Quiz 4 Biochemistry
1/290
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
291 Terms
How wide and deep is the major groove in DNA?
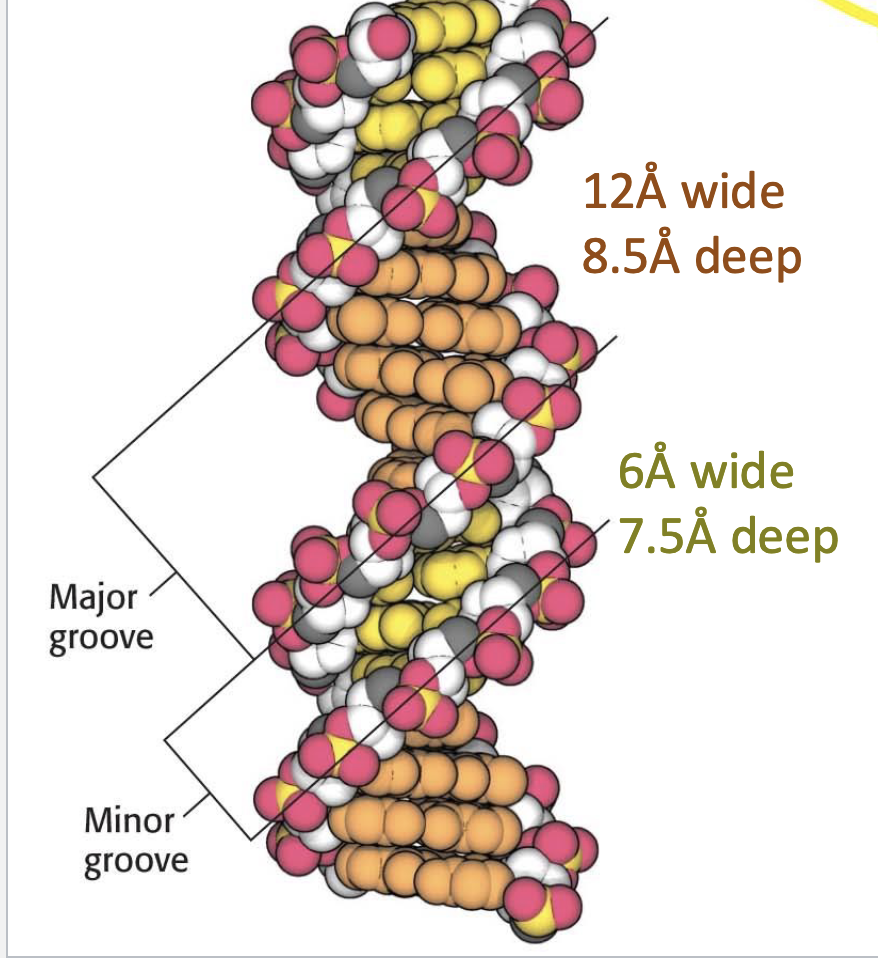
12 Å wide and 8.5 Å deep.
How wide and deep is the minor groove in DNA?
6 Å wide and 7.5 Å deep.
Why is the major groove more commonly used by DNA-binding proteins?
It is wider and exposes more specific base-pair information, making it easier for proteins to recognize DNA sequences.
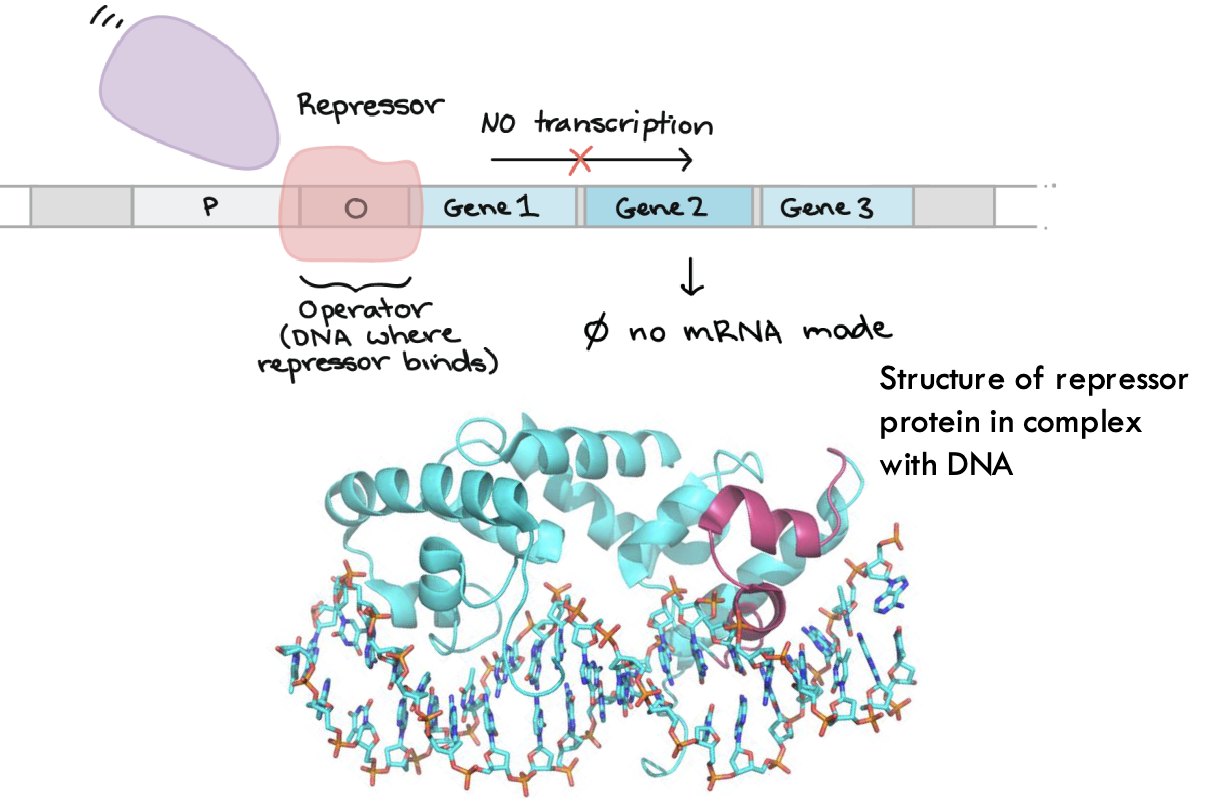
What happens when a repressor protein binds to the DNA operator?
Transcription is blocked, and no mRNA is produced.
What type of base-pair information is visible in the major groove?
Hydrogen bond donors/acceptors and methyl groups unique to each base pair (e.g., A-T vs. G-C).
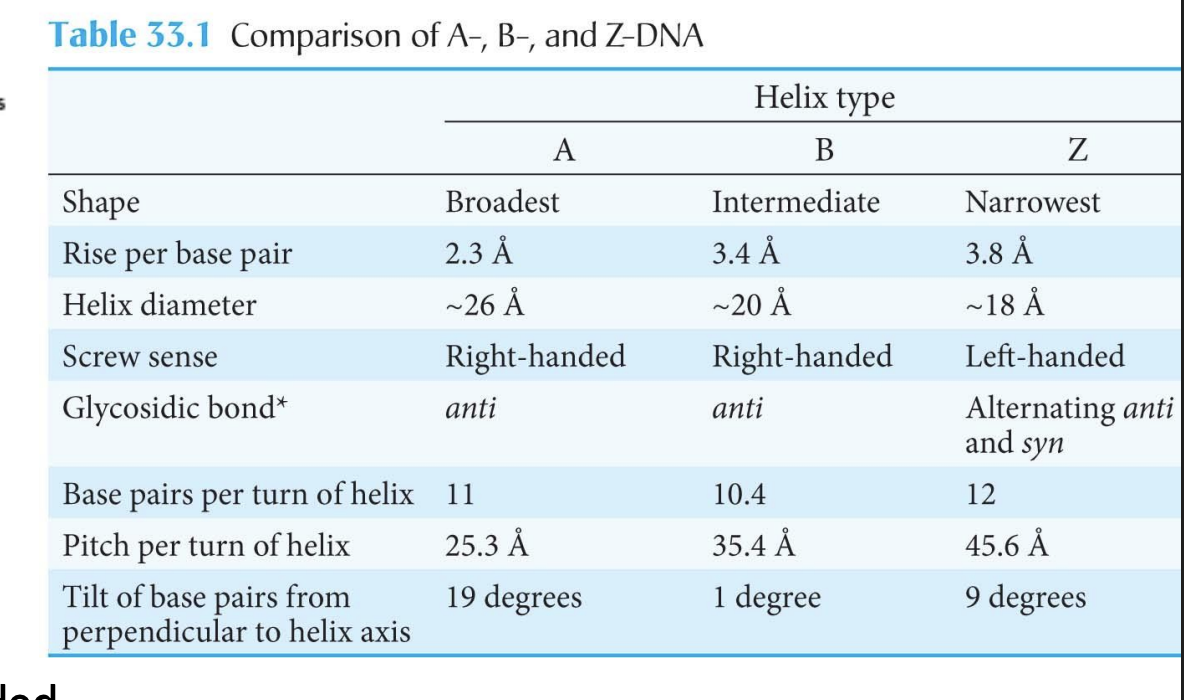
What are the three main forms of the DNA helix?
A-DNA, B-DNA (most common), and Z-DNA.
Example: B-DNA is the typical form in cells, with right-handed twist and 10.4 base pairs per turn.
What is the tilt of base pairs in A, B, and Z forms of DNA?
A-DNA: 19°
B-DNA: 1°
Z-DNA: 9°
Example: The nearly perpendicular base tilt in B-DNA allows stable interactions with proteins.
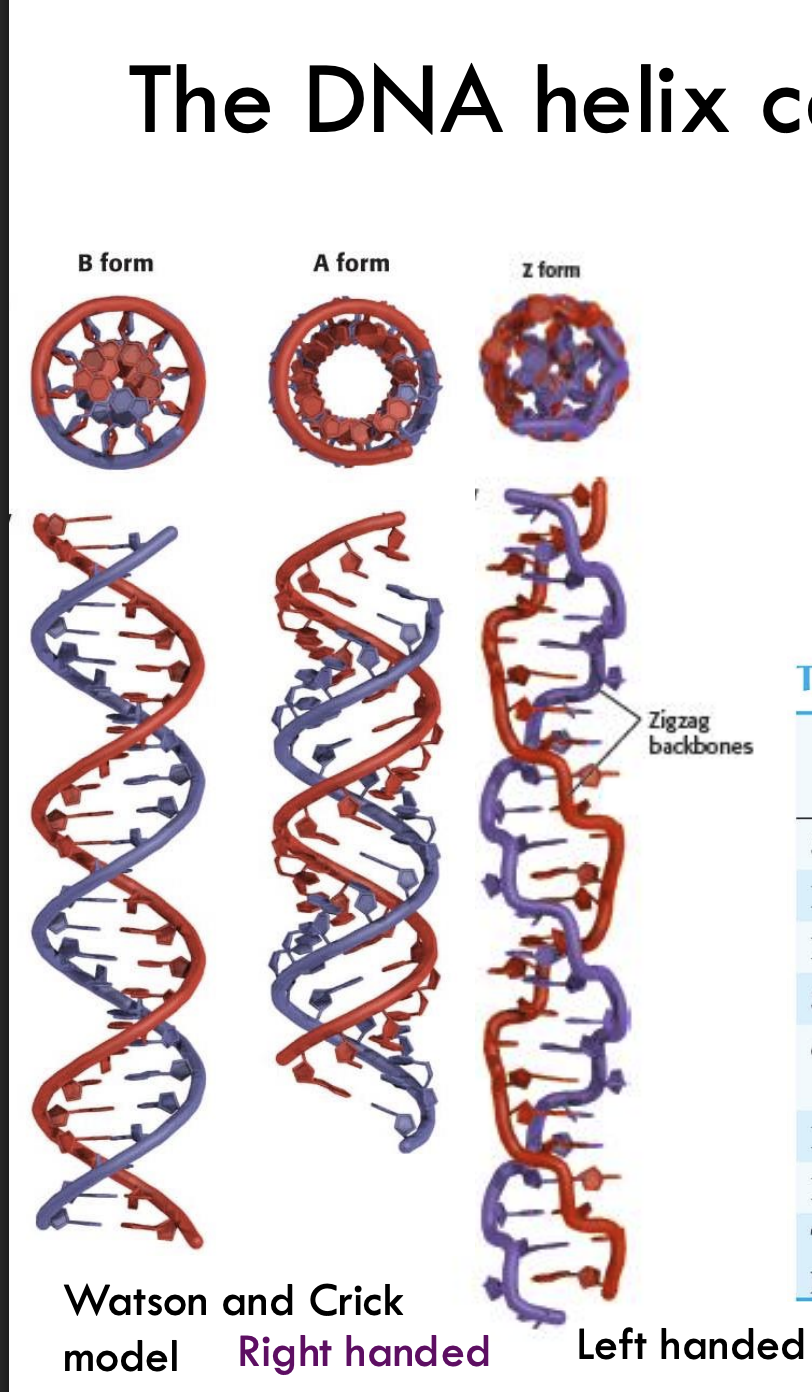
Which glycosidic bond orientations are found in A, B, and Z-DNA?
A & B: anti
Z: alternating anti and syn
Example: In Z-DNA, guanines are anti and cytosines are syn, creating a zig-zag backbone.
How do helix diameters compare among A, B, and Z-DNA?
A-DNA: ~26 Å (broadest)
B-DNA: ~20 Å
Z-DNA: ~18 Å (narrowest)
What determines major vs. minor grooves in DNA?
The distance between sugar-phosphate backbones:
Shorter distance = minor groove
Larger distance = major groove
Example: Proteins often bind in the major groove due to easier access to base-specific information.
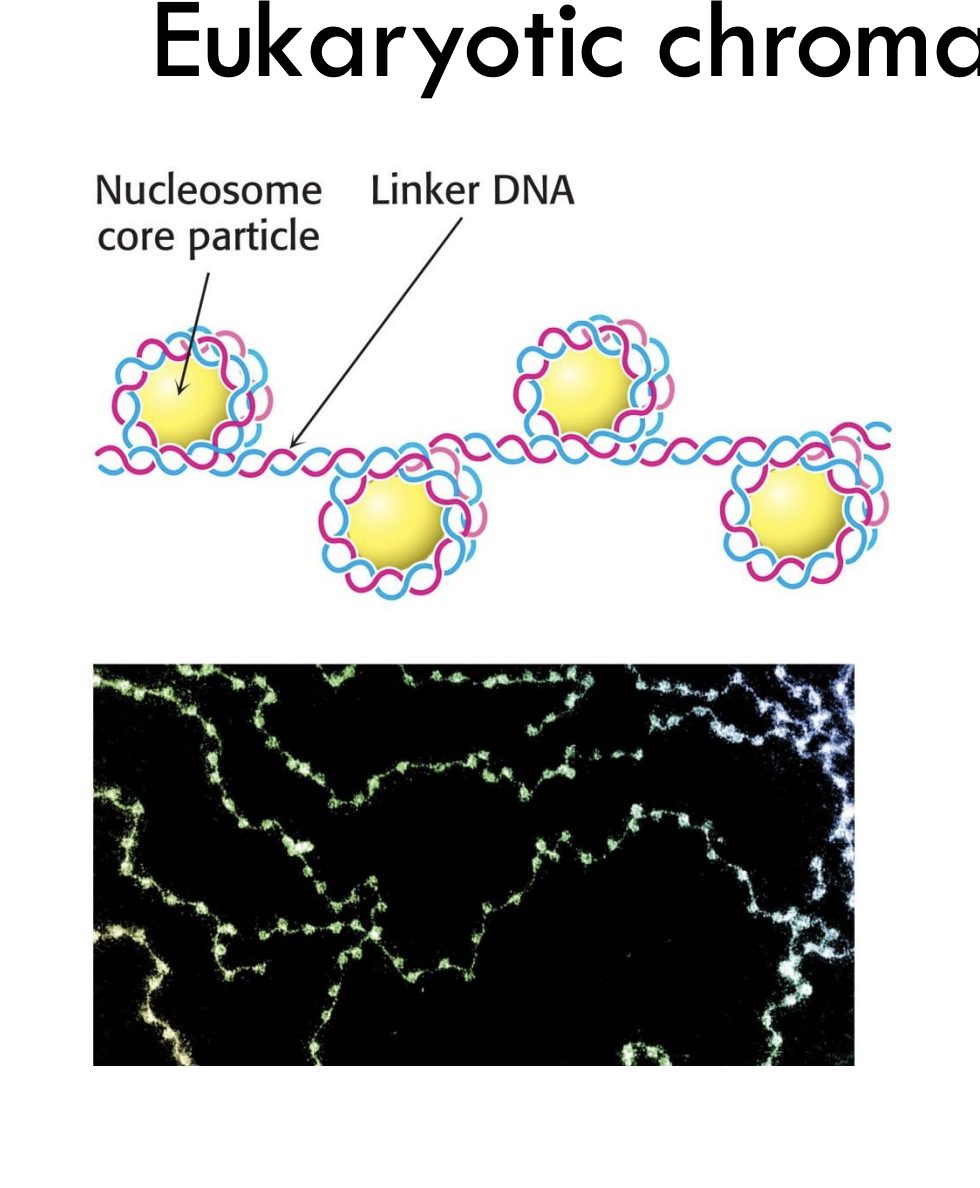
What is the basic unit of eukaryotic chromatin?
The nucleosome, which includes DNA wrapped around a histone core.
Example: Each nucleosome contains ~147 base pairs of DNA.
What connects nucleosomes in chromatin?
Linker DNA connects adjacent nucleosome core particles.
What is the approximate diameter of the nucleus where chromosomes are stored?
A: About 5 micrometers (µm).
Example: This space contains highly packed and organized chromatin in the form of chromosomes.
How many base pairs of DNA are wrapped around a nucleosome core?
About 146 base pairs.
Example: Each "bead" on the chromatin string contains ~146 bp of DNA wrapped around the histone core.
What histone proteins make up the nucleosome core, and how many of each?
The core consists of 8 histones: two each of H2A, H2B, H3, and H4.
Example: H3 and H4 form a tetramer, and H2A-H2B form two dimers.

What is the role of histones in chromatin?
They organize and compact DNA by allowing it to wrap around them, forming nucleosomes.
Example: This compaction helps fit DNA into the 5 μm diameter nucleus.
Is histone binding to DNA random?
No, histone binding is sequence-dependent.
Example: It favors regions rich in AT base pairs.
How does staggering AA, AT, or TT dinucleotides at 10 bp intervals affect nucleosome binding?
It narrows the minor groove and bends the DNA, making it easier to wrap around the histone core.
Why do AT-rich regions bend more easily around nucleosomes?
AT base pairs are more flexible and allow tighter bending of DNA, facilitating nucleosome wrapping.
Example: Alternating AT/GC sequences help DNA adopt the curved shape around the histone.
What are histone tails rich in, and why is that important?
Lysine and arginine, which are positively charged and help interact with negatively charged DNA.
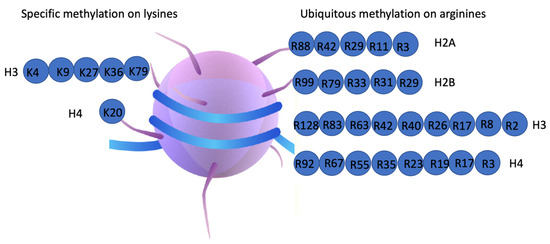
Which histones make up the nucleosome core and how many of each are present?
H2A, H2B, H3, and H4 — two copies of each.
Example: H3 and H4 form a tetramer; H2A and H2B form two dimers.
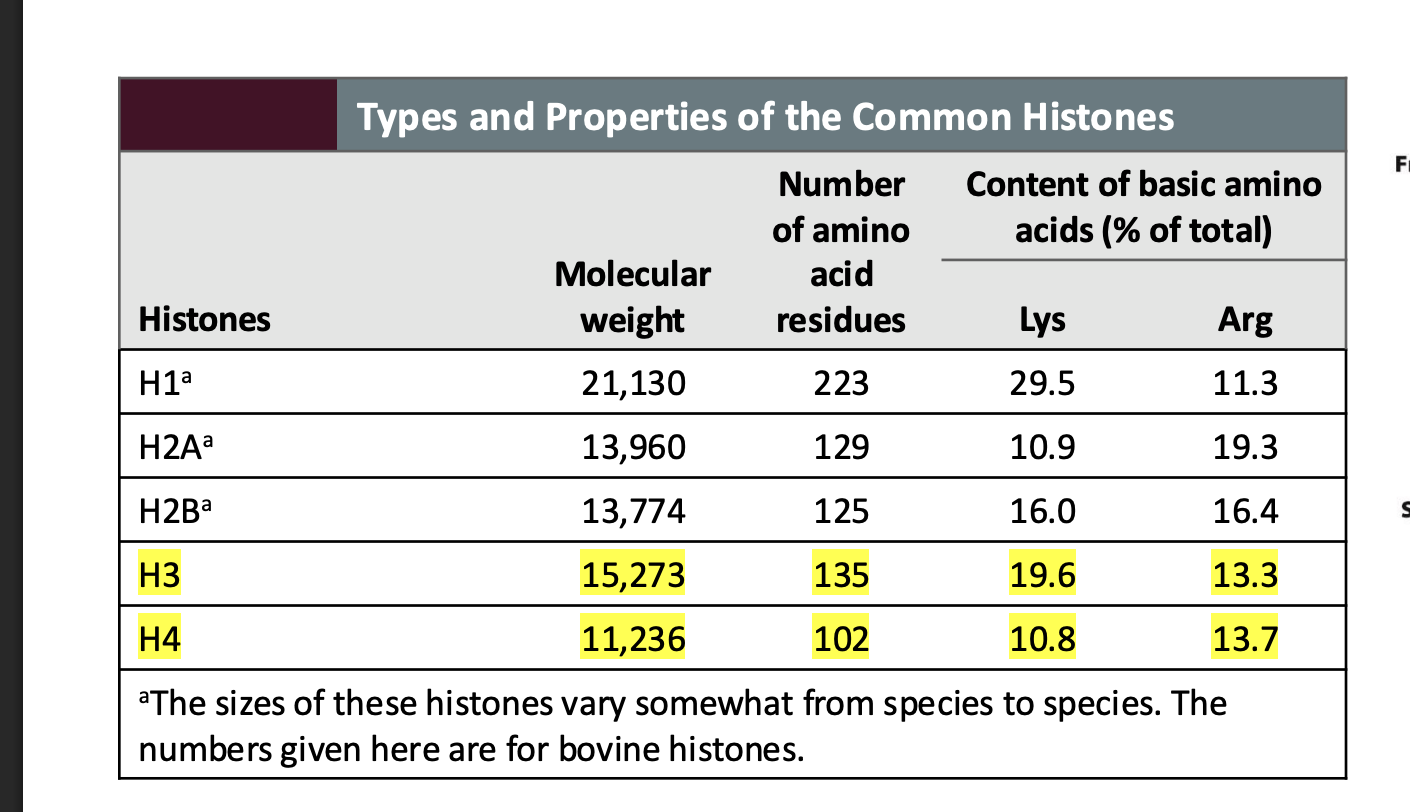
Which histone has the highest lysine content?
H1, with 29.5% lysine.
Example: H1 is known as the linker histone and helps compact DNA between nucleosomes.
What are common post-translational modifications of histones?
Methylation, acetylation, ADP-ribosylation, phosphorylation, glycosylation, sumoylation, and ubiquitination.
Example: Acetylation of lysines in histone tails is associated with active transcription.
What is the basic level of DNA compaction called?
The "beads on a string" structure, which compacts DNA about 7-fold.
Example: This structure consists of nucleosomes connected by ~50 bp linker DNA.
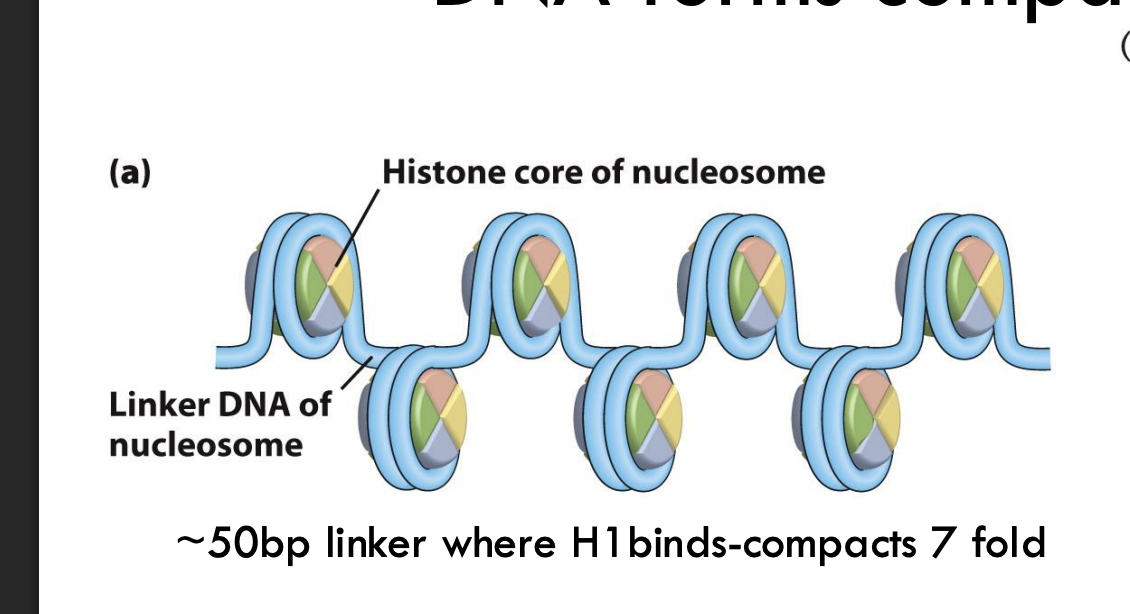
Which histone binds to linker DNA and helps compact DNA further?
Histone H1.
Example: H1 binding results in 7-fold compaction at the nucleosome level.
What is the next level of compaction beyond nucleosomes?
The 30-nm fiber, which compacts DNA approximately 100-fold.
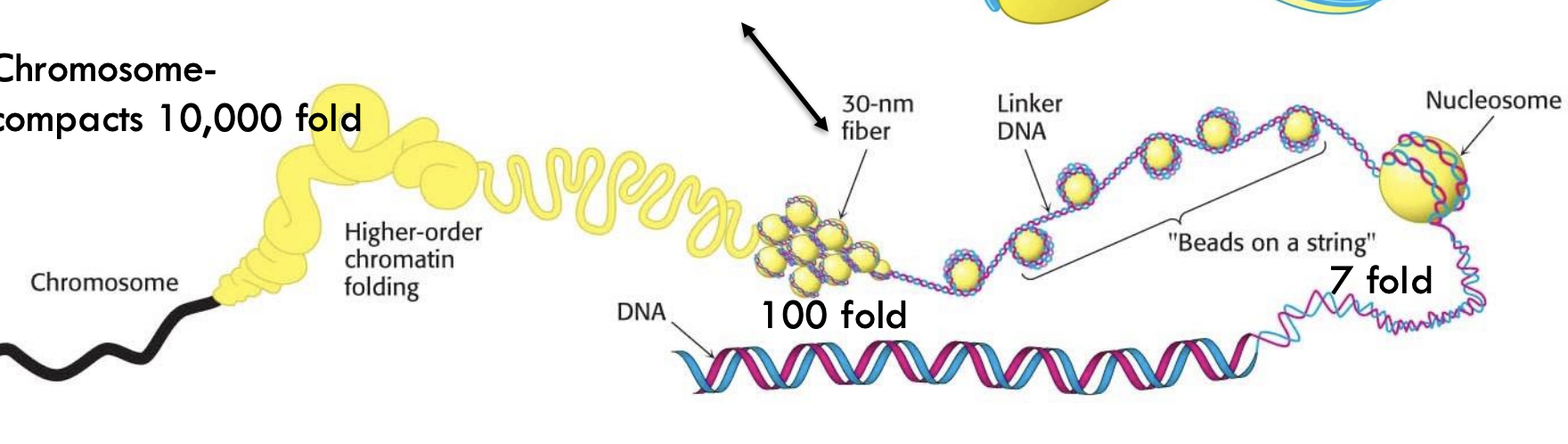
How much total compaction occurs to form a chromosome?
Around 10,000-fold compaction.
What is the role of higher-order chromatin folding?
It organizes the 30-nm fiber into even more compact structures, contributing to full chromosome formation.
Is DNA packing uniform across the genome?
No, packing is transcription-specific, and areas with fewer H1 histones are often more transcriptionally active.
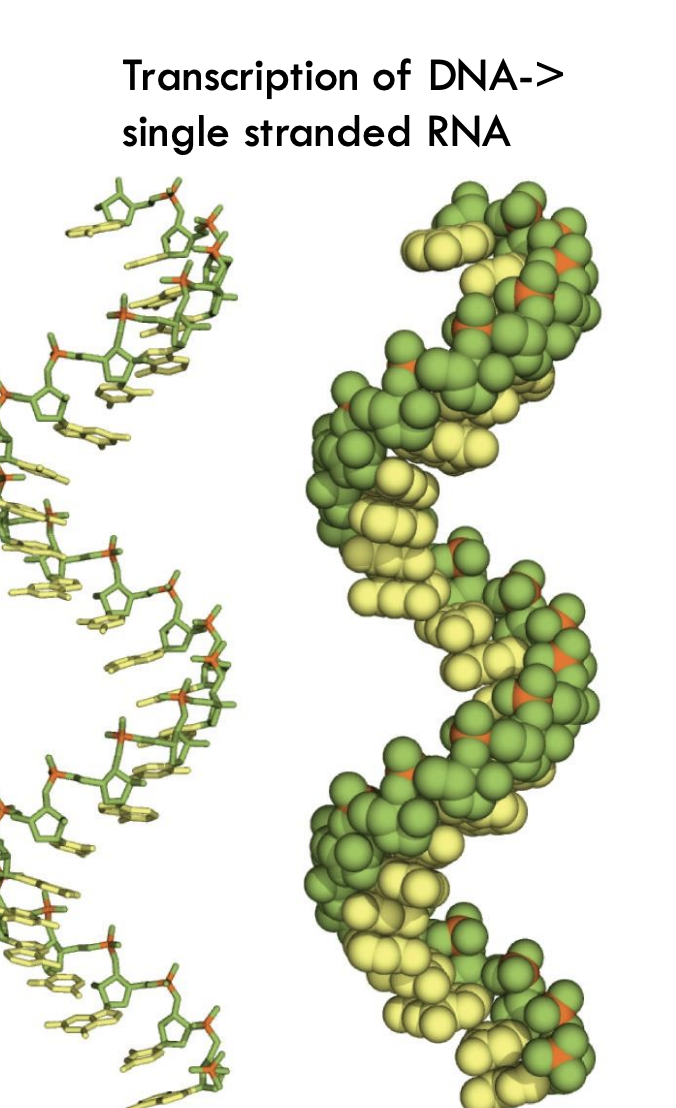
What is the structure of RNA after transcription?
RNA is single-stranded but can form secondary structures with right-handed helical conformation.
What unusual base pairing is allowed in RNA?
G-U base pairing (wobble pairing) is allowed.
Example: This helps tRNA recognize multiple codons.
What type of base interactions are preferred in RNA secondary structure?
Purine–purine interactions are preferred.
Example: Purines (adenine and guanine) stabilize RNA folding through stacking.
What is a monocistronic mRNA?
An mRNA that codes for only one protein.
Example: Common in eukaryotes.
What is a polycistronic mRNA?
An mRNA that codes for multiple proteins.
Example: Found in bacteria and archaea (e.g., lac operon in E. coli).
Does RNA have a single, standard secondary structure?
No, RNA does not have a standard secondary structure; it can fold into diverse shapes depending on sequence and interactions.
What are common RNA secondary structural elements?
Hairpins, bulge loops, interior loops, and junctions.
Example: tRNA has all of these to support its L-shaped tertiary structure.
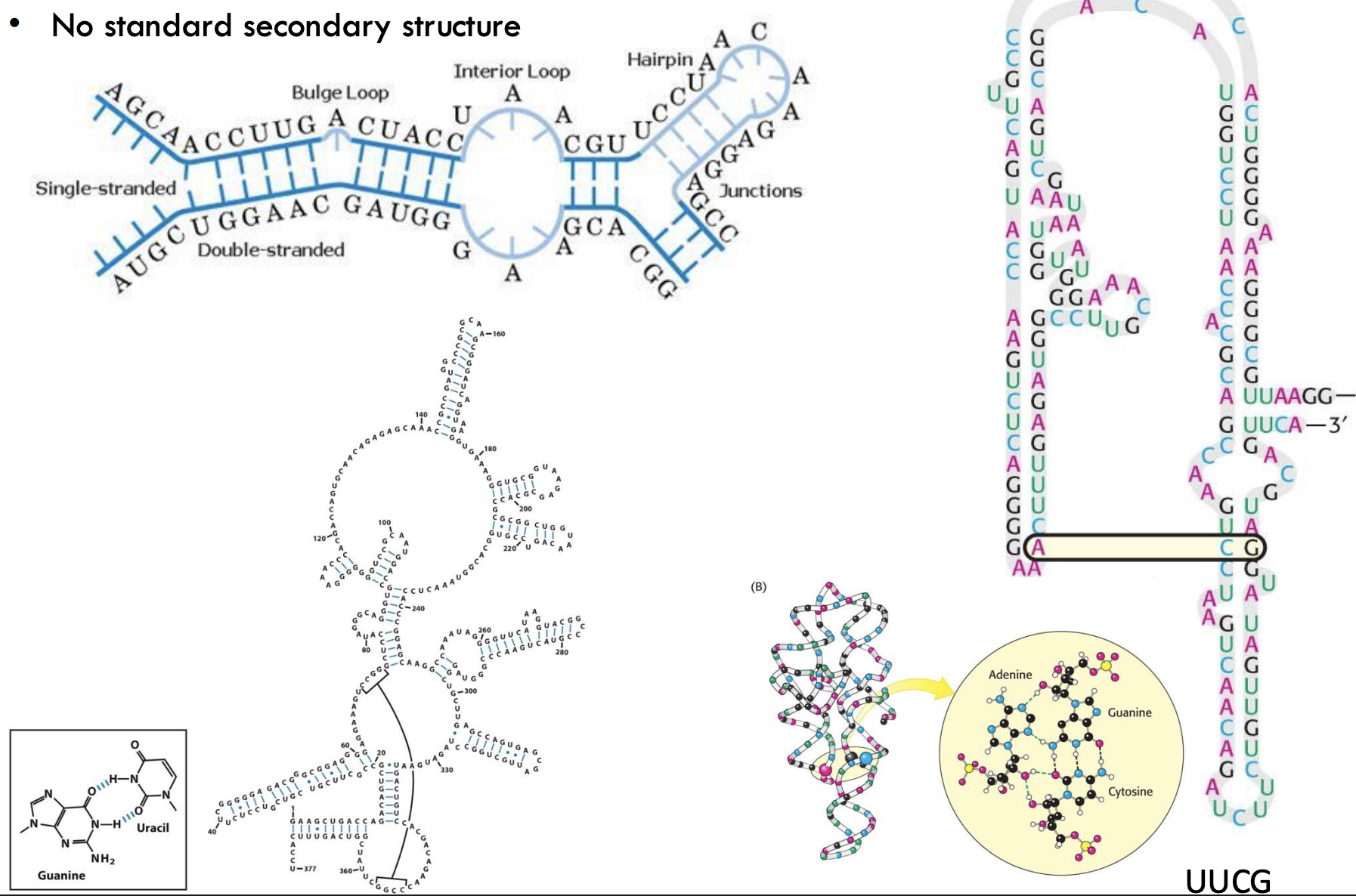
What type of base pairing is shown in secondary RNA structure diagrams?
Watson-Crick (A-U, G-C) and noncanonical like G-U (wobble pairs).
What is a hairpin loop in RNA?
A region where complementary bases pair to form a stem, while unpaired bases create a loop at the top.
Example: Common in mRNA regulation and tRNA structure.
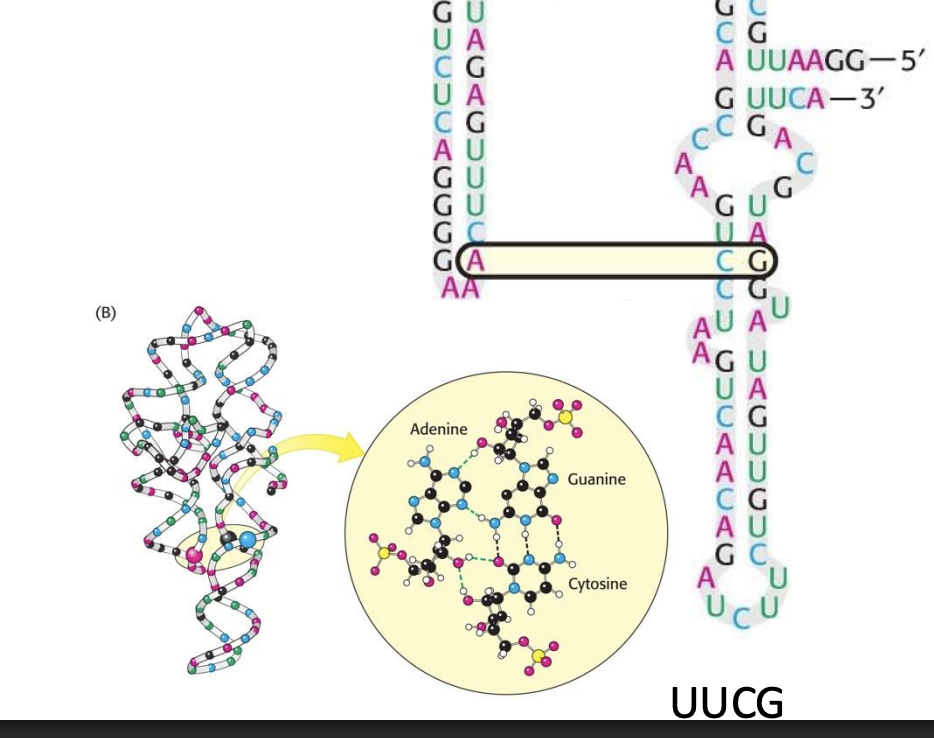
What base sequence is shown forming a hairpin with internal pairing on this slide?
The sequence UUCG, a classic tetraloop that stabilizes RNA hairpins.
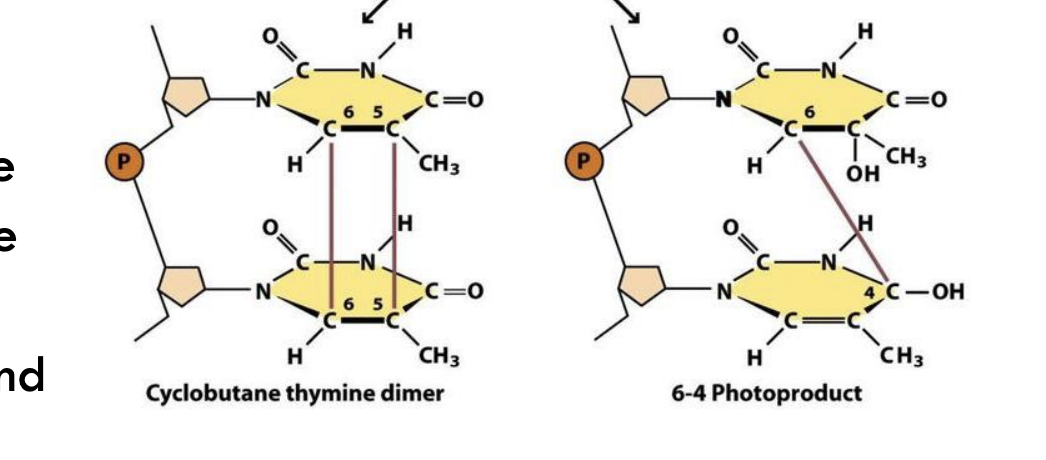
What type of damage does UV light cause to DNA?
UV light induces pyrimidine dimerization, especially forming thymine dimers.
Example: Cyclobutane thymine dimers or 6–4 photoproducts can distort the DNA helix.
Why is UV-induced DNA damage significant?
It is a primary cause of skin cancers due to mutation accumulation in skin cells.
What does ionizing radiation (e.g., X-rays, γ-rays) do to DNA?
It causes ring opening and strand breaks, which are difficult to repair.
Can all radiation-induced DNA damage be repaired?
No, some damage can be repaired, but others result in mutations that contribute to aging and cancer.
What are two specific types of thymine dimers shown in the diagram?
Cyclobutane thymine dimer
6–4 photoproduct
Example: Both are caused by UV light and distort the DNA structure.
What is one way to treat cancer by targeting DNA?
By modifying DNA to prevent replication and transcription, leading to cell death.
What is cisplatin and how does it work?
Cisplatin is a chemotherapeutic agent that binds DNA by replacing its chloride atoms with nitrogens from adjacent purines (like adenine or guanine).
How does cisplatin disrupt DNA function?
It distorts the DNA structure, blocking replication and transcription, which leads to cell death.
Why are carbohydrates named that way?
Because many have the general formula (CH₂O)ₙ, meaning they are hydrates of carbon.
What are carbohydrates made from in plants?
From CO₂ and H₂O through photosynthesis.
Example: Cellulose is produced this way.
What is the molecular weight range of carbohydrates?
From small (glyceraldehyde, 90 g/mol) to large (amylopectin, >200,000,000 g/mol).
What are two main energy-related functions of carbohydrates?
Energy source: glucose
Energy storage: glycogen, starch
Name two non-energy roles of carbohydrates.
Structure/protection: chitin, cellulose, connective tissue
Cell signaling: as part of informational molecules or glycoproteins
Which amino acids use carbohydrates as carbon sources?
Isoleucine (Ile), Leucine (Leu), Valine (Val), Alanine (Ala)
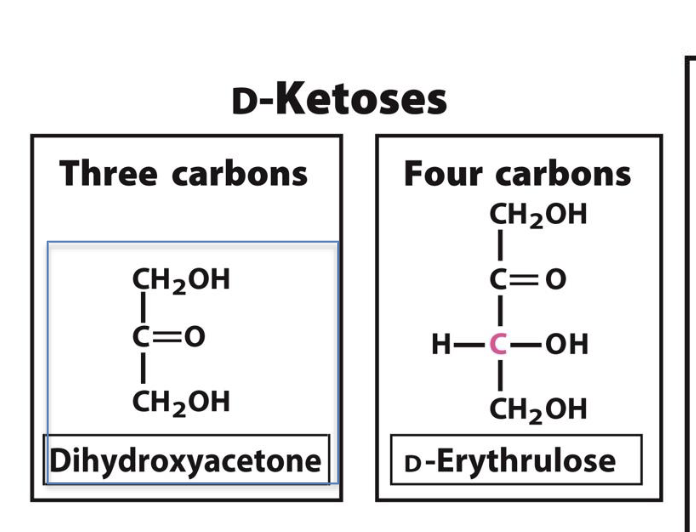
What is the difference between an aldose and a ketose?
Aldose has an aldehyde group (e.g., D-glyceraldehyde)
Ketose has a ketone group (e.g., dihydroxyacetone)
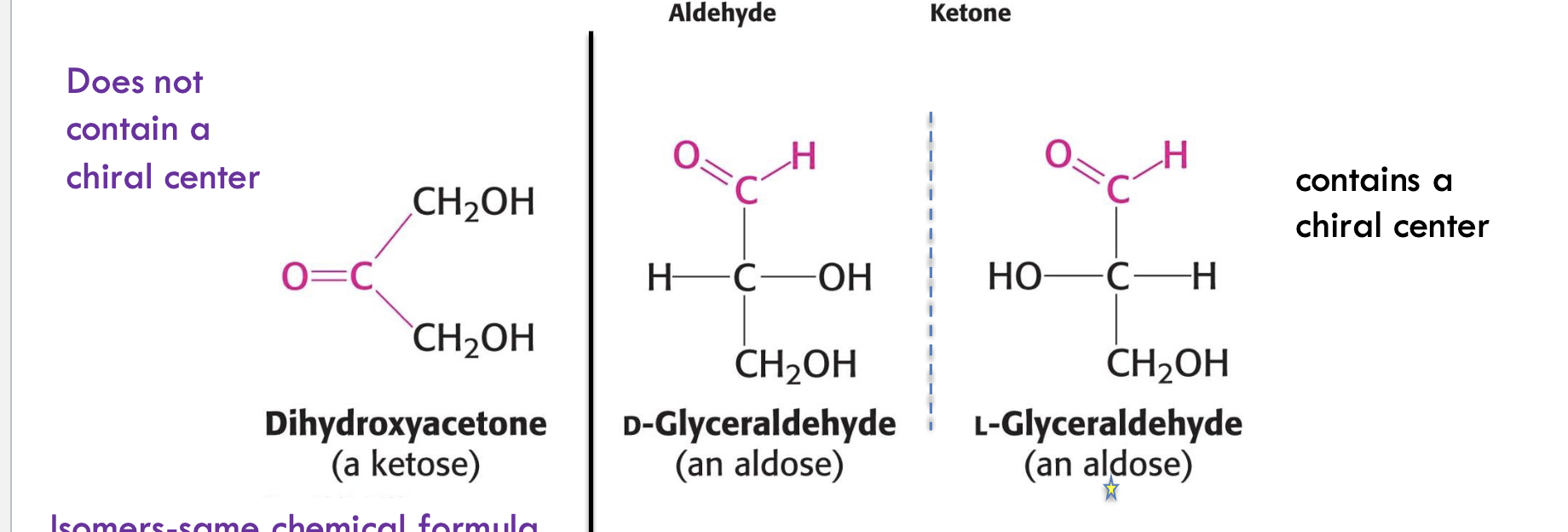
How are carbohydrates classified by size?
Monosaccharides: single sugar (e.g., D-glucose)
Oligosaccharides: 2–10 sugars (e.g., sucrose)
Polysaccharides: more than 10 sugars
What determines the basic name of a carbohydrate?
The number of carbon atoms + the suffix -ose.
Example:
3 carbons = triose
5 carbons = pentose
6 carbons = hexose
What two functional groups must all monosaccharides have?
A carbonyl group (aldehyde or ketone)
Two or more hydroxyl (-OH) groups
How many stereoisomers does a monosaccharide with n chiral centers have?
It has 2ⁿ stereoisomers.
Example: Glyceraldehyde has 1 chiral center → 2¹ = 2 stereoisomers (D and L forms).
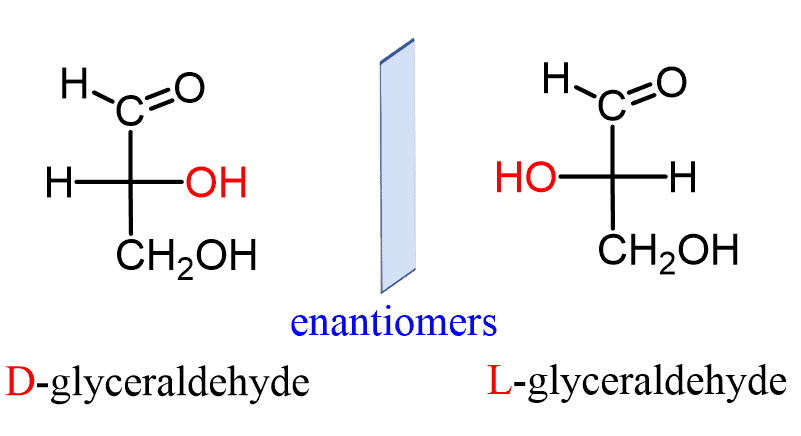
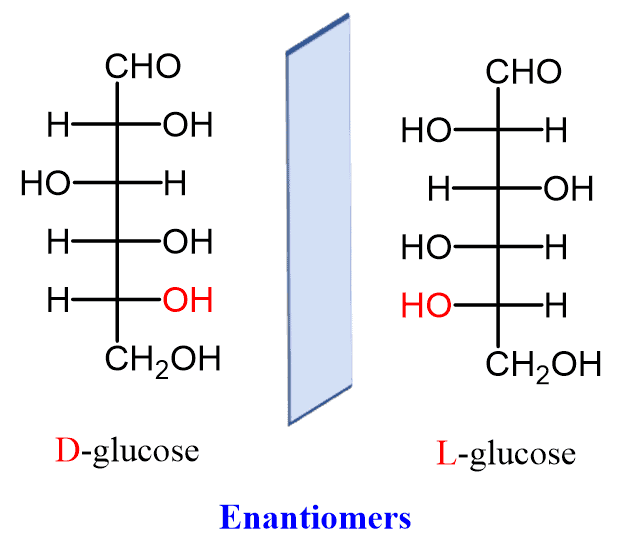
What defines a D-isomer in carbohydrates?
The OH group on the chiral center farthest from the carbonyl is on the right.
What defines an L-isomer in carbohydrates?
The OH group on the chiral center farthest from the carbonyl is on the left.
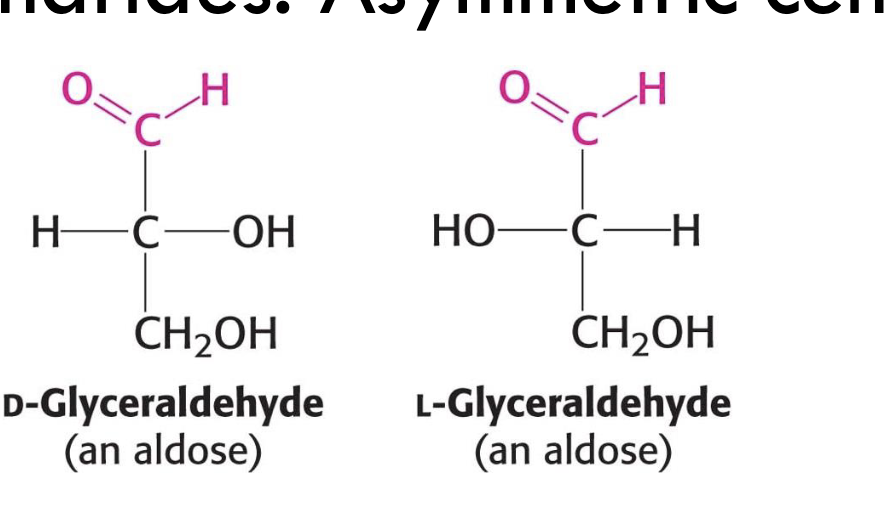
What are D-Glyceraldehyde and L-Glyceraldehyde examples of?
They are aldotrioses and enantiomers (mirror-image stereoisomers).
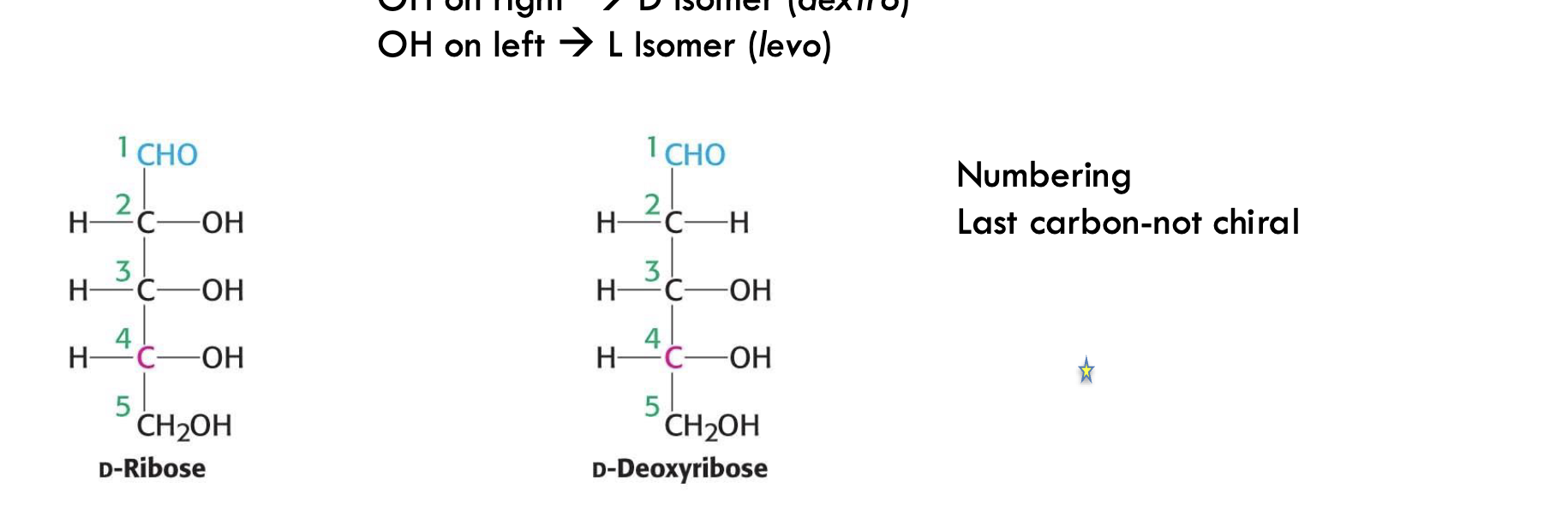
Is the last carbon in a monosaccharide usually chiral?
No, the last carbon (often CH₂OH) is not chiral.
What’s the structural difference between D-ribose and D-deoxyribose?
D-deoxyribose lacks the -OH group at carbon 2, making it a deoxy sugar.
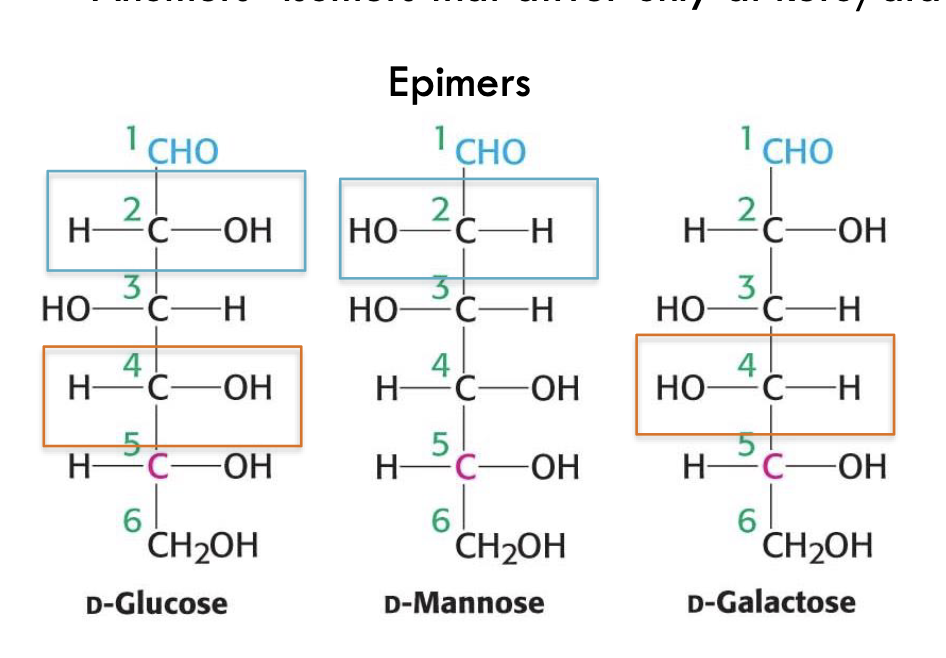
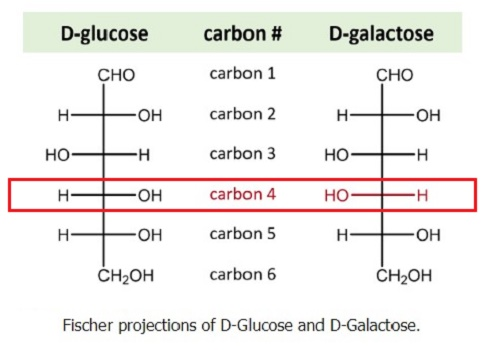
What are epimers?
Isomers that differ at only one chiral carbon.
Examples:
D-glucose vs. D-mannose → epimers at C-2
D-glucose vs. D-galactose → epimers at C-4
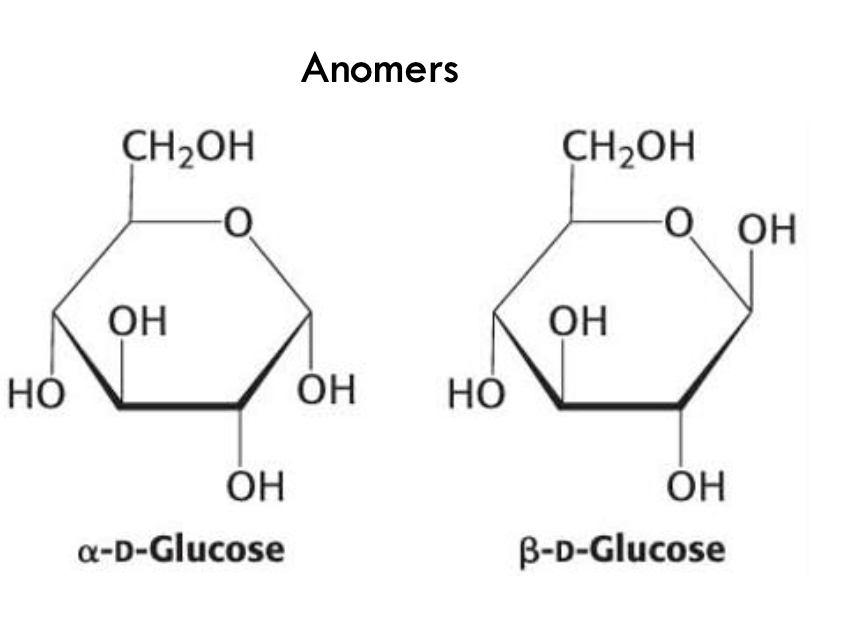
What are anomers?
Isomers that differ only at the carbonyl-derived carbon (anomeric carbon) in cyclic form.
Example: α-D-glucose vs. β-D-glucose.
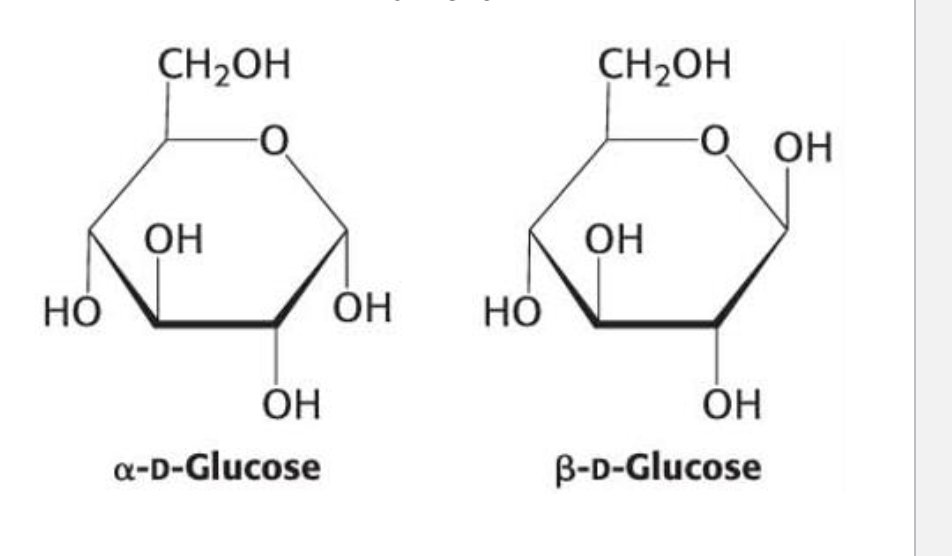
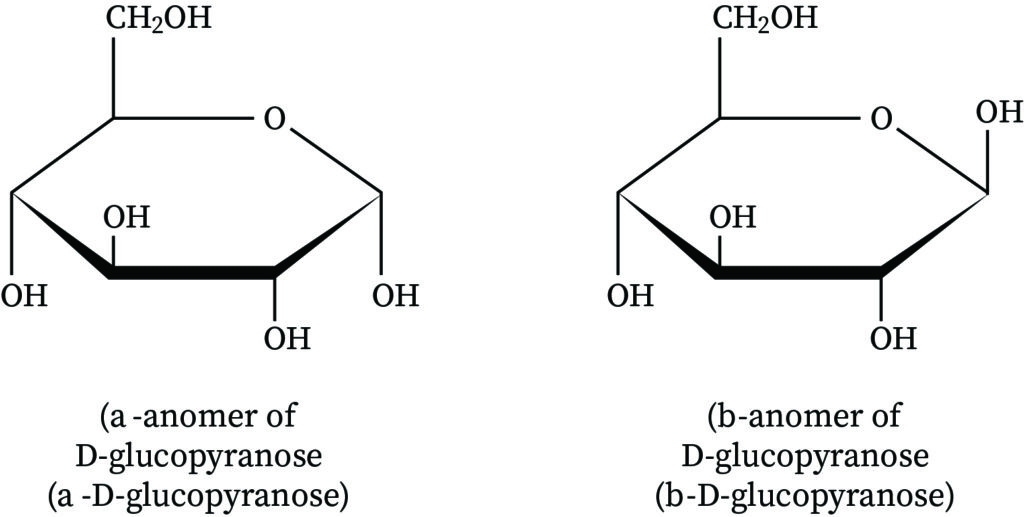
What defines α- and β-anomers of glucose?
α-anomer: OH on the anomeric carbon is opposite the CH₂OH group
β-anomer: OH on the anomeric carbon is same side as CH₂OH
What defines a sugar as a D-aldose?
The OH group on the chiral center farthest from the carbonyl is on the right.
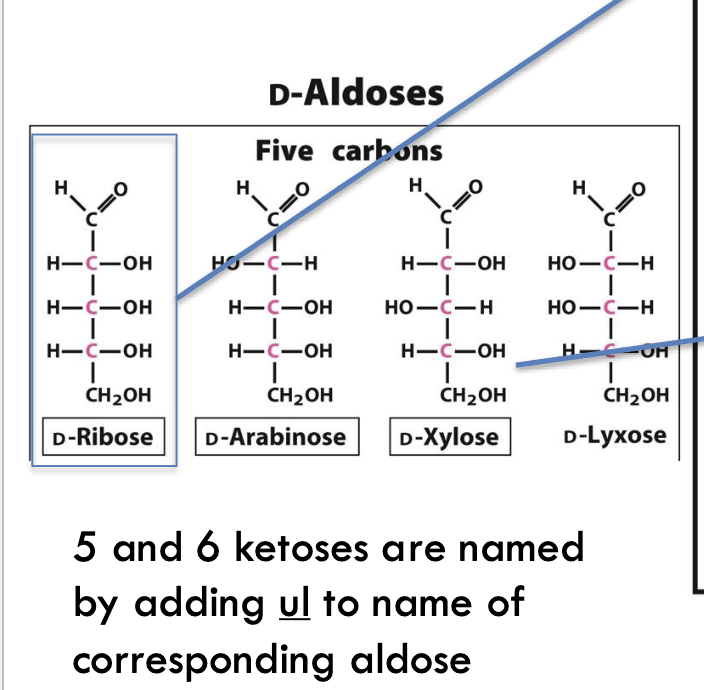
How many D-aldoses exist with 3–6 carbons?
15 D-aldoses
1 with 3C, 2 with 4C, 4 with 5C, and 8 with 6C
What are diastereoisomers?
Stereoisomers that are not mirror images of each other.
Example: D-glucose and D-galactose differ at more than one carbon.
Name three 6-carbon D-aldoses.
D-Glucose
D-Mannose
D-Galactose
Which carbon determines D vs. L configuration in aldoses?
The second-to-last chiral carbon (penultimate carbon).
Rule: OH on right = D; OH on left = L.
What is the simplest D-ketose?
Dihydroxyacetone, a 3-carbon ketose (no chiral centers).
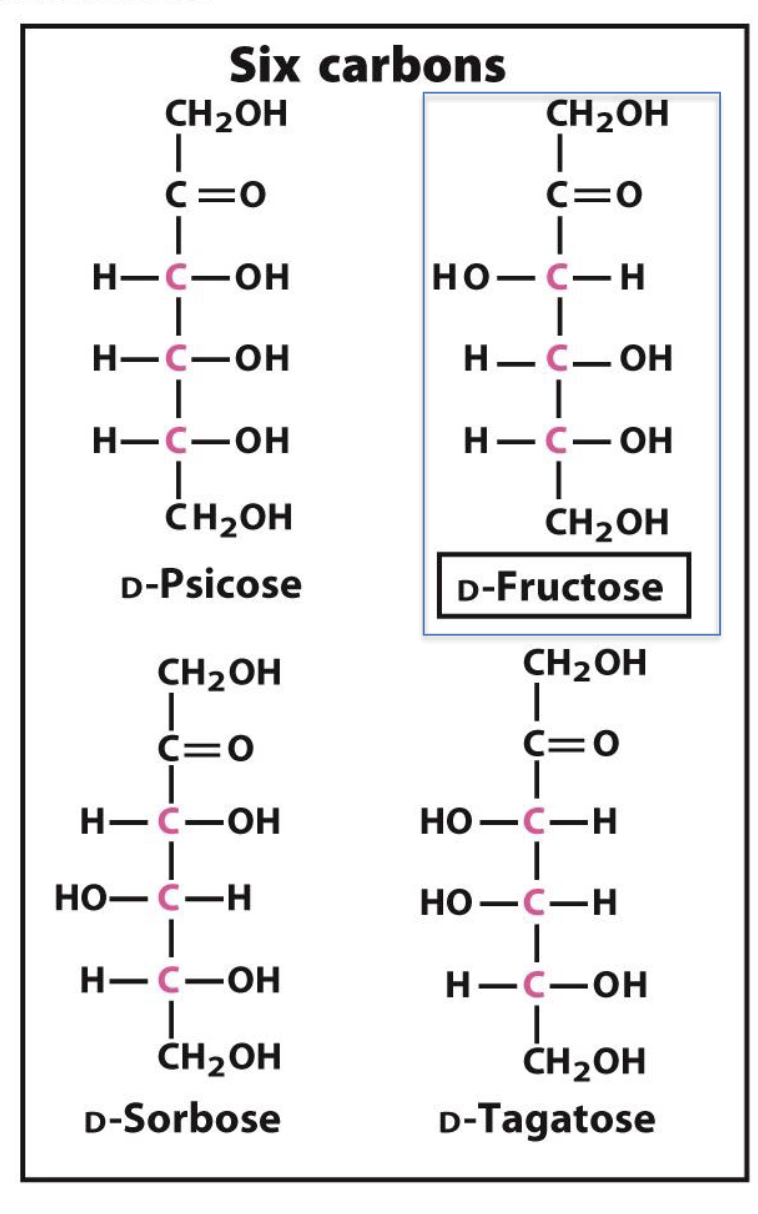
How are 5- and 6-carbon ketoses named?
By adding "-ulose" to the name of the corresponding aldose.
Example: D-ribose → D-ribulose, D-xylose → D-xylulose
What is the 6-carbon ketose form of D-glucose?
D-fructose
Which 5-carbon D-ketoses are shown?
D-ribulose
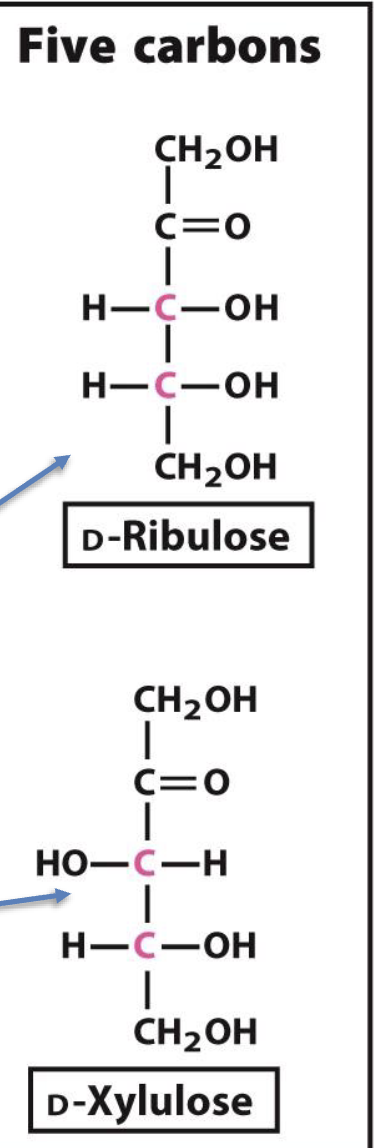
D-xylulose
Name two 6-carbon D-ketoses other than fructose.
D-sorbose
D-psicose
At what size do monosaccharides typically form cyclic structures?
When they contain five or more carbon atoms.
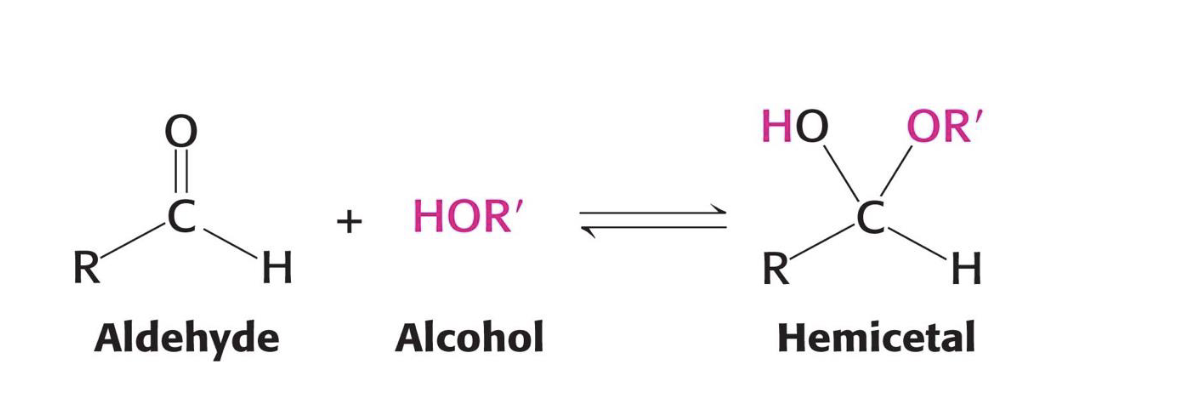
What forms when an aldehyde reacts with an alcohol in a sugar molecule?
A hemiacetal forms.
Example: Aldoses like glucose cyclize this way.
What forms when a ketone reacts with an alcohol in a sugar molecule?
A hemiketal forms.
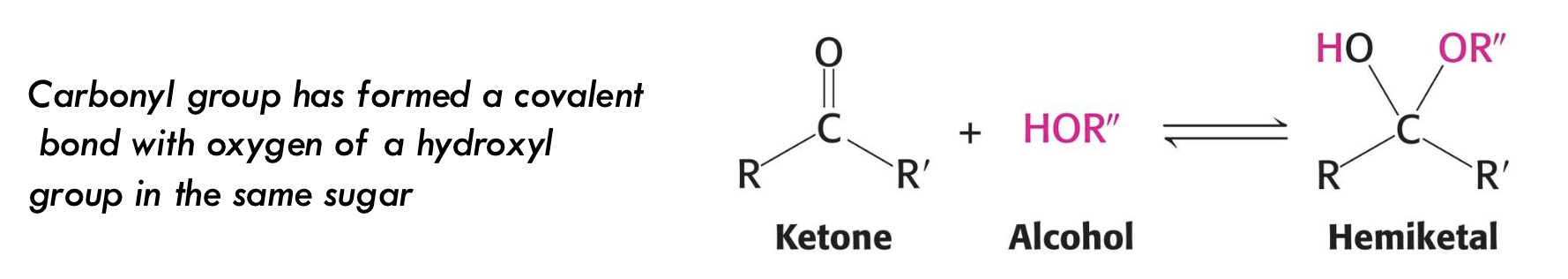
Example: Ketoses like fructose cyclize this way.
What drives the cyclization of sugars into ring forms?
A nucleophilic attack of a hydroxyl group on the carbonyl group (either aldehyde or ketone) within the same molecule.
What type of reaction forms cyclic glucose (glucopyranose)?
Hemiacetal formation from the reaction between an aldehyde (C1) and an internal alcohol (C5 OH).
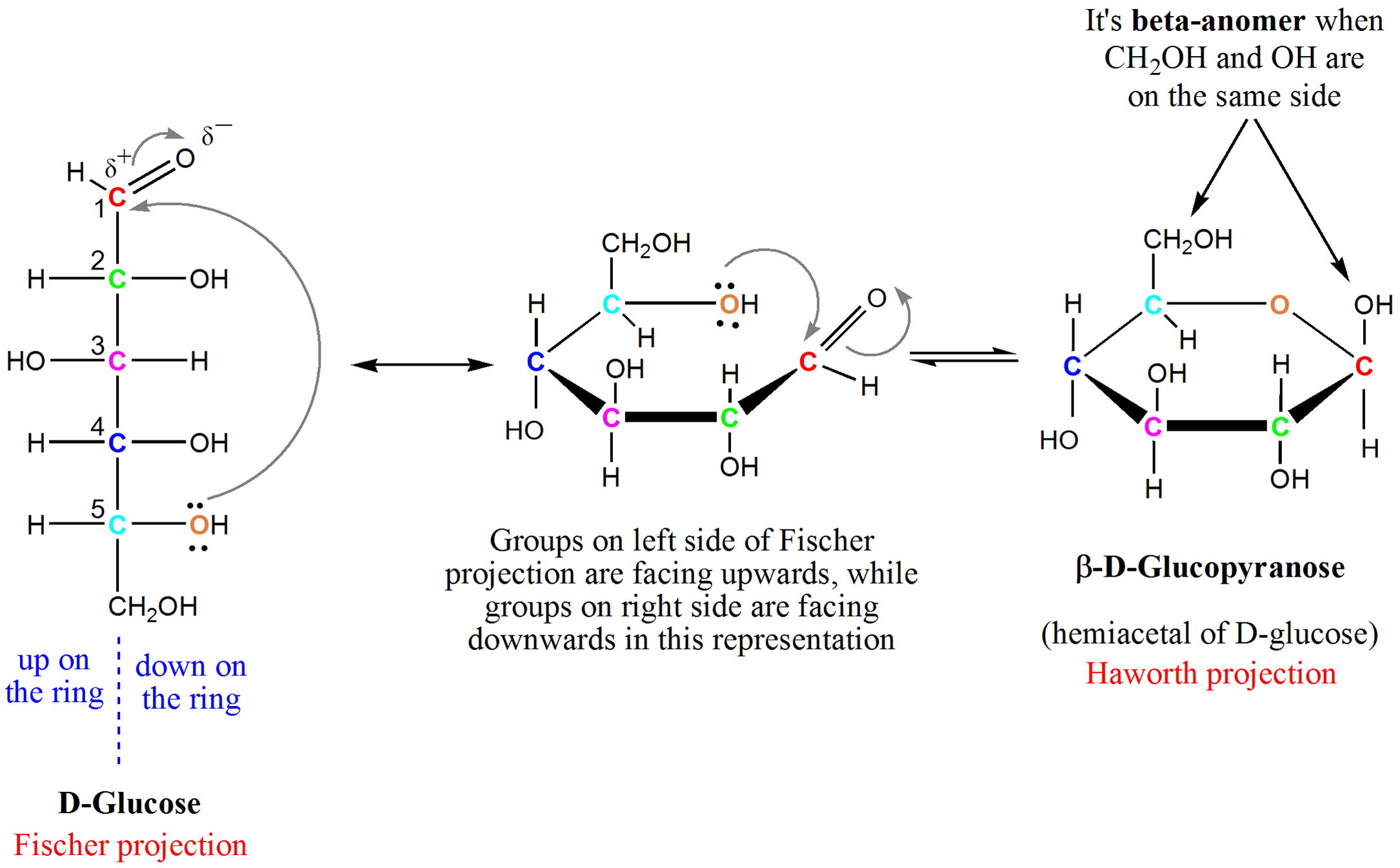
What are the two cyclic forms of D-glucose called?
α-D-Glucopyranose: OH on C1 is down
β-D-Glucopyranose: OH on C1 is up
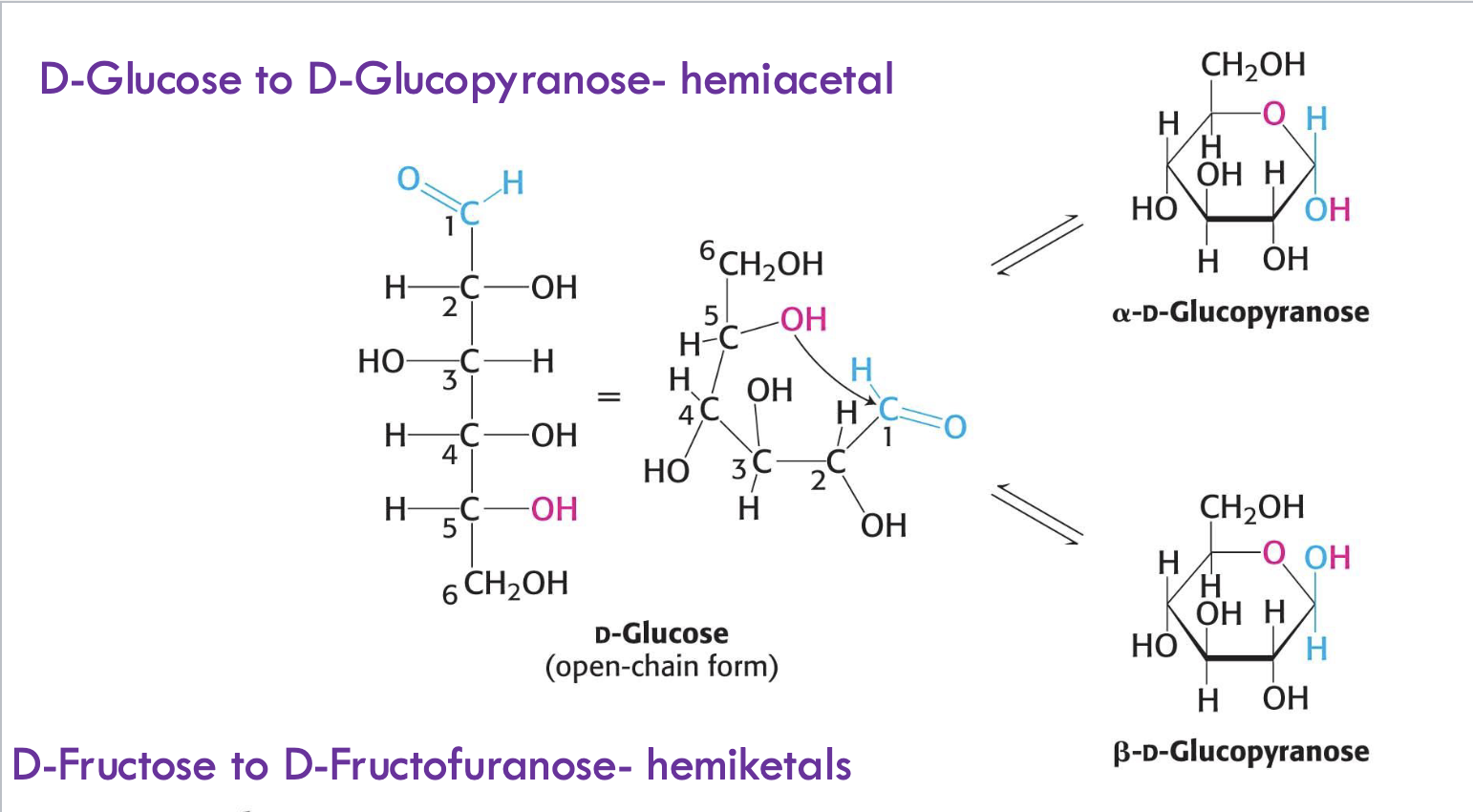
What type of reaction forms cyclic fructose (fructofuranose)?
Hemiketal formation from the reaction between a ketone (C2) and an internal alcohol (C5 OH).
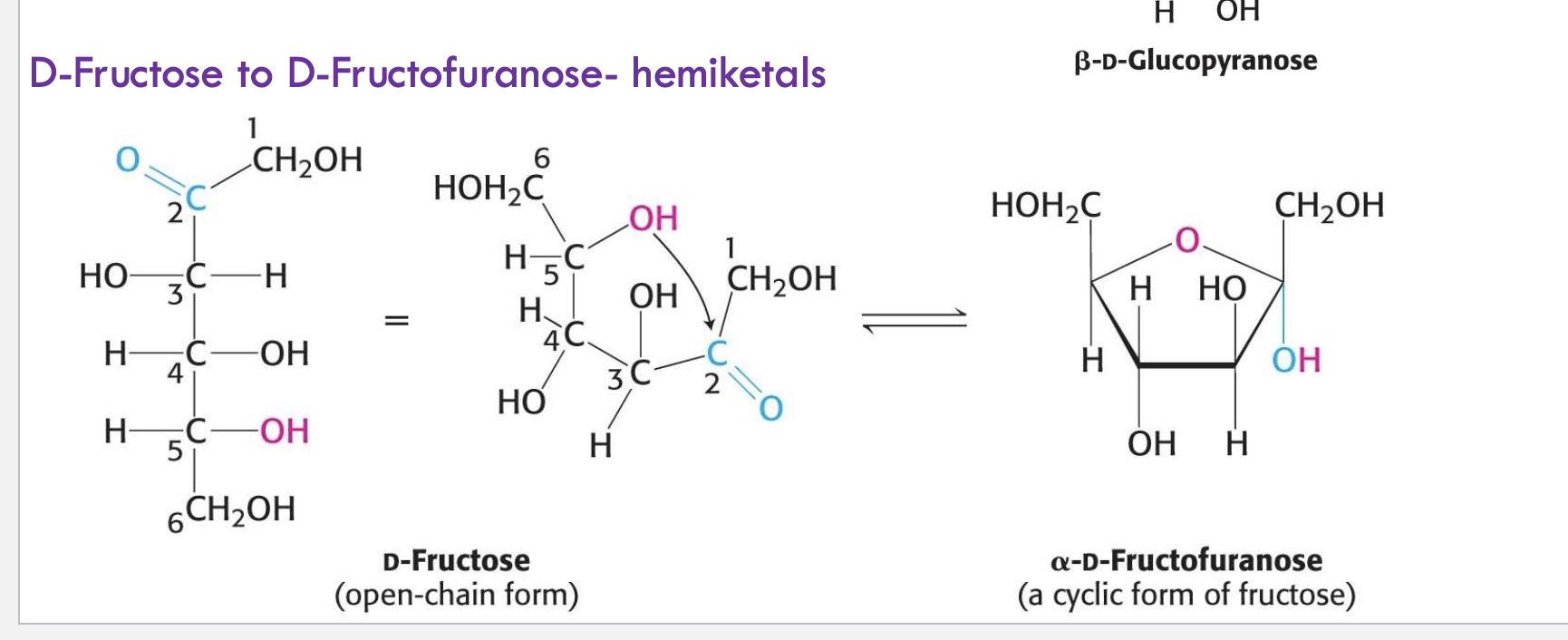
What is the ring size of cyclic glucose vs. cyclic fructose?
Glucose forms a 6-membered ring (pyranose)
Fructose forms a 5-membered ring (furanose)
What are anomers?
Diastereomers that differ only at the anomeric carbon—the carbon derived from the carbonyl (C1 in aldoses) after cyclization.
How do you distinguish between α- and β-anomers of D-glucose?
α-anomer: OH on the anomeric carbon (C1) is on the opposite side of the ring from the CH₂OH group at C6
β-anomer: OH on C1 is on the same side as CH₂OH
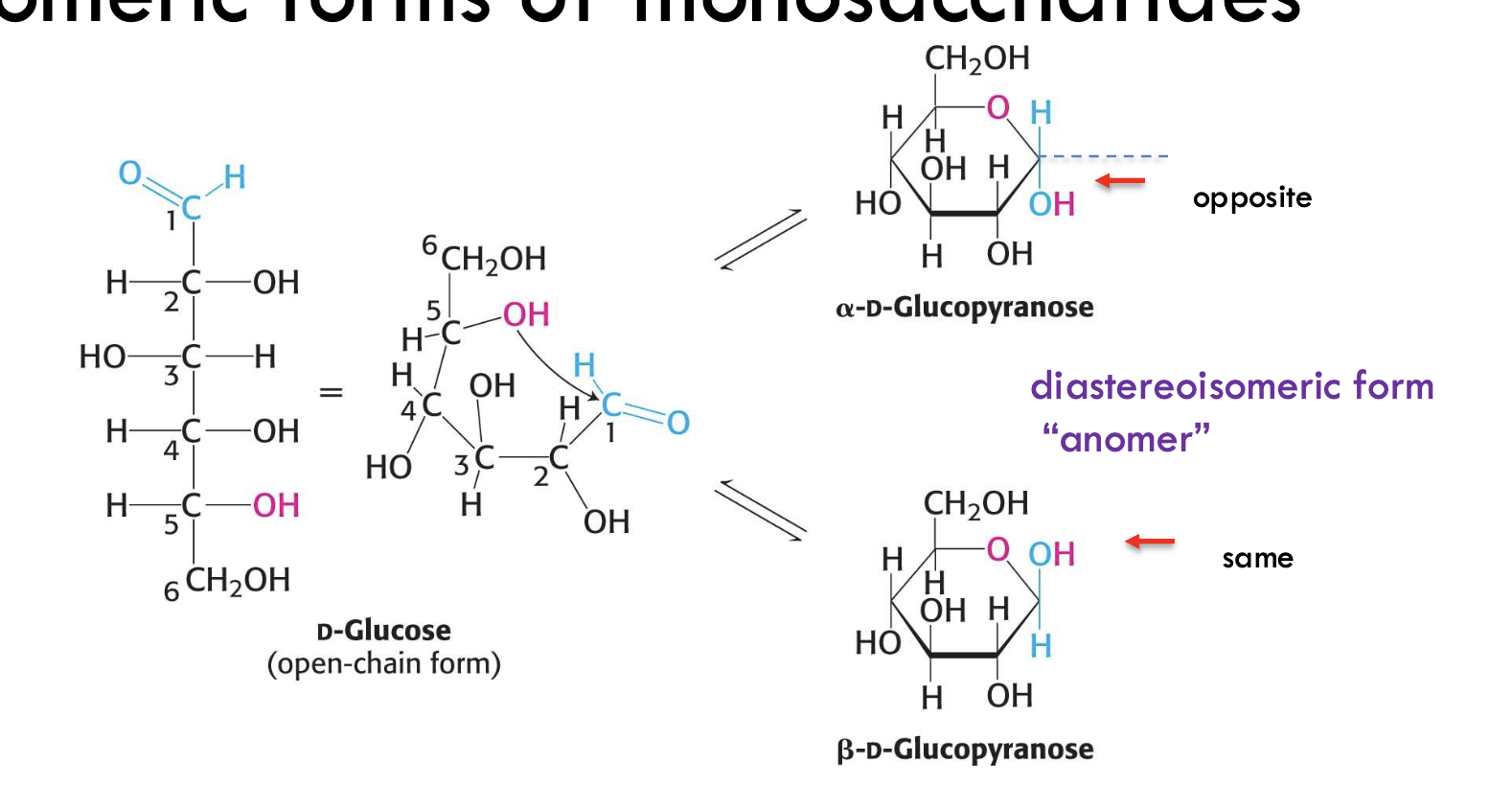
What determines if a hydroxyl group on the anomeric carbon is α or β?
Its position relative to the exocyclic carbon (C6) in the ring form of the sugar.
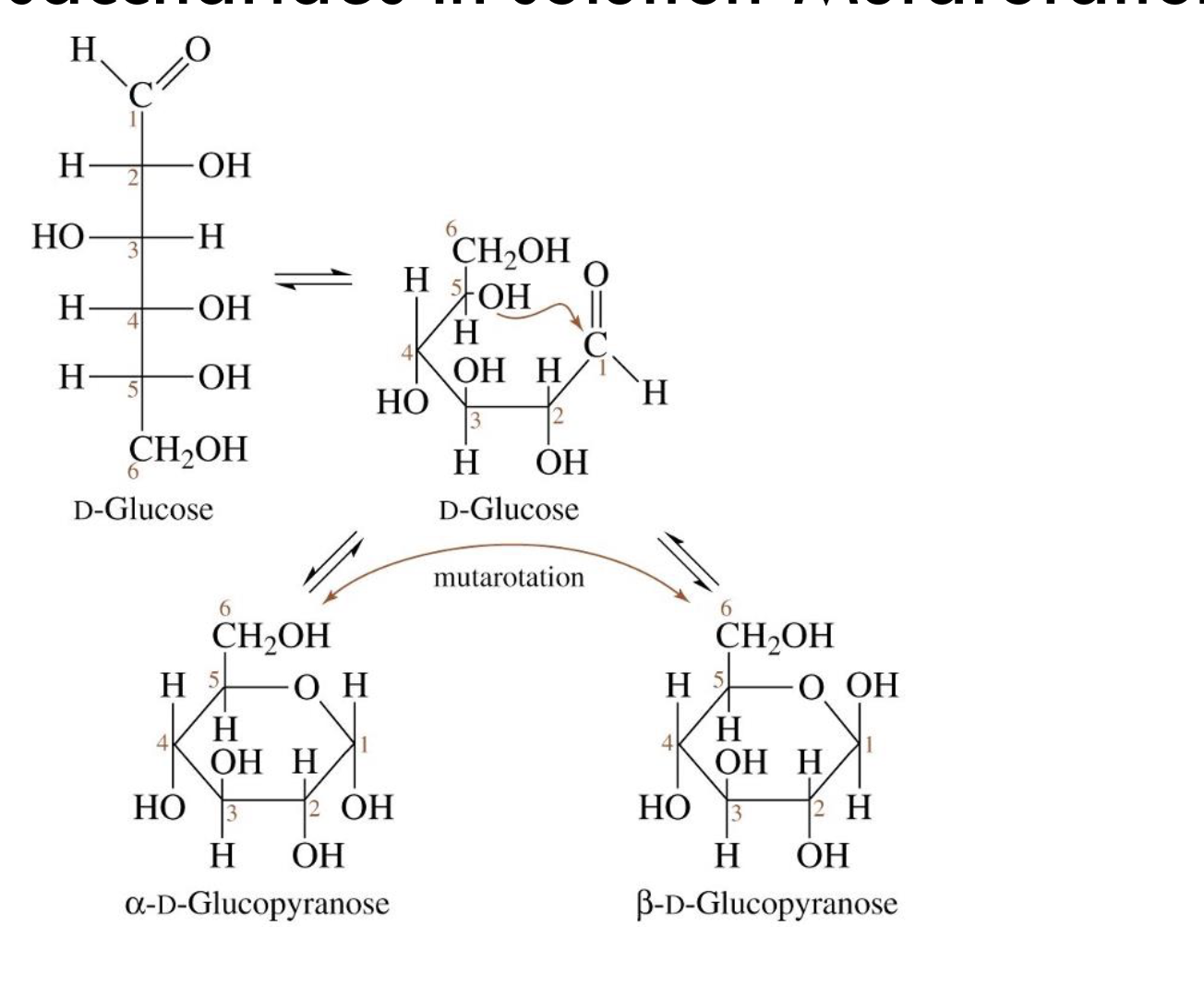
What is mutarotation?
The interconversion between α- and β-anomers of a sugar in solution via the open-chain form.
What forms of D-glucose exist in solution at equilibrium?
~1/3 α-D-glucopyranose
~2/3 β-D-glucopyranose
A small amount of linear D-glucose
Why does β-D-glucopyranose predominate at equilibrium?
It is more stable due to less steric hindrance (equatorial OH at anomeric carbon).
What is a glycosidic bond?
A covalent bond between the anomeric carbon of one sugar and the hydroxyl group of another.
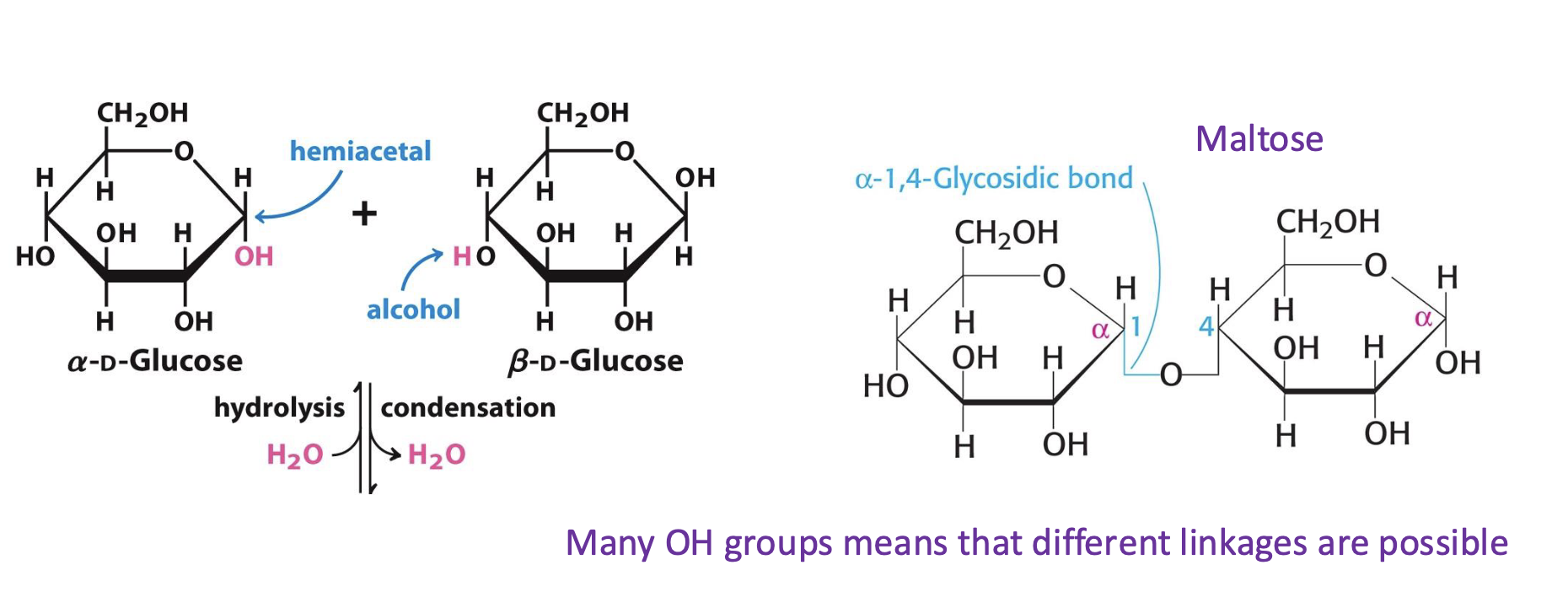
How is the disaccharide maltose formed?
By condensation of two glucose molecules via an α(1→4) glycosidic bond.
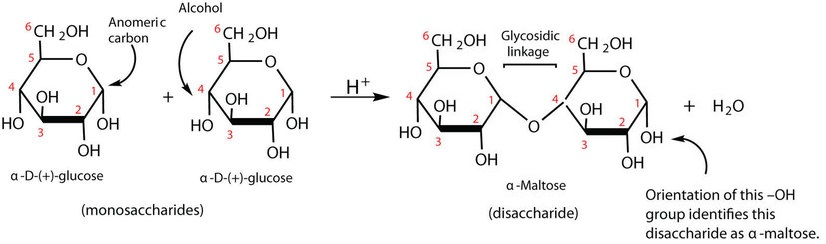
What is the systematic name of maltose?
α-D-glucopyranosyl-(1→4)-D-glucopyranose
Why are multiple glycosidic linkages possible between sugars?
Because sugars contain many hydroxyl (OH) groups, enabling different combinations and branching.
What is the reducing end of a sugar?
The end of a disaccharide or polysaccharide that has a free anomeric carbon, which can open to the linear form and act as a reducing agent.
What effect does forming a glycosidic bond have on sugar reactivity?
It renders that sugar unit nonreducing by locking the anomeric carbon in an acetal (or ketal) form.
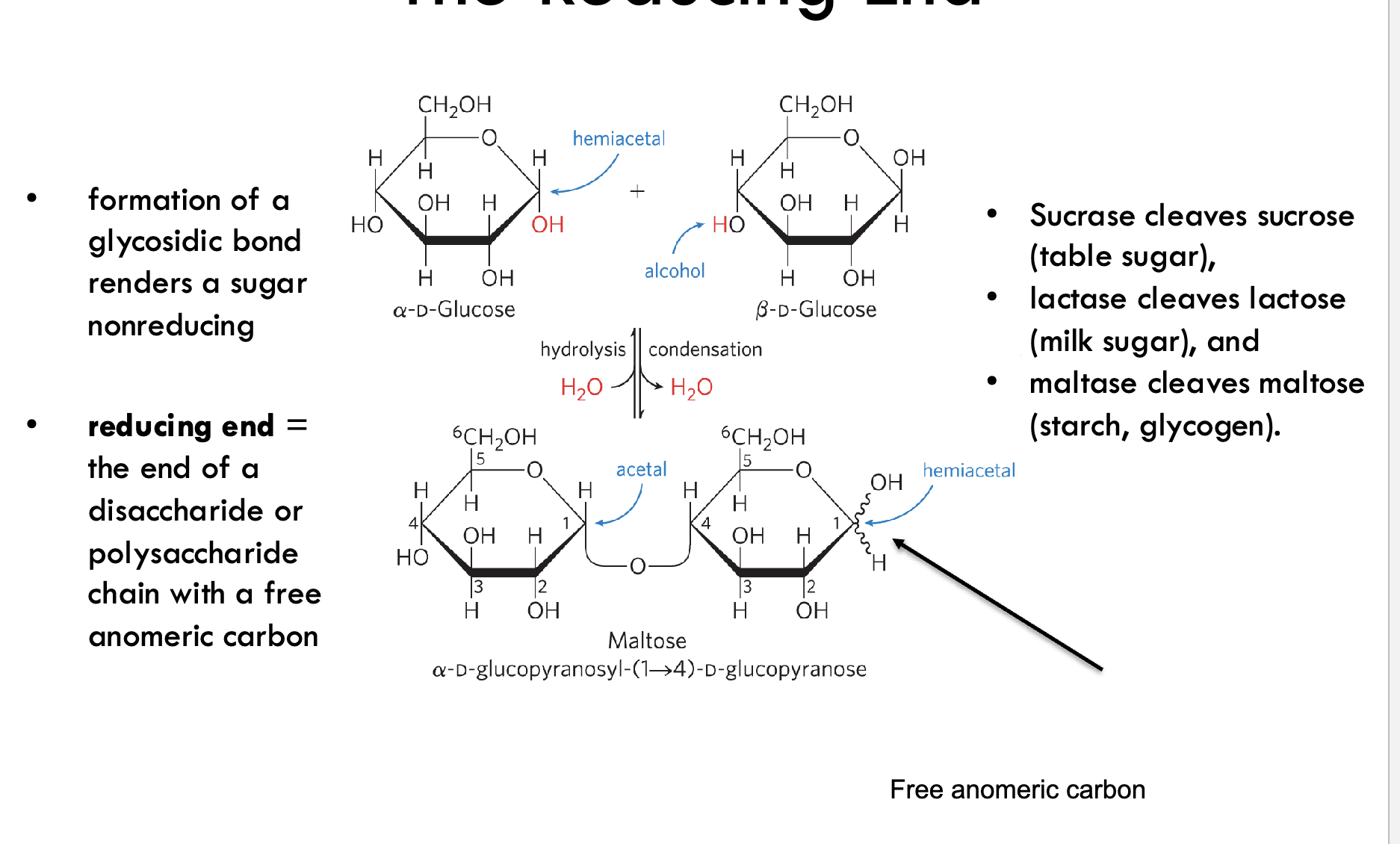
What type of glycosidic bond is found in maltose?
An α(1→4) glycosidic bond between two glucose units.
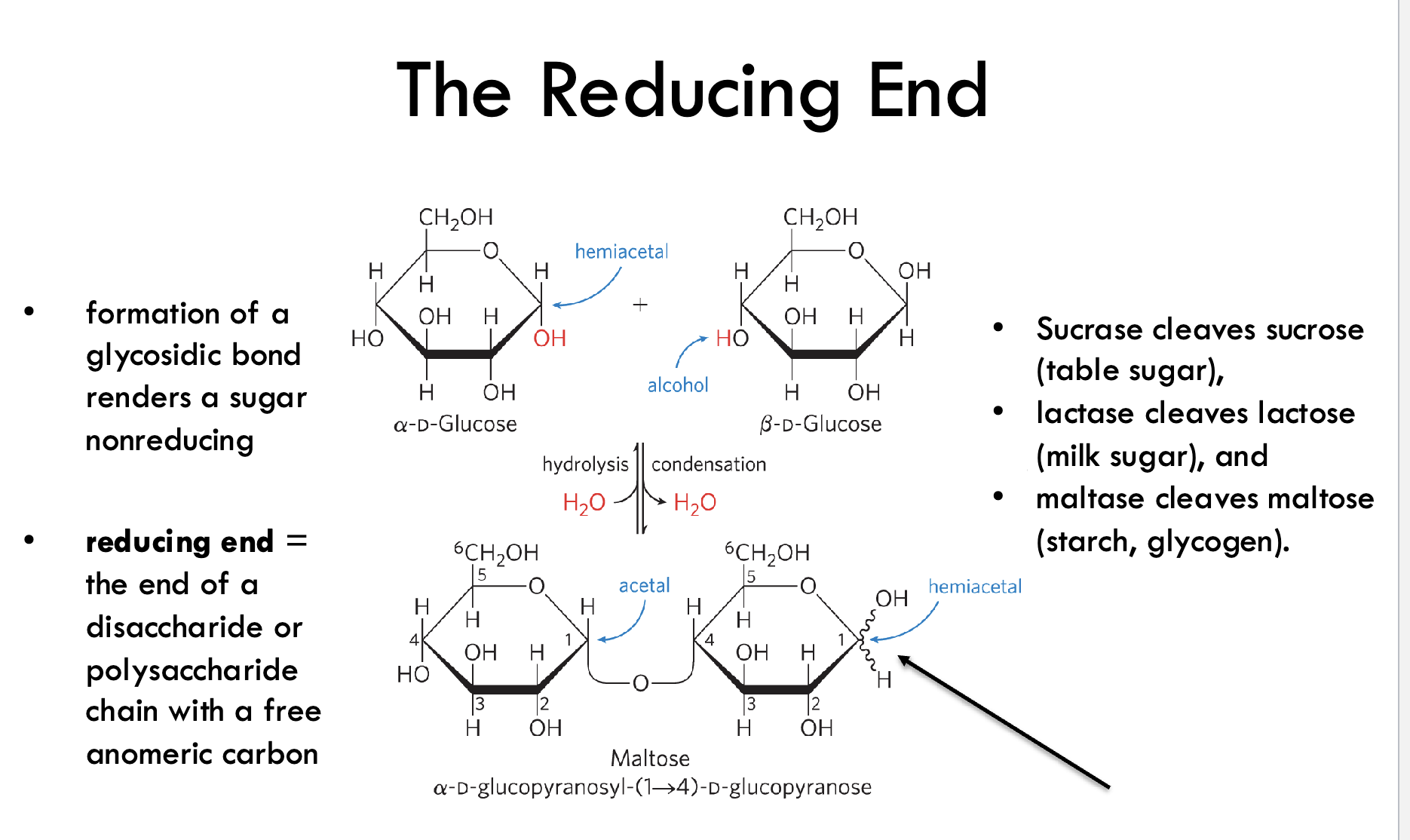
Which enzymes cleave common disaccharides?
Sucrase: cleaves sucrose
Lactase: cleaves lactose
Maltase: cleaves maltose (from starch or glycogen)
What are polysaccharides?
Carbohydrate polymers made up of 20 or more monosaccharide units.
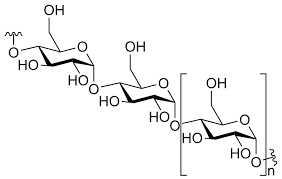
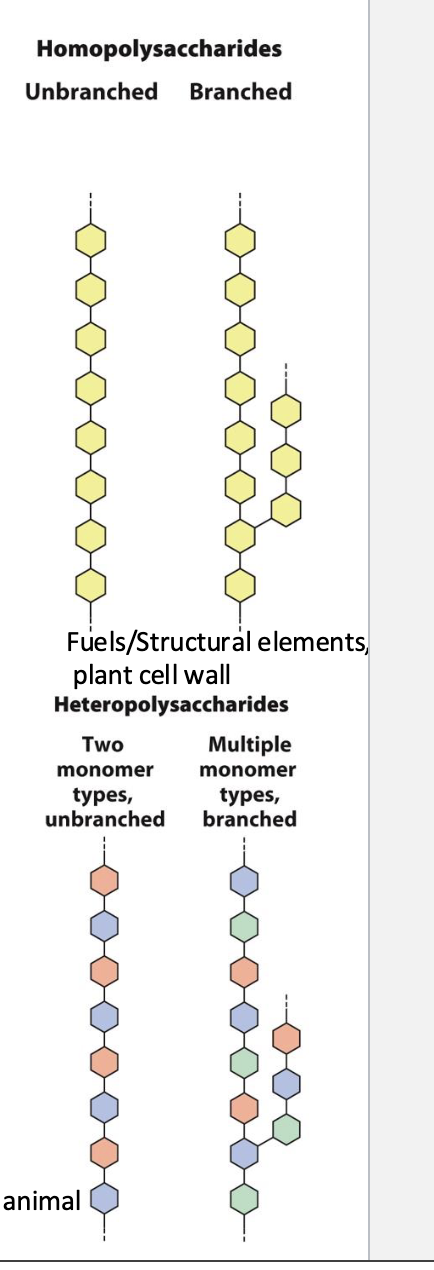
What is the difference between homopolysaccharides and heteropolysaccharides?
Homopolysaccharides: contain only one type of monomer
Heteropolysaccharides: contain two or more types of monomers