A&P Fluid Electrolyte Acid-Base Homeostasis
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
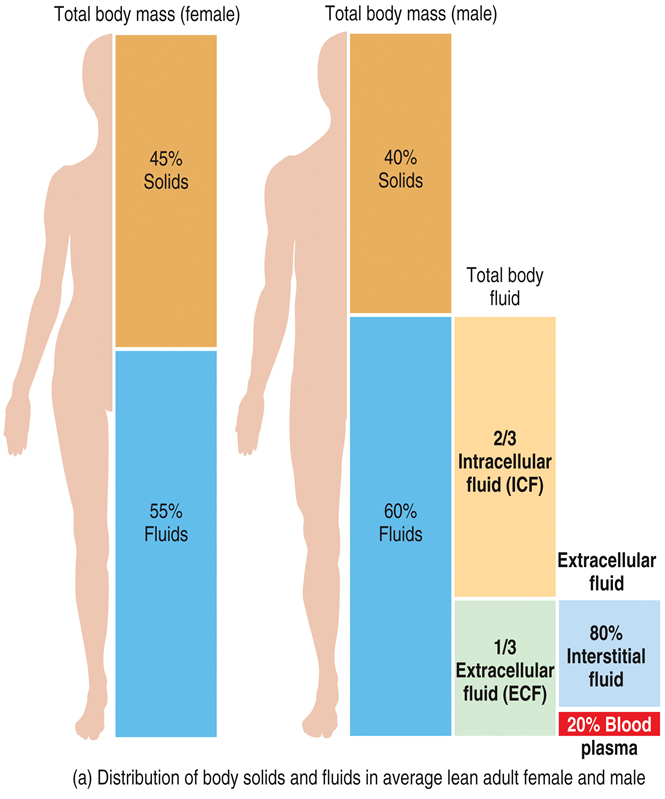
In adults, body fluids make up
◦between 55% and 65% of total body mass
Body fluids are present in two main compartments
inside cells intracellular (2/3) and outside cells extracellular (1/3)
Intracellular fluid is
cytosol
Extracellular fluid is
◦interstitial fluid (80%) and blood plasma (20%)
PHOTO OF MAN AND WOAMN MAKEUP
Intracellular and extracellular
The plasma membrane OF CELLS
◦separates intracellular fluid from interstitial fluid
Blood vessel walls divide the
◦interstitial fluid from blood plasma
Capillary walls are thin enough to
◦allow exchange of water and solutes between blood plasma and interstitial fluid
pHOTO BLOOD CAPILLARY AND TISSUE CELLS
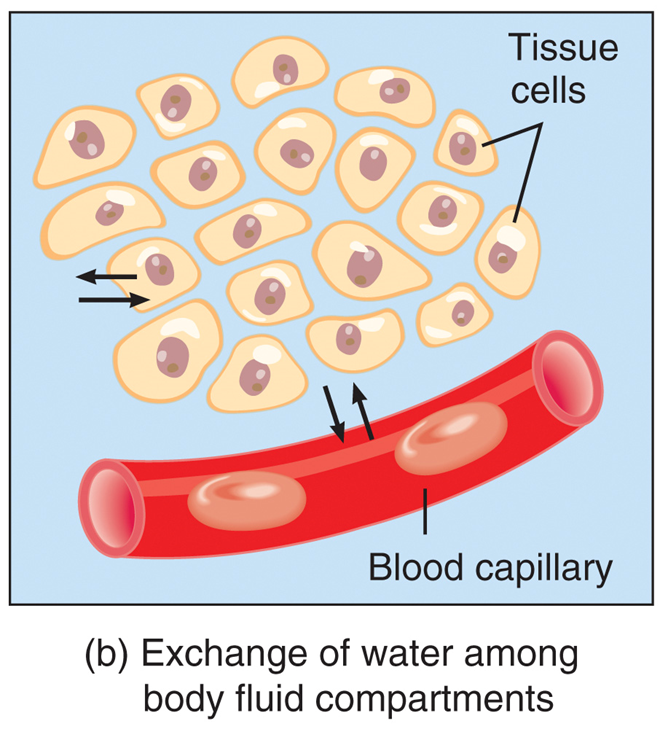
Filtration, reabsorption, diffusion, and osmosis allow
◦continuous exchange of water and solutes among body fluid compartments
The balance of inorganic compounds that dissociate into ions (electrolytes) is closely related to
◦fluid balance
The body gains water by
◦ingestion and metabolic synthesis
The body loses water via
◦urination, perspiration, exhalation, and in feces
Volume of water gain and loss
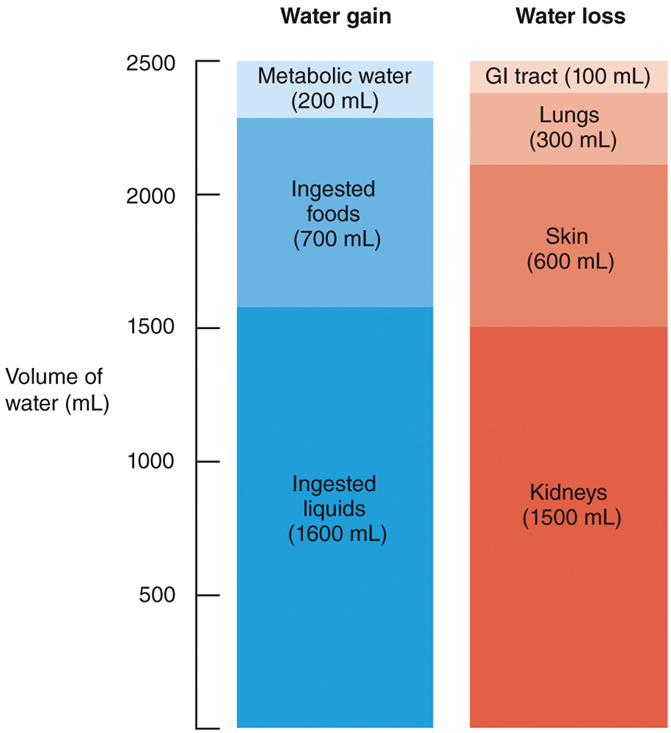
•The level of aerobic respiration determines the volume
•of metabolic water formed
•The amount of water formed is
•directly proportional to the amount of ATP produced
•When water loss is greater than water gain,
•dehydration occurs, leading to increased thirst
Fluid Compartments and Fluid Homeostasis: The Thirst Response
Photo
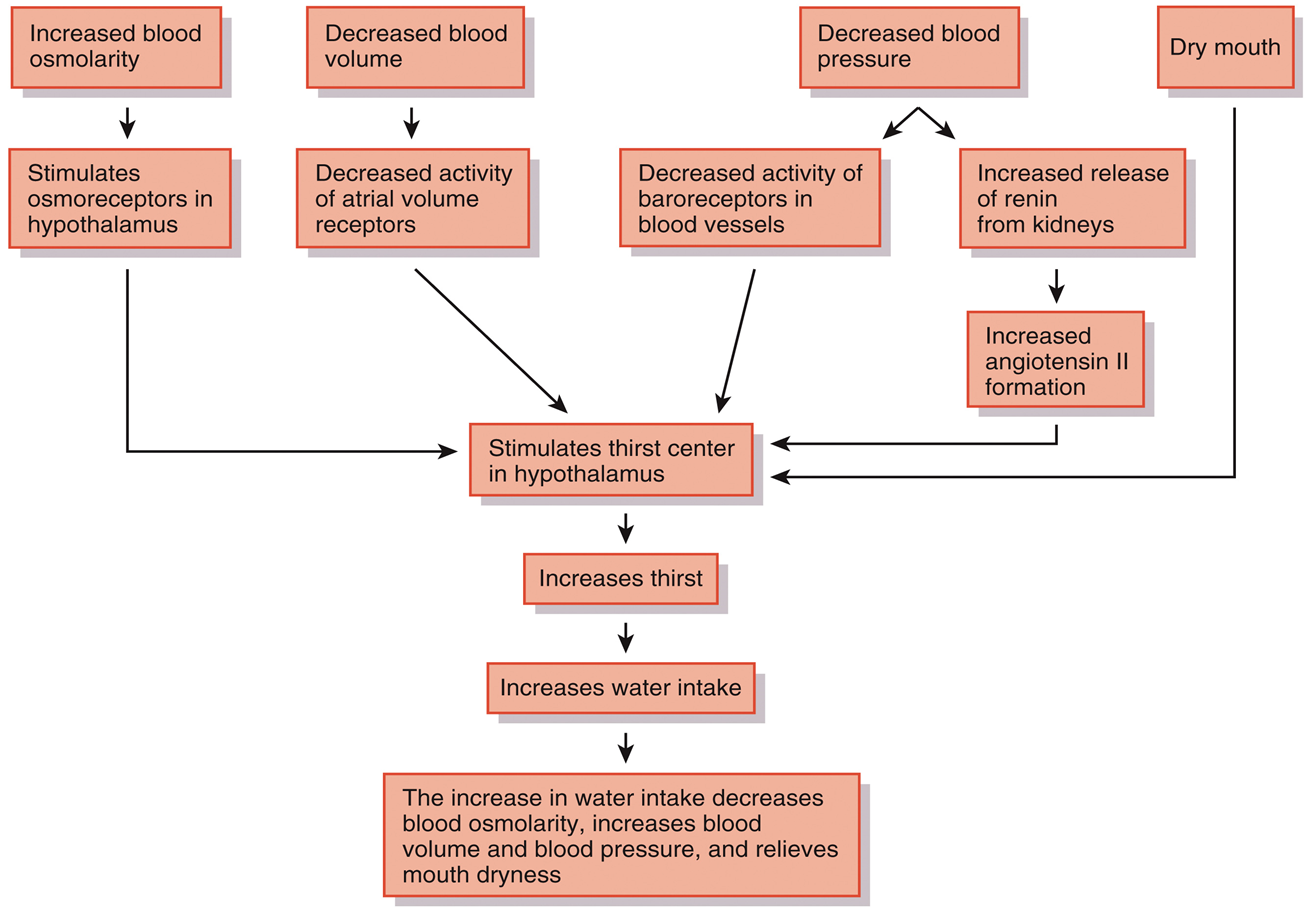
•Elimination of excess body water occurs through
Urine production
•The amount of urinary salt loss is
•the main factor determining body fluid volume
•The two main solutes in urine are
•sodium ions and chloride ions
•Wherever solutes go, water follows
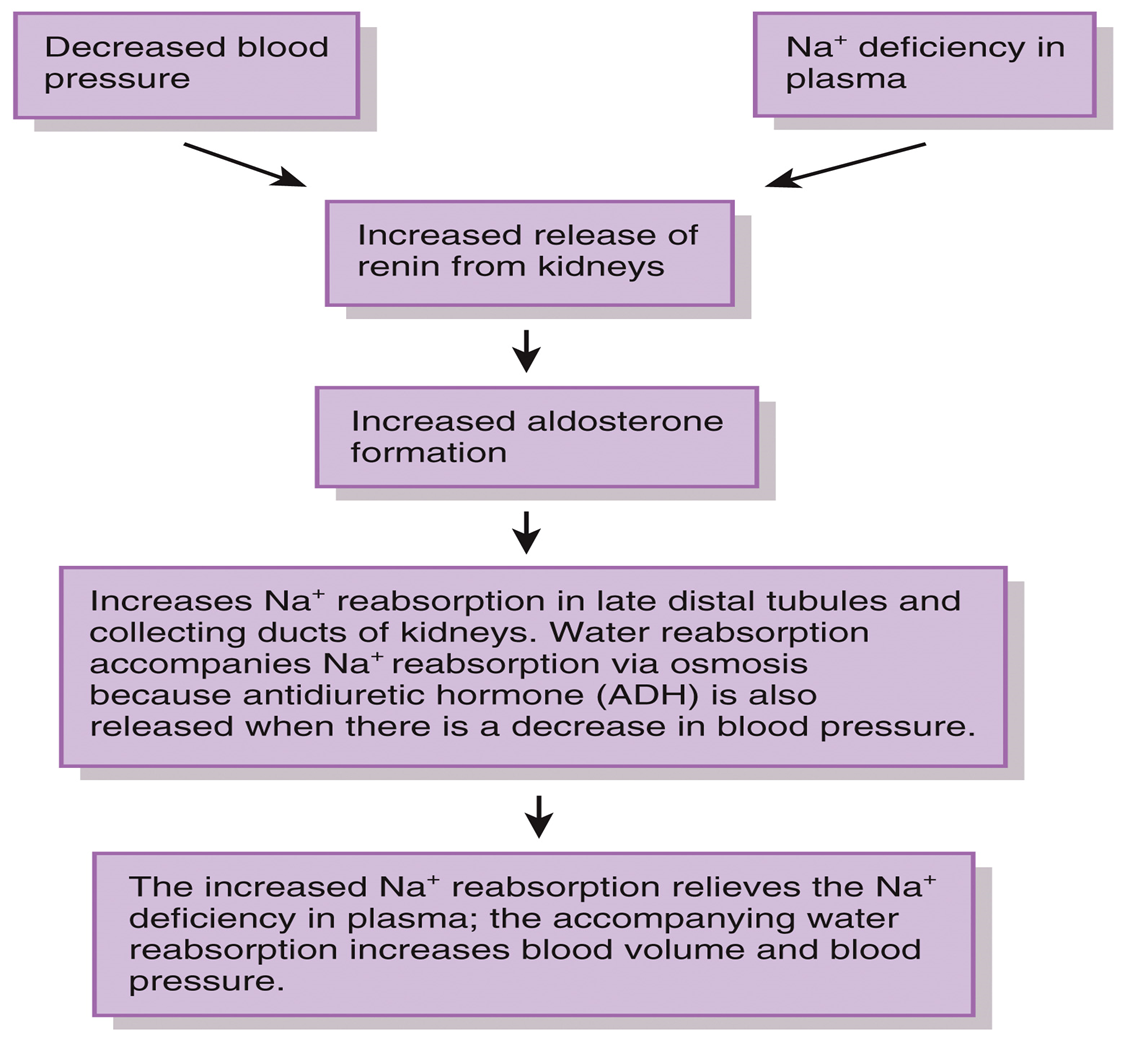
•Three major hormones control renal sodium and chloride ions
•Angiotensin II
•Aldosterone
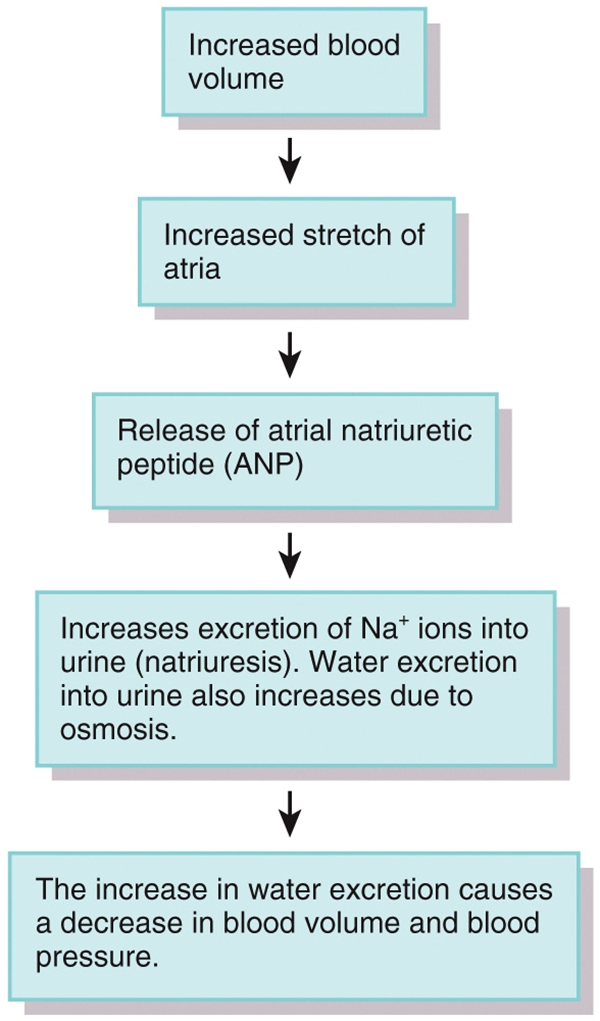
•Atrial natriuretic peptide (ANP)
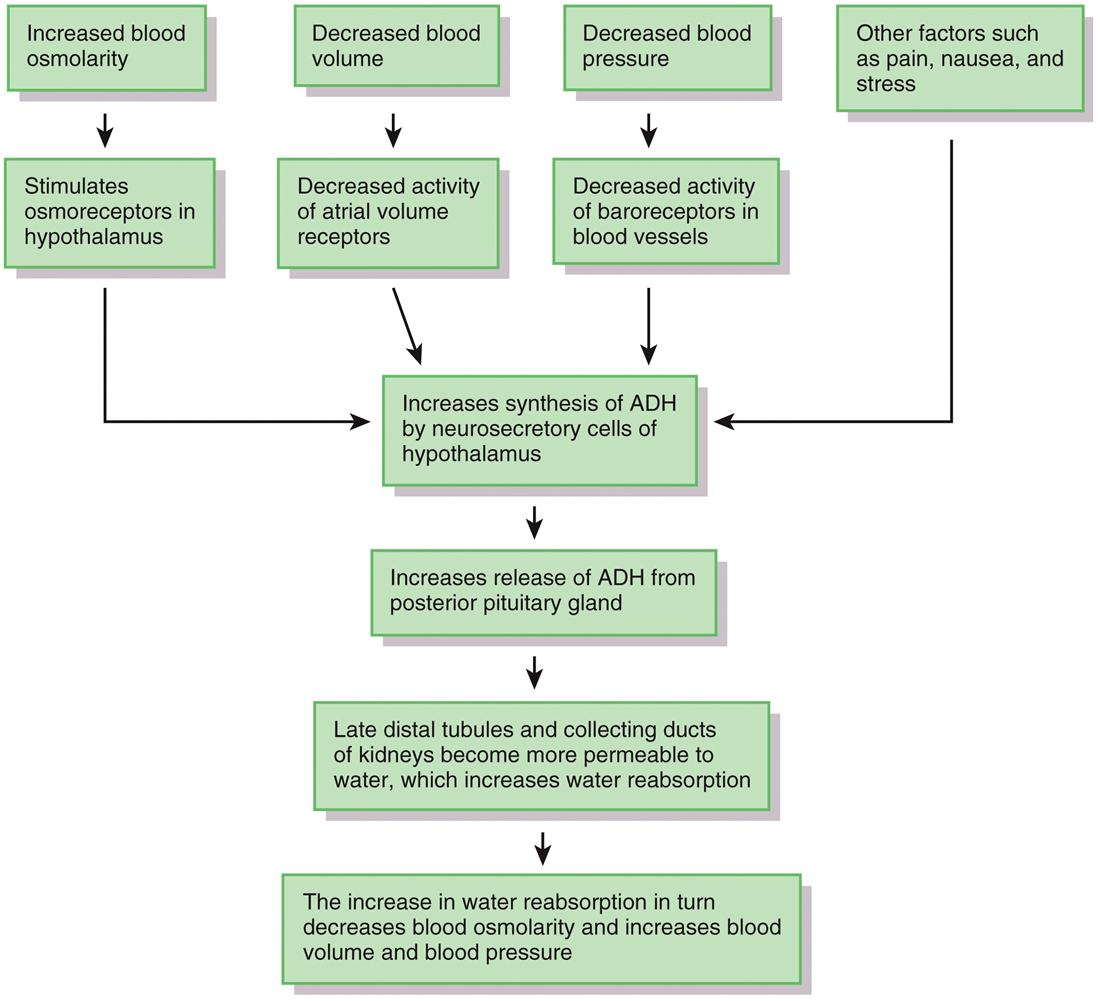
•The major hormone that regulates water loss is
•antidiuretic hormone (ADH)
flow chart regulation of water and solute loss adh photo antidiuretic
Regulation of Water and Solute Loss: Aldosterone
flow chart photo
Regulation of Water and Solute Loss: ANP atrial natriuretic peptide
flow chart
•When the extracellular fluid is isotonic to the cells of the body,
•they do not shrink or swell
•changes in the osmolarity of the extracellular fluids (as with dehydration or over-hydration) can cause
•the cells of the body to shrink or swell
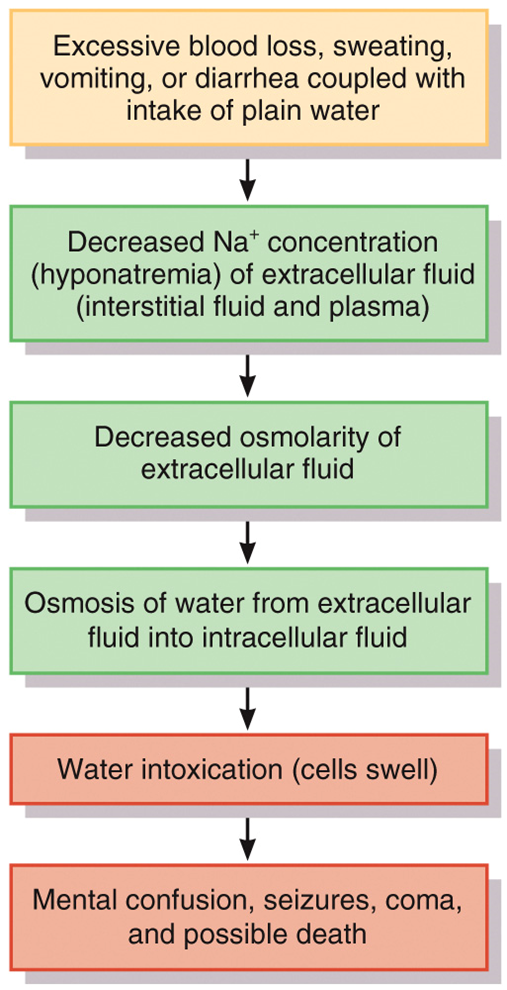
Water intoxication occurs when
excess body water causes cells to swell dangerously
This may occur when a person consumes water faster than the kidneys can excrete it
Water intoxication flow chart photo
Ions formed when
◦electrolytes dissociate and dissolve:
◦Electrolytes in Body Fluids
Ions formed when electrolytes dissociate and dissolve:
◦Control osmosis of water between fluid compartments
◦Help maintain the acid-base balance
◦Carry electrical current
◦Serve as cofactors
Electrolytes in Body Fluids Photo
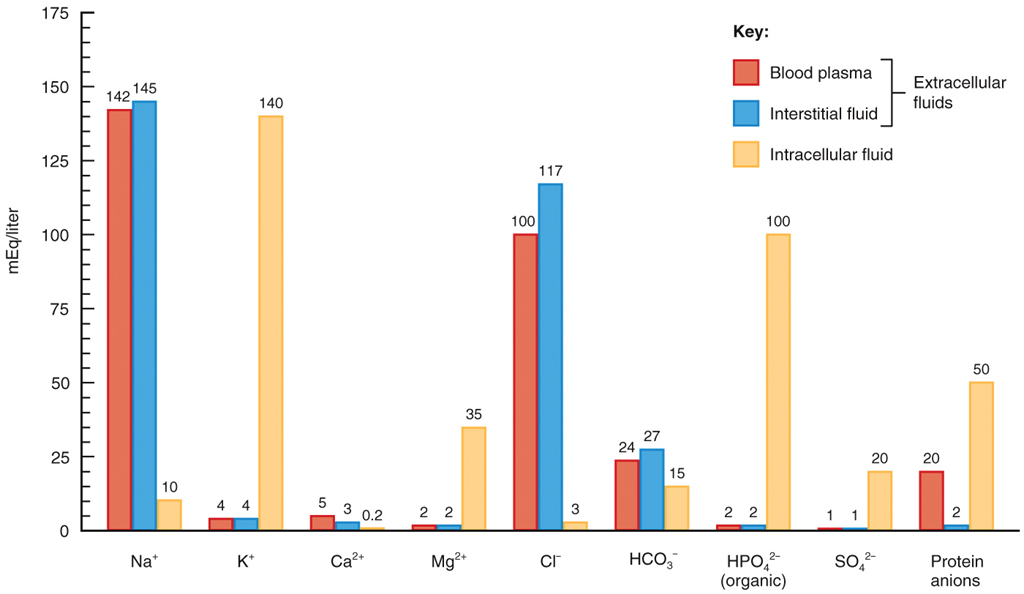
◦Blood plasma contains ___ protein ions;
interstitial fluid contains ____
Many
only a few
•most abundant cation in extracellular fluid
Sodium
•the major extracellular anion
Chloride
Sodium is used for
•Used for impulse transmission, muscle contraction, fluid, and electrolyte balance
•Its level is controlled by aldosterone, ADH, and ANP
Chloride helps regulate
•Helps regulate osmotic pressure between compartments
•Forms HCl in the stomach
•most abundant cation in intracellular fluid
•Potassium
•important plasma ion
Bicarbonate
•Potassium:
•Involved in fluid volume, impulse conduction, muscle contraction, and regulating pH
•Mineralocorticoids (mainly aldosterone) regulate the plasma level
•Bicarbonate:
•Major member of the plasma acid-base buffer system
•Kidneys reabsorb or secrete it for final acid-base balance
most abundant mineral in the body
Calcium
•occurs as calcium phosphate salt
Phosphate
•an intracellular cation
MAGNESIUM
•Calcium:
•Structural component of bones and teeth
•Used for blood coagulation, neurotransmitter release, muscle tone, excitability of nerves and muscles
•Level in plasma regulated by parathyroid hormone
•Phosphate:
•Used in the buffer system
•Regulated by parathyroid hormone and calcitriol
•Magnesium:
•Blood plasma, interstitial fluid, and intracellular fluid have different concentrations of electrolytes and protein ions
•Blood plasma contains many protein ions; interstitial fluid contains only a few
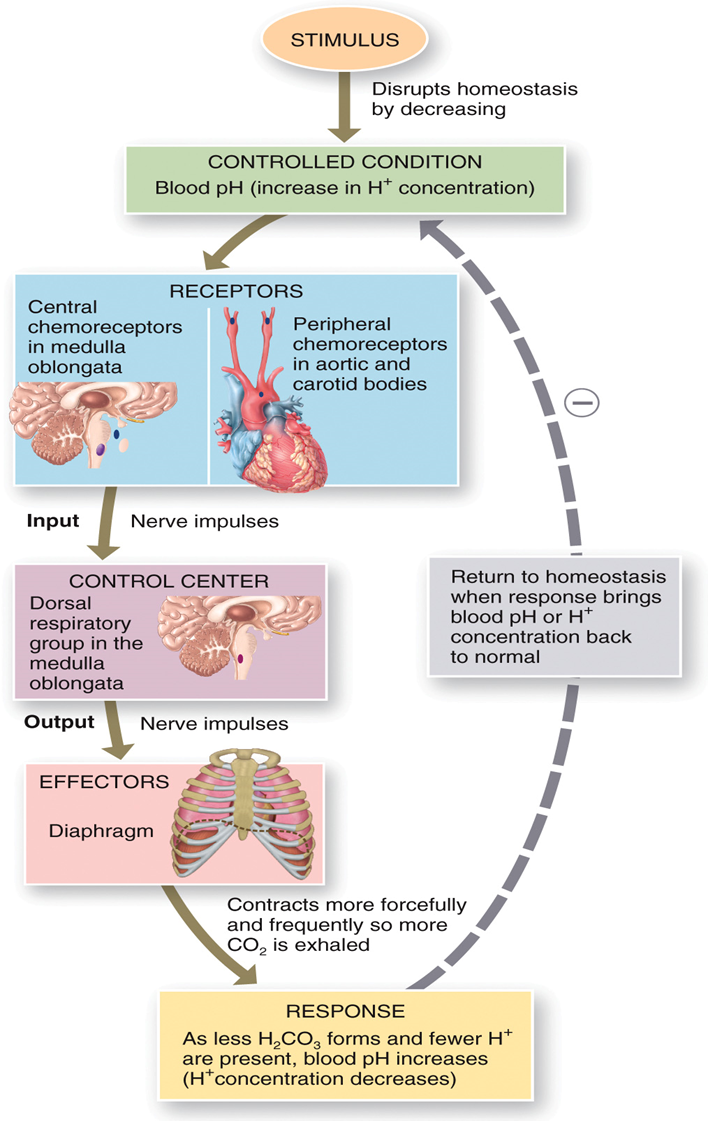
•The pH of arterial blood ranges from
•7.35 to 7.45
•Mechanisms that maintain PH
•Kidney excretion of H+
•Exhalation of carbon dioxide
•Buffer systems
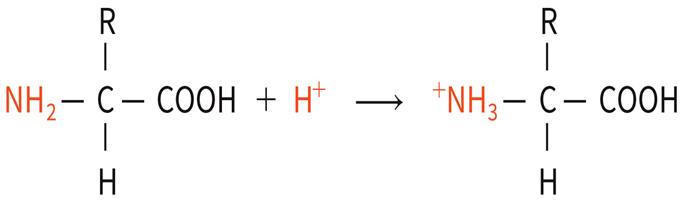
Equation photos release hydrogen ion
Accept hydrogen ion photo
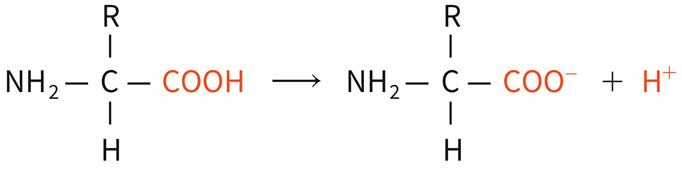
◦Buffer systems include: Protein buffer system:
◦most abundant in intracellular fluid and blood plasma. When pH rises, the COOH group dissociates to act like an acid.
When pH falls,
◦the free amino group dissociates to act like a base
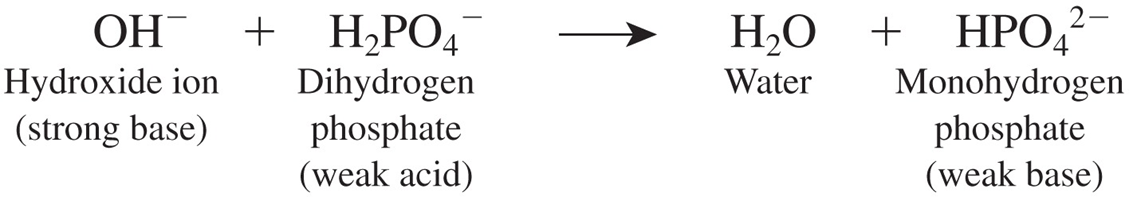
Carbonic acid-bicarbonate buffer system:
this is based on the bicarbonate ion (HCO3-) which acts as a weak base and carbonic acid (H2CO3) which acts as a weak acid
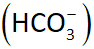
If the pH falls, the
bicarbonate ion removes excess h+
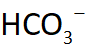
ACID BASE BALANCE EQUATION
HYDROGEN ION AND BICARBONATE ION =
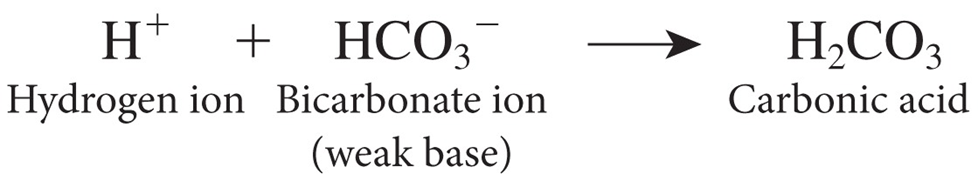
Exhalation of carbon dioxide: CO2 mixes with
water in the blood to form carbonic acid (H2C O3).
Exhaling CO2 leads to
less acid production and a rise in pH whereas retaining CO2 leads to more acid production and a drop in pH
aCID BASE BALANCE EQUATION PHOTO CO2 AND H20
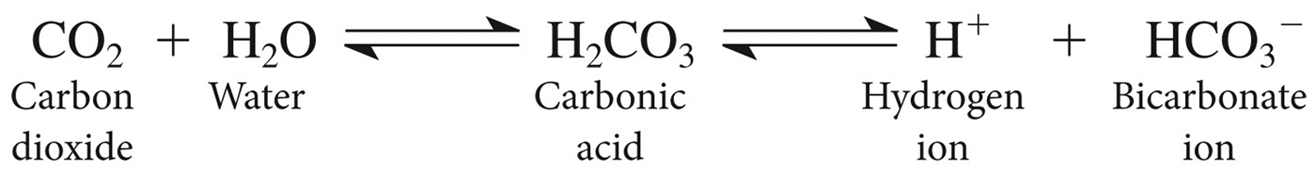
STIMULUS AND HOMEOSTASIS PHOTO
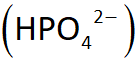
Phosphate buffer system: PHOSPHATE are the ions used PHOTO/ EQUATION
this system acts similarly to the carbonic acid-bicarbonate buffer system
◦Kidney excretion of H+:
◦Excreting H+ in the urine removes nonvolatile acids
◦The proximal convoluted tubules and collecting ducts of the kidneys secrete H+ into the tubular fluid
Buffer systems
Most consist of a weak acid and its salt, which functions as a weak base. They prevent drastic changes in body fluid pH.
Proteins buffer system
The most abundant buffers in body cells and blood. Hemoglobin inside red blood cells is a good buffer.
Carbonic acid-
bicarbonate buffer system
Important regulator of blood pH. The most abundant buffers in extracellular fluid (ECF).
Phosphates buffer system
Important buffers in intracellular fluid and urine.
Exhalation of CO2
With increased exhalation of CO2 pH rises (fewer H+). With decreased exhalation of CO2, pH falls (more H+)
Kidneys
Renal tubules secrete H+ into urine and reabsorb HCO3- so it is not lost in urine
•Acidosis blood pH is
below 7.35
•Respiratory acidosis
• blood pH drops due to excessive retention of CO2 leading to excess H2CO3
•Metabolic acidosis:
• arterial blood levels of HCO3– falls
•Alkalosis blood pH is
above 7.45
•Respiratory alkalosis:
•blood pH rises due to excessive loss of CO2 as in hyperventilation
•Metabolic alkalosis:
•arterial blood levels of HCO3– rises
DEFINITION:
Respiratory acidosis
Increased PCO2 (above 45 mmHg) and decreased p H (below 7.35) if no compensation
Cause of Respiratory acidosis
Hypoventilation due to emphysema, pulmonary edema, trauma to respiratory center, airway obstructions, or dysfunction of muscles of respiration.
Compensatory mechanism of Respiratory acidosis
Renal: increased excretion of H+; increased reabsorption of H C O3—. If compensation is complete, p H will be within normal range but PCO2 will be high.
Definition Respiratory alkalosis
Decreased PC O2 (below 35 mmHg) and increased p H (above 7.45) if no compensation.
Causes of Respiratory alkalosis
Hyperventilation due to oxygen deficiency, pulmonary disease,
cerebrovascular accident (CVA), or severe anxiety.
Compensatory mechanism Respiratory alkalosis
Renal: decreased excretion of H+; decreased reabsorption of H C O3 . If compensation is complete, pH will be within normal range but Pc o2 will be low.
Metabolic acidosis
definition
Decreased H C O3_ (below 22 mEq/liter) and decreased pH (below 7.35) if no compensation.
Metabolic acidosis
causes
Loss of bicarbonate ions due to diarrhea, accumulation of acid
(ketosis), renal dysfunction.
Metabolic acidosis
compensatory mechanism
Respiratory: hypoventilation, which slows loss of C O2. If compensation is complete, pH will be within normal
range but H C O3− will be high.
Metabolic alkalosis
definition
Increased H C O3− (above 26 mEq/ liter) and increased pH (above 7.45) if no compensation.
Metabolic alkalosis
causes
Loss of acid due to vomiting, gastric suctioning, or use of certain diuretics; excessive intake of alkaline drugs.
Metabolic alkalosis
compensatory mechanism
Respiratory: hypoventilation, which slows loss of C O2. If compensation is complete, pH will be within normal range but HCO3− will be high
•Significant differences exist between adults and infants with respect to
•fluid distribution, regulation of fluid and electrolyte balance, and acid-base homeostasis
•Factors that influence distribution:
•Metabolic rate
•Functional development of the kidneys
•Body surface area
•Breathing rate
•Ion concentrations
•Older adults often have impaired ability to
•maintain fluid, electrolyte and acid-base balance
•Older adults often suffer from
•dehydration, hypernatremia, hypokalemia, and acidosis
Sodium (Na+) deficiency and signs and symptoms
Hyponatremia may be due to decreased sodium intake; increased sodium loss through vomiting, diarrhea, aldosterone deficiency, or taking certain diuretics; and excessive water intake.
Muscular weakness; dizziness, headache, and hypotension; tachycardia and shock; mental confusion, stupor, and coma.
Sodium (Na+) excess and signs and symptoms
Hypernatremia may occur with dehydration, water deprivation,
or excessive sodium in diet or intravenous fluids; causes
hypertonicity of ECF, which pulls water out of body cells into ECF,
causing cellular dehydration.
Intense thirst, hypertension, edema, agitation, and convulsions.
Chloride (CI_) deficiency and signs and symptoms
Hypochloremia may be due to excessive vomiting, overhydration, aldosterone deficiency, congestive heart failure, and therapy with certain diuretics such as furosemide (Lasix®).
Muscle spasms, metabolic alkalosis, shallow respirations, hypotension, and tetany.
Chloride (CI_) excess name and causes signs and symptoms
Hyperchloremia may result from dehydration due to water loss or water deprivation; excessive chloride intake; or severe renal failure, hyperaldosteronism, certain types of acidosis, and some drugs.
Lethargy, weakness, metabolic acidosis, and rapid, deep breathing. certain types of acidosis, and some drugs.
Potassium (K+) deficiency signs and symptoms
Hypokalemia may result from excessive loss due to vomiting or diarrhea, decreased potassium intake, hyperaldosteronism, kidney disease, and therapy with some diuretics.
Muscle fatigue, flaccid paralysis, mental confusion, increased urine output, shallow respirations, and changes in electrocardiogram, including flattening of T wave.
Potassium (K +) excess signs and symptoms
Hyperkalemia may be due to excessive potassium intake, renal failure, aldosterone deficiency, crushing injuries to body tissues, or transfusion of hemolyzed blood.
Irritability, nausea, vomiting, diarrhea, muscular weakness; can cause death by inducing ventricular fibrillation.
Calcium (C a2 +) deficiency signs and symptoms
Hypocalcemia may be due to increased calcium loss, reduced calcium intake, elevated phosphate levels, or hyperparathyroidism.
Numbness and tingling of fingers; hyperactive reflexes, muscle cramps, tetany, and convulsions; bone fractures; spasms of laryngeal muscles that can cause death by asphyxiation.
Calcium (C a2 +) excess signs and symptoms
Hypercalcemia may result from hyperparathyroidism, some cancers, excessive intake of vitamin D, and Paget's disease of bone.
Lethargy, weakness, anorexia, nausea, vomiting, polyuria, itching, bone pain, depression, confusion, paresthesia, stupor, and coma.
Phosphate (HP04 2-)
deficiency and signs and symptoms
Hypophosphatemia may occur through increased urinary losses, decreased intestinal absorption, or increased utilization.
Confusion, seizures, coma, chest and muscle pain, numbness and tingling of fingers, decreased coordination, memory loss, and lethargy.
Phosphate (HP04 2-) excess and signs and symptoms
Hyperphosphatemia occurs when kidneys fail to excrete excess phosphate, as in renal failure; can also result from increased intake of phosphates or destruction of body cells, which releases phosphates into blood.
Anorexia, nausea, vomiting, muscular weakness, hyperactive reflexes, tetany, And tachycardia.