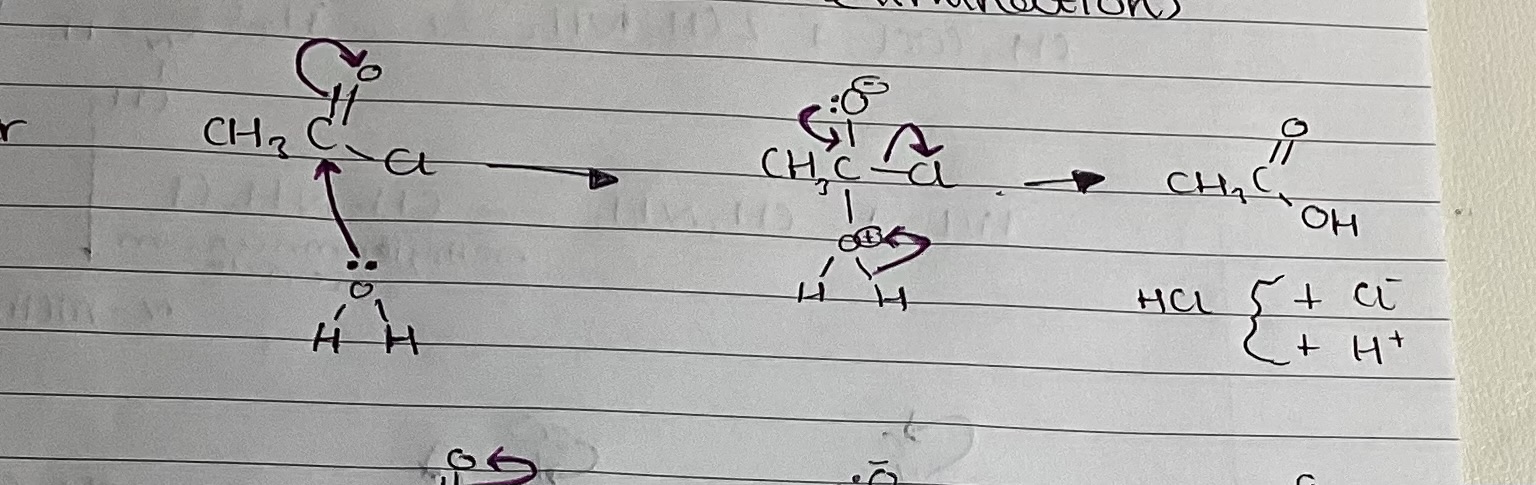
show mechanism with alcohol
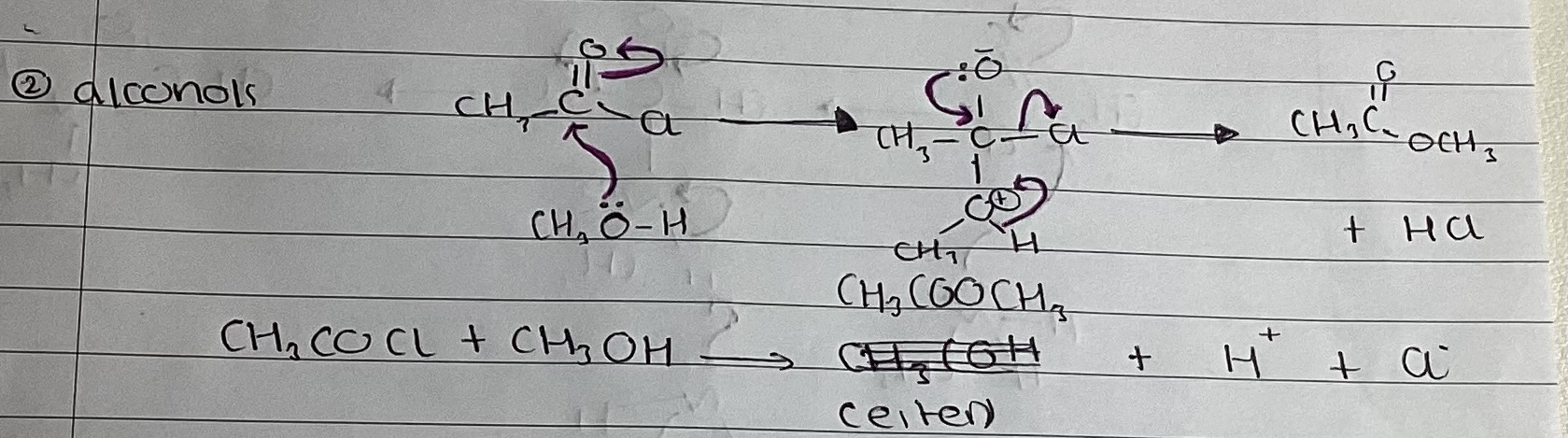
show mechanism with ammonia
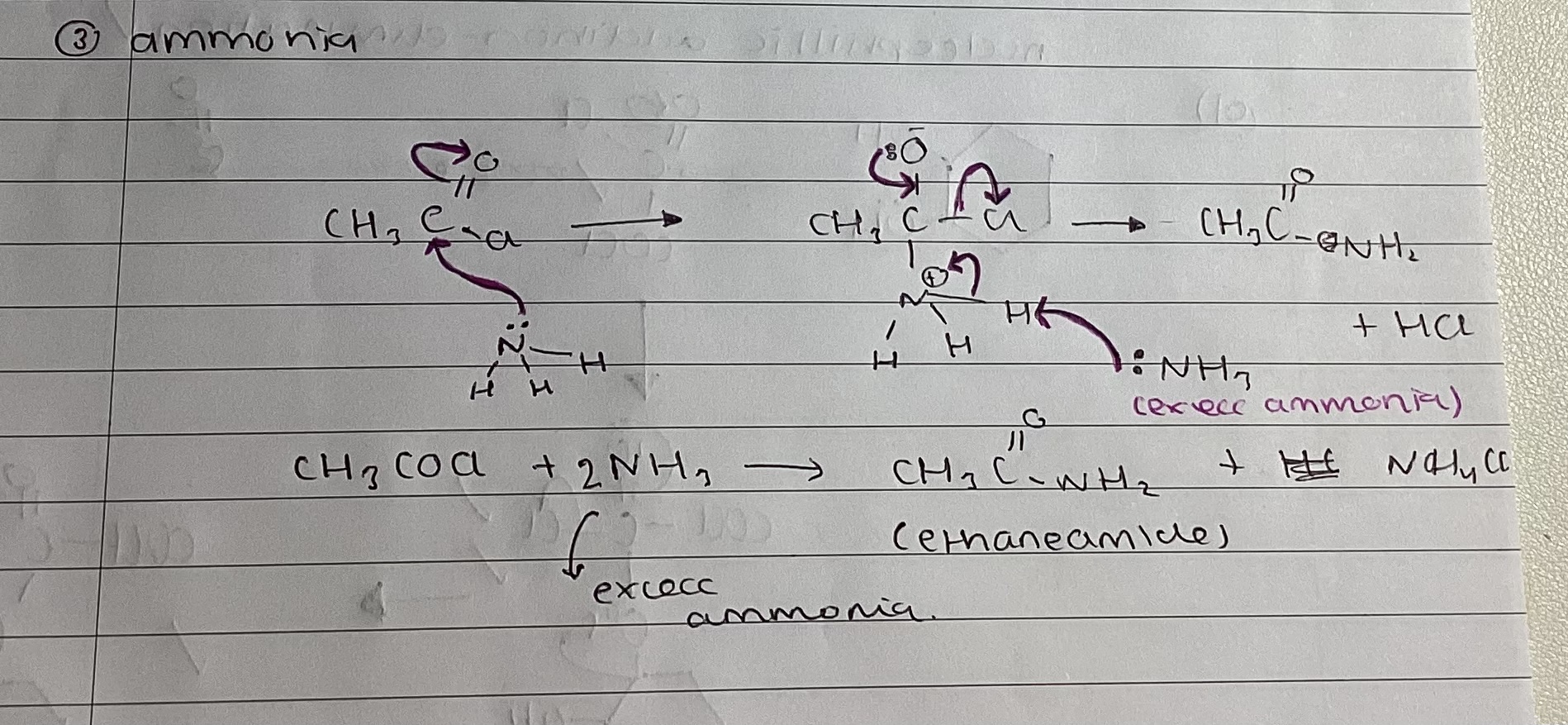
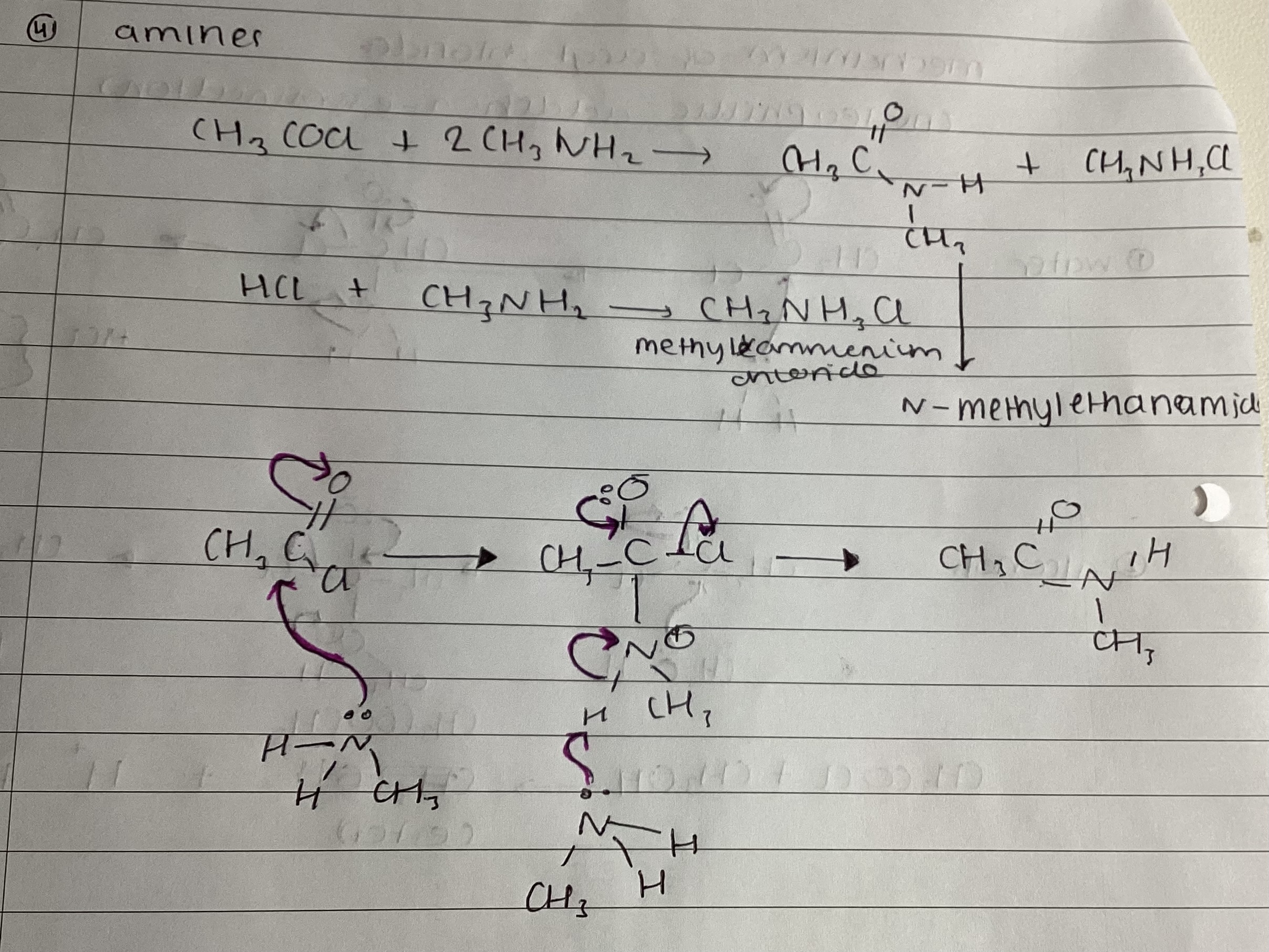
show mechanism with amines
draw ethanoic anhydride
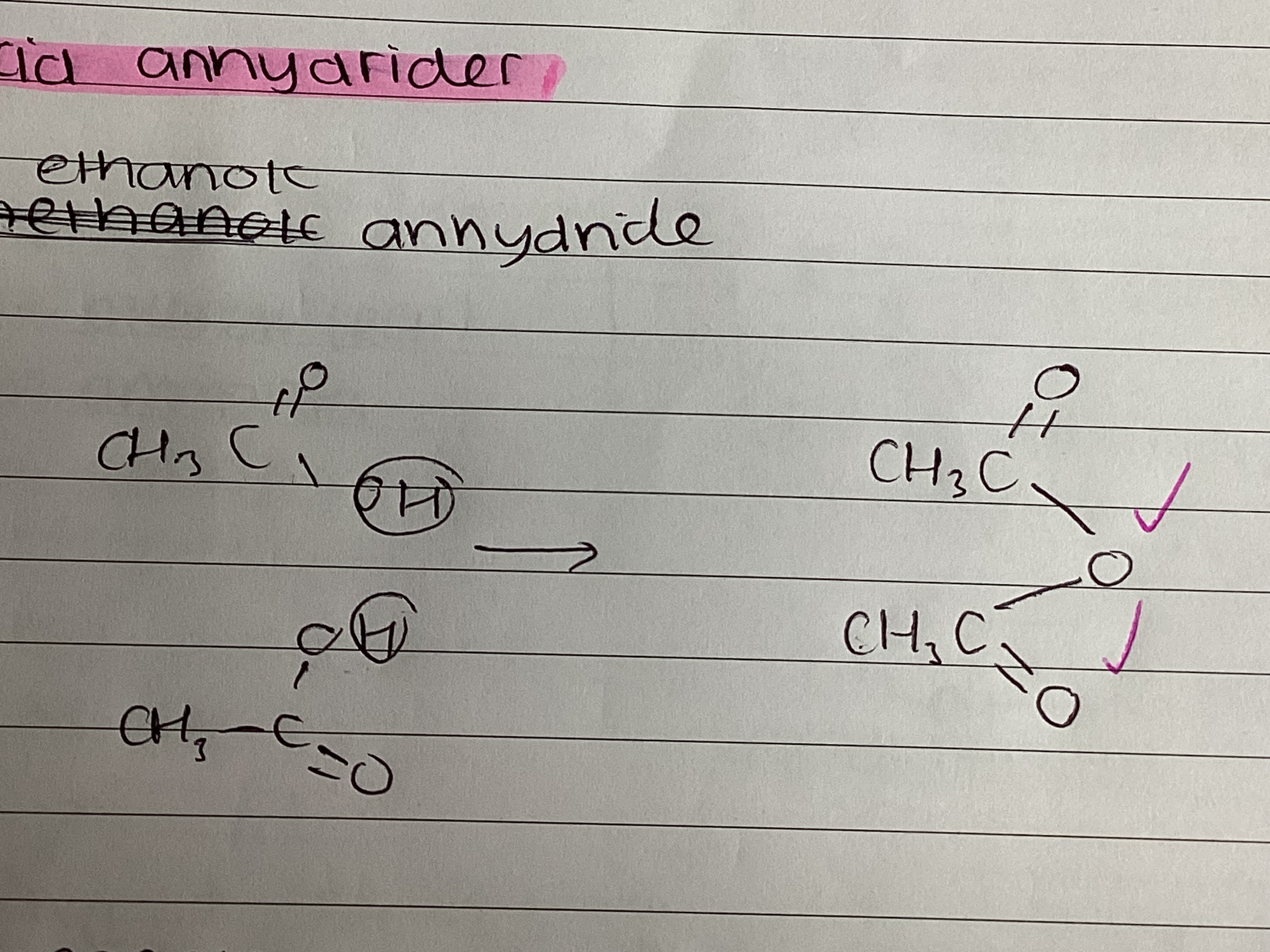
state a reaction between ethanoic anhydride and water
(CH3CO)2O + H2O → 2CCH3COOH
ethanoic anhydride and methanol
(CH3CO)2O + CH3OH → CH3COOCH3 + CH3COOH
ethanoic anhydride and ammonia
(CH3CO)2O + 2NH3 → CH3CONH2 + CH3COONH4
ethanoic anhydride and methylamine
(CH3CO)2O + 2CH3NH2 → CH3CONHCH3 + CH3COONH3
what is CH3COONHCH3 called
n-methyl ethanamide