Structure of Water and Hydrogen Bonding - Topic 1.1
1/26
Earn XP
Description and Tags
1. What is the chemical structure of water? 2. How does the chemical structure of water result in polarity. 3. How does water's polarity result in cohesion and adhesion through hydrogen bond interactions? 4. What chemical characteristics of water result from its cohesive and adhesive properties? 5. How do living systems depend on the chemical properties of water?
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What determines the properties of a molecule? How does this relate to the components of water?
The subcomponents of biological molecules.
Water is composed of two elements, oxygen and hydrogen, in a 1:2 ratio, respectively.
What is a covalent bond?
It’s the bond type in which atoms share electrons
What are the bond types that are involved in a water molecule?
Covalent bonds, because oxygen is sharing electrons with hydrogen.
Why is sharing not done equally in a water molecule? What does this result in?
Because oxygen is more electronegative compared to hydrogen, resulting in an unequal sharing of electrons between oxygen and hydrogen. (The electrons go closer to the oxygen, making oxygen more negative and hydrogen more positive).
This results in something called polarity
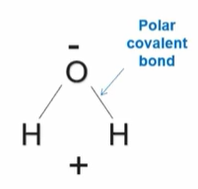
What is polarity that happens in covalent bonds?
It’s when there are differences in atomic electronegativities.
What is another term for a covalent bond that results in polarity.
A polar covalent bond.
What is a hydrogen bond?
A weak bond interaction between the negative and positive regions of two separate molecules.
Since water is polar, what can it do with hydrogen bonds?
It can form hydrogen bonds with other water molecules or with other molecules that have a charge.
What is cohesion?
When 2 of the SAME molecules form hydrogen bonds with each other.
An example of cohesion:
A water molecule bonding with another water molecule
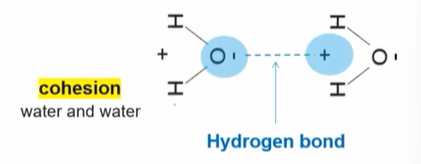
Significance of this picture:
Cohesion
The molecules are positioned so that the negative side of one water molecule is positioned near the positive side of the other, so they can form a hydrogen bond in between.
What is adhesion?
When 2 DIFFERENT molecules form hydrogen bonds with each other.
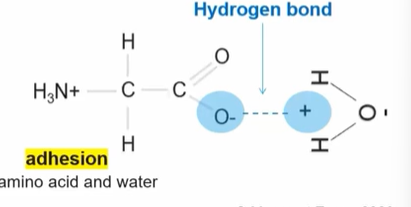
Significance of this picture:
Adhesion
The amino acid also has a dual polarity property, so the negative side of the amino acid is positioned near the hydrogen’s positive side, which allows a hydrogen bond to be formed between those two.
Why are these properties of water that derive out of its polarity important?
Because life depends on them.
Hydrogen bonds between water molecules can result in what?
Surface tension
Cohesion, adhesion, and surface tension allow for water to demonstrate additional chemical behaviors known as what?
Emergent properties.
What is surface tension?
The result of increased hydrogen bond interactions between water molecules only at the surface
Example of surface tension:
A water droplet on a penny.

How is a leaf floating on the surface tension of water important to living things?
Leaves of aquatic plants oftentimes rest on the surface of water, allowing them to have more access to sunlight for processes like photosynthesis
Also showcases high surface tension

Water’s adhesive property gives water what in its liquid state?
A high solvency ability (it’s not totally a “universal solvent”)
Why is salt being dissolved in water an important property that life depends on?
Because organisms must obtain key nutrients from their environment
Considering that living things are made up of 70% water, dissolved materials in the water allow for easy access by cells.

What does water’s cohesive property allow for?
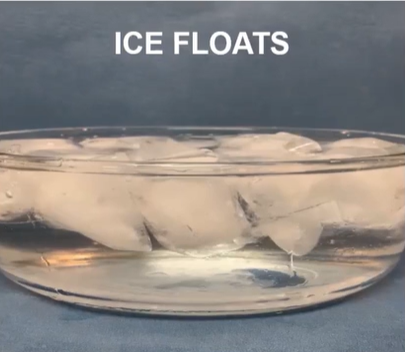
Unique hydrogen bond interactions to occur when water is in a solid state, making ice less dense than liquid water.
How is ice floating to the top of the water and leaving some liquid water underneath an important property for living things?
Because aquatic organisms are still able to live in water in freezing climates because the water will freeze on the surface, leaving liquid water underneath for those organisms to still thrive.
Before water changes chemical state, it can do what?
Its cohesive property allows it to absorb a lot of thermal energy
This helps water resist sudden changes in temperature
High heat capacity
Why do fish and other aquatic organisms depend on high heat capacity?
So that they can maintain an appropriate temperature regulation of their bodies.
What is capillary action?
A result of both the adhesive and cohesive properties of water.
Why is liquid going up a straw when you put the straw in a cup of water important for living things?
Because plants can access water from the soil through this capillary action ability into their roots.