ATOMIC STRUCTURE
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
Where are protons, neutrons and electrons found in the atom?
protons in the nucleus
neutrons in the nucleus
electrons in orbitals
What is the relative mass of protons, neutrons and electrons?
protons= 1
neutrons= 1
electrons= 1/1840 or 0
What is the relative charge of protons, neutrons and electrons?
protons= +1
neutrons= 0
electrons= -1
What is the atomic number?
Z
the number of protons in the nucleus
What is the mass number?
A
the total number of protons and neutrons in the atom
What are isotopes?
atoms with the same number of protons but different numbers of neutrons
What are chemical and physical properties like for isotopes of the same atom?
similar chemical properties as they have the same electronic structure
varying physical properties as they have different masses
What is a mass spectrometer used for?
to determine all the isotopes present in a sample of an element, to identify elements
Why does the time of flight mass spectrometer need to be in a vacuum?
to prevent air particles from ionising
What’re the 4 steps in the time of flight mass spectrometer?
ionisation
acceleration
flight tube
detection
What’re the 2 methods of ionisation?
electron impact
electrospray
When is electron impact ionisation used?
for elements and substances with low formula mass, as electron impact can cause larger organic molecules to fragment
What occurs in electron impact ionisation?
a vaporised sample is injected at low pressure
an electron gun fires high energy electrons at the sample
this knocks out an outer electron
forming positive ions with different charges
When is electrospray ionisation used?
with larger organic molecules as fragmentation does not occur
What occurs in electrospray ionisation?
sample is dissolved in a volatile, polar solvent
injected through a fine needle giving a fine mist or aerosol
the tip of needle has high voltage
at the tip of the needle the sample molecule gains a proton from solvent forming MH+
M(g) + H+ → MH+(g)
solvent evaporates away while the MH+ ions move towards a negative plate
What occurs during acceleration?
positive ions are accelerated by an electric field to a constant kinetic energy
as all particles have the same kinetic energy, the velocity of each particle depends on its mass
lighter particles have a faster velocity and heavier particles have a slower velocity
What occurs in the flight tube?
positive ions with smaller m/z values will have the same kinetic energy as those with larger m/z and will move faster
heavier particles take longer to move through the drift carea
the ions are distinguished by different flight times
t = d/v
What occurs in detection?
ions reach the detector and generate a small current, which is fed into a computer for analysis
current is produced by electrons transferring from the detector to the positive ions
size of the current is proportional to the abundance of the species
What does the mass spectrometer measure?
mass/ charge ratio
abundance
How would you calculate the relative atomic mass?
sum of isotopic mass x relative abundance/ total relative abundance
What would happen if a molecule is put through a mass spectrometer with an electron impact ionisation stage?
breaks up and gives a series of peaks caused by fragments
peak with the largest m/z is equal to the Mr
peak is called the parent peak
What would happen if a molecule is put through a mass spectrometer after electrospray ionisation?
fragmentation will not occur
one peak equal to the mass of the MH+ ion
subtract 1 to get the Mr
What is the A-level model of electronic structure?
principle energy levels numbered 1,2,3,4
sub energy levels labelled s, p, d, f
orbitals hold up to 2 electrons of opposite spin
How many electrons can the s orbital hold?
2
How many electrons can the p orbital hold?
6
How many electrons can the d orbital hold?
10
How many electrons can the f orbital hold?
14
How would you write oxygens electronic structure?
1s2 2s2 2p4
How would you write calciums electronic structure?
1s2 2s2 2p6 3s2 3p6 4s2
What do orbitals represent?
the probabilities of finding an electron at any point within certain spatial distributions around the nucleus
each orbital has its own 3 dimensional space
What is the shape of s sublevels?
spherical
What is the shape of p sublevels?
figure of 8 shaped
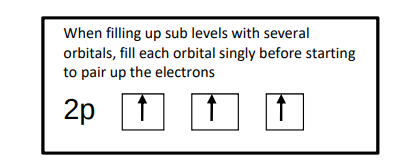
What’re spin diagrams?
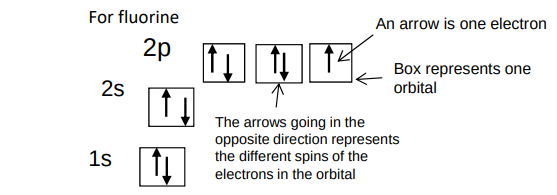
What would a spin diagram for fluorine be?
What’re the different blocks of the periodic table?
s block
p block
d block
What is the s block of the periodic table?
contain elements with their outer electron filling an s-subshell
What is the p block of the periodic table?
contains elements with their outer electron filling a p-subshell
What is the d block of the periodic table?
contains elements with their outer electron filling a d subshell
How are positive ions formed?
when electrons are lost from the outermost shell
How are negative ions formed?
when electrons are gained
What is the electronic structure of chromium?
1s2 2s2 2p6 3s2 3p6 4s1 3d5
What is the electronic structure of copper?
1s2 2s2 2p6 3s2 3p6 4s1 3d10
What is the definition of the first ionisation energy?
the enthalpy change when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge
What is the equation for the first ionisation energy?
H(g) → H+(g) + e-
What is the equation for the second ionisation energy?
the enthalpy change when one mole of gaseous ions with a single positive charge forms one mole of gaseous ions with a double positive charge
What is the equation for the second ionisation energy?
Ti+(g) → Ti2+ + e-
What’re the factors which affect ionisation energy?
the attraction of the nucleus
the distance of the electrons from the nucleus
shielding of the attraction of the nucleus
How does the attraction of the nucleus affect ionisation energy?
the more protons in the nucleus the greater the attraction
How does the distance of the electrons from the nucleus affect ionisation energy?
the bigger the atom, the further the outer electrons are from the nucleus and the weaker the attraction to the nucleus
How does shielding of the attraction of the nucleus affect ionisation energy?
an electron in an outershell is repelled by electrons in complete inner shells, weakening the attraction of the nucleus
Why are second ionisation energies always larger than the first ionisation energy?
as when the first electron is removed a positive ion is formed, which increases the attraction on the remaining electrons so the energy required to remove the next electron is larger
Why has helium got the largest ionisation energy?
its first electron is in the first shell closest to the nucleus and has no shielding effects from inner shells, so has a larger ionisation energy than hydrogen as it has one more proton
Why do first ionisation energies decrease down a group?
as one goes down a group, the outer electrons are found in shells further from the nucleus and are more shielded so the attraction of the nucleus becomes smaller
Why is there a general increase in the first ionisation energy across a period?
as one goes across a period, the electrons are being added to the same shell which has the same distance from the nucleus and the same shielding effect. the number of protons increases, making the effective attraction of the nucleus greater
Why has sodium got a lower first ionisation energy than neon?
sodium will have its outer electron in a 3s shell further from the nucleus and is more shielded, so sodiums outer electron is easier to remove and has a lower ionisation energy
Why is there a small drop from magnesium to alumium?
alumium is starting to fill a 3p sub shell wheras magnesium has its outer electrons in the 3s subshell. the electrons in the 3p subshell are slightly easier to remove because the 3p electrons are higher in energy and are also slightly shielded by the 3s electrons
Why is ther a small drop from phosphorus to sulfur?
with sulfur there are 4 electrons in the 3p subshell and the 4th is starting to fill the firs 3p orbital. when the second electron is added to a 3p orbital there is a slight repulsion between the 2 negatively charged electrons which makes the second electron easier to remove
What happens if a graph of second ionisation energies is plotted?
similar pattern to the first ionisation energy graph
all elements will have shifted to the left
group 1 elements are now the peaks of the graph